Analysis of Lipids in the Medulla of Japanese Hair and Their Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Hair Samples
2.4. Determination of the Unsaturated Lipid Content of the Medulla
2.5. Determination of the Unsaturated Lipid Content in the Medulla of Hair with Lipid Content Reduced by Acetone or Chloroform Treatment
2.6. Changes in the Glossiness of Hair with Reduced Lipid Content in the Medulla
2.7. Analysis of Hair Lipids Using TLC
2.8. Lipid Spectral Analysis Using Micro-ATR FTIR
2.9. Statistical Analysis
3. Results
3.1. Determination of the Unsaturated Lipid Content of the Medulla
3.2. Determination of the Unsaturated Lipid Content in Hair with Lipid Content Reduced by Acetone and Chloroform Treatment
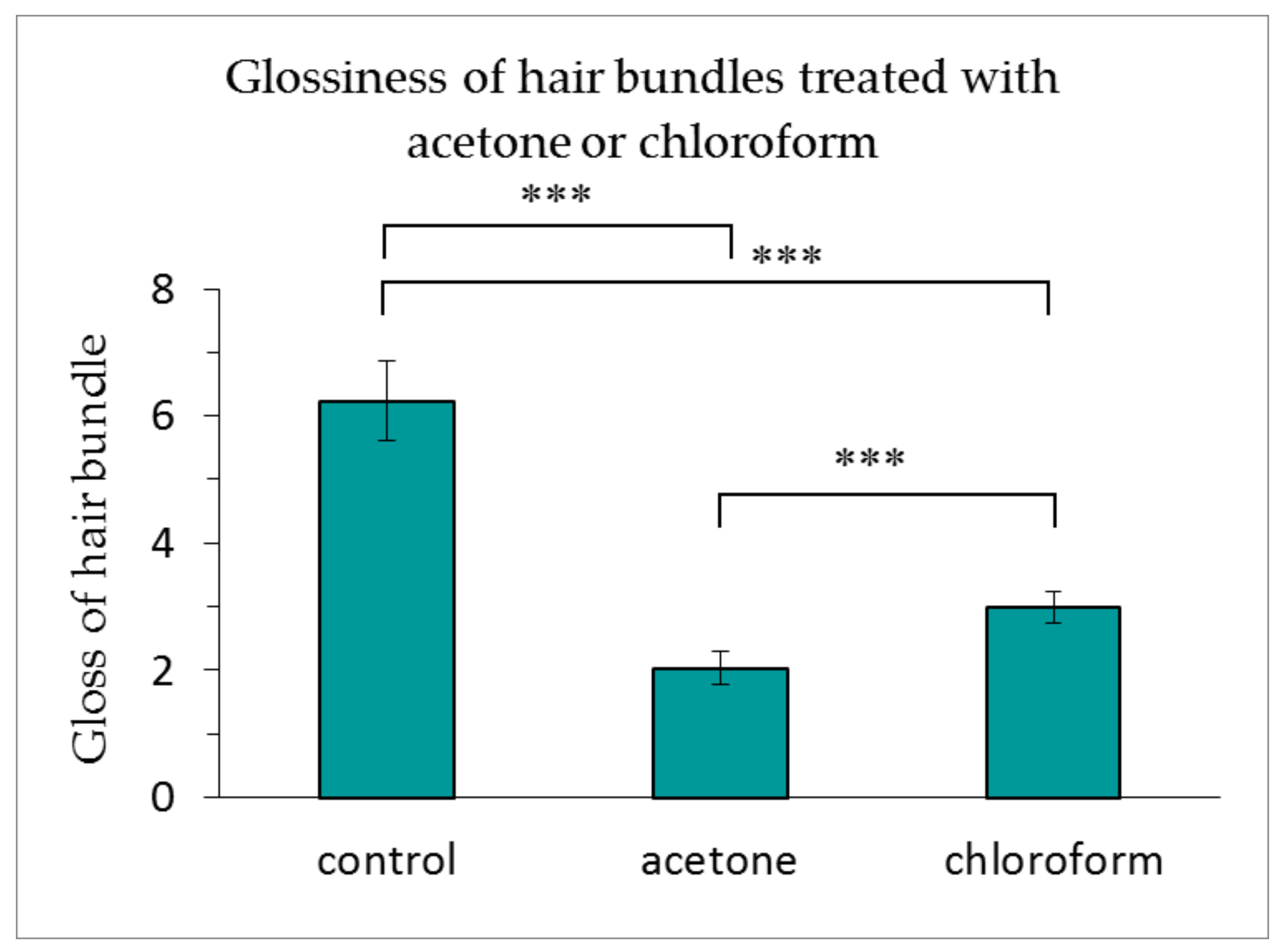
3.3. Changes in the Glossiness of Hair with the Lipids Removed from the Medulla
3.4. Hair Lipid Analysis Using TLC
3.5. Spectral Analysis of Lipids Using Second Derivative Micro-ATR FTIR
3.6. Spectral Analysis of Micro-ATR FTIR
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Robins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nomura, Y. Doubutsumou No Keitaikansatsu. Hokkaido Univ. Collect. Sch. Acad. Pap. 1995, 2, 3–11. (In Japanese) [Google Scholar]
- Clement, J.L.; Hagege, R.; Le Pareaux, A.; Carteaud, J.P. Ultrastructural study of the medulla of mammalian hairs. Scan. Electron Microsc. 1981, Pt 3, 377–382. [Google Scholar]
- Satoh, N. Mechanism of beautifulness of hair and the structure factors. J. Surf. Sci. Soc. Jpn. 2006, 27, 480–484. (In Japanese) [Google Scholar] [CrossRef]
- Nagase, S.; Shibuichi, S.; Ando, K.; Kariya, E.; Satoh, N. Influence of internal structures of hair fiber on hair appearance. I. Light scattering from the porous structure of the medulla of human hair. J. Cosmet. Sci. 2002, 53, 89–100. [Google Scholar] [PubMed]
- Morioka, K. Hair Follicle. Differentiation under the Electron Microscope. An Atlas; Academic Press: Tokyo, Japan, 2005; pp. 25–43. [Google Scholar]
- Brown, F.M. The microscopy of mammalian hair for anthropologists. Proc. Am. Philosoph. Soc. 1942, 85, 250–274. [Google Scholar]
- Hutchinson, P.E.; Thompson, J.R. The size and form of the medulla of human scalp hair is regulated by the hair cycle and cross-sectional size of the hair shaft. Br. J. Dermatol. 1999, 140, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Deedrick, D.W.; Koch, S.L. Microscopy of hair Part I: A practical guide and manual for human hairs. Forensic. Sci. Commun. 2004, 6, 1. [Google Scholar]
- De Cássia Comis Wagner, R.; Kiyohara, P.K.; Silveira, M.; Joekes, I. Electron microscopic observations of human hair medulla. J. Microsc. 2007, 226, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Parakkal, P.F.; Matoltsy, A.G. A study of the differentiation products of the hair follicle cells with the electron microscope. J. Invest. Dermatol. 1964, 43, 23–34. [Google Scholar] [CrossRef]
- Deedrick, D.W.; Koch, S.L. Microscopy of hair Part II: A practical guide and manual for animal hairs. Forensic. Sci. Commun. 2004, 6, 3. [Google Scholar]
- Roth, S.I. Hair and nail. In Ultrastructure of Normal and Abnormal Skin; Zelickson, A.S., Ed.; Lea & Febinger: Philadelphia, PA, USA, 1967; pp. 105–131. [Google Scholar]
- Rogers, G.E.; Kuczek, E.S.; Mackinnon, P.J.; Presland, R.B.; Fietz, M.J. Special biochemical features of the hair follicle. In The Biology of Wool and Hair, Structure and Function of the Hair Follicle; Rogers, G.E., Reis, P.J., Ward, K.A., Marshall, R.C., Eds.; Springer Book Archive., Springer International Publishing AG: Berlin, Germany, 1989; pp. 69–85. [Google Scholar]
- Swift, J.A. The electron histochemistry of cysteine-containing proteins in thin transverse sections of human hair. J. R. Microsc. Soc. 1968, 88, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.L.; Le Pareux, A.; Ceccaldi, P.F. Contribution to hair medulla study (Author transl.). Ann. Dermatol. Venereol. 1981, 108, 849–857. [Google Scholar] [PubMed]
- Matolsty, A.G. A study of the medullary cells of hair. Exp. Cell Res. 1953, 5, 98–110. [Google Scholar]
- Langbein, L.; Yoshida, H.; Praetzel-Wunder, S.; Parry, D.A.; Schweizer, J. The keratins of the human beard hair medulla: The riddle in the middle. J. Investig. Dermatol. 2010, 130, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.I.; Rivett, D.E.; Tucker, D.J.; Hudson, A.H. Analysis of the intercellular and membrane lipids of wool and other animal fibers. Text. Res. J. 1989, 59, 109–113. [Google Scholar] [CrossRef]
- Mazukawa, Y.; Narita, H.; Imokawa, G. Characterization of the lipid composition at the proximal root regions of human hair. J. Cosmet. Chem. 2005, 56, 1–16. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Integral lipids of mammalian hair. Comp. Biochem. Physiol. B Comp. Biochem. 1989, 92, 759–761. [Google Scholar] [CrossRef]
- Weitkamp, A.W.; Smiljanic, A.M.; Rothman, S. The free fatty acids of human hair fat. J. Am. Chem. Soc. 1947, 69, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, G.; Ji, C.; Hoptroff, M.; Jones, A.; Collins, L.Z.; Janssen, H.G. Gas chromatography-mass spectrometry and Raman imaging measurement of squalene content and distribution in human hair. Anal. Bioanal. Chem. 2016, 408, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S. Integral hair lipid in human hair follicle. J. Dermatol. Sci. 2011, 64, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mahrle, G.; Orfanos, C.E. The spongious keratin and the medulla of human scalp hair. Arch. Derm. Res. 1971, 241, 305–316. [Google Scholar] [CrossRef]
- Kreplak, L.; Briki, F.; Duvault, Y.; Doucet, J.; Merigoux, C.; Leroy, F.; Lévêque, J.L.; Miller, L.; Carr, G.L.; Williams, G.P.; et al. Profiling lipids across Caucasian and Afro-American hair transverse cuts, using synchrotron infrared microspectrometry. Int. J. Cosmet. Sci. 2001, 23, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Analysis of ultraviolet radiation wavelengths causing hardening and reduced elasticity of collagen gels in vitro. Cosmetics 2018, 5, 14. [Google Scholar] [CrossRef]
- Maeda, K.; Yamazaki, J.; Okita, N.; Shimotori, M.; Igarashi, K.; Sano, T. Mechanism of cuticle hole development in human hair due to UV-radiation exposure. Cosmetics 2018, 5, 24. [Google Scholar] [CrossRef]
- Maeda, D. Method for Producing Organic Compound Modified Inorganic Filler and Organic Compound Modified Inorganic Filler. Japan Patent Application No. WO2016166823A1, 31 April 2016. [Google Scholar]
- Tipson, R.S. Functional groups in carbohydrates and their derivatives. In Infrared Spectrosc. Carbohydr—A Review of the Literature; Natl. Bur. Stand. Monogr. 110; U. S. Department of Commerce: Washington, DC, USA, 1968; pp. 5–8. [Google Scholar]
- Amankwa, E. Raman and Surface-Enhanced Raman Spectroscopy of Fatty Acids and Lipids. 2016. Available online: http://urn.fi/urn:nbn:fi:uef-20170094 (accessed on 17 March 2108).
- Lee, D.H.; Condrate, R.A.; Lacourse, W.C. FTIR spectral characterization of thin film coatings of oleic acid on glasses. Part II Coatings on glass from different media such as water, alcohol, benzene and air. J. Mater. Sci. 2000, 35, 4961–4970. [Google Scholar] [CrossRef]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Dermatoendocrinol. 2009, 1, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Walde, P. Giant vesicle formation from oleic acid/sodium oleate on glass surfaces induced by adsorbed hydrocarbon molecules. Langmuir 2002, 18, 10509–10511. [Google Scholar] [CrossRef]
- Fukuda, H.; Goto, A.; Yoshioka, H.; Goto, R.; Morigaki, K.; Walde, P. Electron spin resonance study of the pH-induced transformation of micelles to vesicles in an aqueous oleic acid/oleate system. Langmuir 2001, 17, 4223–4231. [Google Scholar] [CrossRef]
- Nawa, E.; Nishigaki, Y.; Yamamoto, D.; Shioi, A. Rhythmic shape change of a vesicle under a pH gradient. Soft Matter 2013, 9, 7832–7842. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshida, S. Distribution of glycolipid and unsaturated fatty acids in human hair. Lipids 2014, 49, 905–917. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ymazaki, J.; Maeda, K. Analysis of Lipids in the Medulla of Japanese Hair and Their Function. Cosmetics 2018, 5, 27. https://doi.org/10.3390/cosmetics5020027
Ymazaki J, Maeda K. Analysis of Lipids in the Medulla of Japanese Hair and Their Function. Cosmetics. 2018; 5(2):27. https://doi.org/10.3390/cosmetics5020027
Chicago/Turabian StyleYmazaki, Jun, and Kazuhisa Maeda. 2018. "Analysis of Lipids in the Medulla of Japanese Hair and Their Function" Cosmetics 5, no. 2: 27. https://doi.org/10.3390/cosmetics5020027
APA StyleYmazaki, J., & Maeda, K. (2018). Analysis of Lipids in the Medulla of Japanese Hair and Their Function. Cosmetics, 5(2), 27. https://doi.org/10.3390/cosmetics5020027





