Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products
Abstract
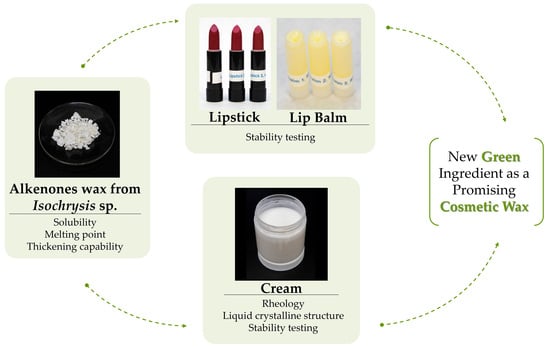
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Solubility
2.2.2. Melting Point Determination
2.2.3. Thickening Capability Test
2.2.4. Lipstick and Lip Balm Formulation
2.2.5. Cream Formulation
2.2.6. Viscosity, Thixotropy, and Rheology
2.2.7. Liquid Crystalline Structure
2.2.8. Stability Testing
3. Results and Discussions
3.1. Determination of Solubility and Thickening Capability Test
3.2. Melting Point Determination
3.3. Lipstick and Lip Balm Stability
3.4. Viscosity, Thixotropy and Rheology
3.5. Liquid Crystalline Structure
3.6. Cream Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Lenick, A.J., Jr. Naturals and Organics in Cosmetics: Trends and Technology; Allured Business Media: Carol Stream, IL, USA, 2010; pp. 1–5. ISBN 978-1-932633-71-9. [Google Scholar]
- Sahota, A. Sustainability: How the Cosmetic Industry is Greening Up; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Organic Cosmetics Market Research. Available online: https://www.cosmeticsandtoiletries.com/networking/news/company/Organic-Cosmetics-Market-Research-372973201.html (accessed on 18 May 2018).
- Rao, S. Green and sustainable ingredients from biotransformation and biofermentation. In Harry’s Cosmetology, 9th ed.; Rosen, M.R., Ed.; Chemical Publishing Co., Inc.: Los Angeles, CA, USA, 2015; Volume 2, pp. 643–653. ISBN 978-0-8206-01779. [Google Scholar]
- Pande, A.; Majeed, M. Multi-functional botanicals for topical applications. In Harry’s Cosmetology; Rosen, M.R., Ed.; Chemical Publishing Co., Inc.: Los Angeles, CA, USA, 2015; Volume 2, pp. 655–695. ISBN 978-0-8206-01779. [Google Scholar]
- Offredo, H. Marine ingredients for skin care: An ocean of resources. In Harry’s Cosmetology; Rosen, M.R., Ed.; Chemical Publishing Co., Inc.: Los Angeles, CA, USA, 2015; Volume 2, pp. 891–907. ISBN 978-0-8206-01779. [Google Scholar]
- O’Neil, G.W.; Carmichael, C.A.; Goepfert, T.J.; Fulton, J.M.; Knothe, G.; Pui Ling Lau, C.; Lindell, S.R.; Mohammady, N.G.E.; Van Mooy, B.A.S.; Reddy, C.M. Beyond fatty acid methyl esters: Expanding the renewable carbon profile with alkenones from Isochrysis sp. Energy Fuels 2012, 26, 2434–2441. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Williams, J.R.; Wilson-Peltier, J.; Knothe, G.; Reddy, C.M. Experimental protocol for biodiesel production with isolation of alkenones as coproducts from commercial isochrysis algal biomass. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, G.W.; Williams, J.R.; Craig, A.M.; Nelson, R.K.; Gosselin, K.M.; Reddy, C.M. Accessing monomers, surfactants, and the queen bee substance by acrylate cross-metathesis of long-chain alkenones. J. Am. Oil Chem. Soc. 2017, 94, 831–840. [Google Scholar] [CrossRef]
- García, J.L.; Vicente, M.D.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Mourelle, L.M.; Gomez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Susilowati, R.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J.-L. Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone tahiti. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 1–6. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Culler, A.R.; Williams, J.R.; Burlow, N.P.; Gilbert, G.J.; Carmichael, C.A.; Nelson, R.K.; Swarthout, R.F.; Reddy, C.M. Production of jet fuel range hydrocarbons as a coproduct of algal biodiesel by butenolysis of long-chain alkenones. Energy Fuels 2015, 29, 922–930. [Google Scholar] [CrossRef]
- Candelilla Wax and Substitution Options. Available online: https://www.brenntag.com/media/documents/bsi/product_data_sheets/life_science/koster_kuenen_food_waxes/candelilla_wax_alternatives.pdf (accessed on 1 April 2018).
- Demand for Natural Wax Increases. Available online: https://www.icis.com/resources/news/2008/08/18/9149059/demand-for-natural-wax-increases/ (accessed on 1 Arpil 2018).
- Global Candelilla Wax Industry 2016 Market Research Report. Available online: http://www.marketresearchstore.com/report/global-candelilla-wax-industry-2016-market-research-report-63520 (accessed on 1 April 2018).
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Mahlia, T.M.I.; Kusumo, F. Optimization of extraction of lipid from Isochrysis galbana microalgae species for biodiesel synthesis. Energy Source Part A 2017, 39, 1167–1175. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; Vishakha, J.; Brinda, S.; Anil, K.; Kumar, G.V. Microalgae-based pharmaceuticals and nutraceuticals: An emerging field with immense market potential. ChemBioEng Rev. 2017, 4, 257–272. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Haimeur, A.; Guéno, F.; Meskini, N.; Tremblin, G. Marine microalgae used as food supplements and their implication in preventing cardiovascular diseases. OCL 2015, 22, D409. [Google Scholar] [CrossRef]
- Callens, C.; Ceulemans, J.; Ludwig, A.; Foreman, P.; Remon, J.P. Rheological study on mucoadhesivity of some nasal powder formulations. Eur. J. Pharm. Biopharm. 2003, 55, 323–328. [Google Scholar] [CrossRef]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological assessment of the nature of interactions between mucoadhesive polymers and a homogenised mucus gel. Biomaterials 1998, 19, 1083–1092. [Google Scholar] [CrossRef]
- Tadros, T.; Leonard, S.; Taelman, M.; Verboom, C.; Wortel, V. Correlating the structure and rheology of liquid crystalline phases in emulsions. Cosmet. Toilet. 2006, 121, 89–94. [Google Scholar]
- Tadros, T.T. Formulations: In Cosmetic and Personal Care; de Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Pajdzik, L.A.; Glazer, A.M. Three-dimensional birefringence imaging with a microscope tilting-stage. I. Uniaxial crystals. J. Appl. Crystallogr. 2006, 39, 326–337. [Google Scholar] [CrossRef]
- Bioavailability. Available online: https://cosmetic-industry.com/en/bioavailability.html (accessed on 1 May 2018).
- Wichers, J.; Abbott, S. Formulating for EfficacyTM, Help and Manual. 1.3.07. Available online: https://www.jwsolutionssoftware.com/ (accessed on 5 June 2018).
- Liu, W.; Wang, X.; Lai, W.; Yan, T.; Wu, Y.; Wan, M.; Yi, J.; Matsui, M.S. Sunburn protection as a function of sunscreen application thickness differs between high and low spfs. Photodermatol. Photoimmunol. Photomed. 2012, 28, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Teramura, T.; Mizuno, M.; Asano, H.; Naito, N.; Arakane, K.; Miyachi, Y. Relationship between sun-protection factor and application thickness in high-performance sunscreen: Double application of sunscreen is recommended. Clin. Exp. Dermatol. 2012, 37, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Dweck, A.C.; Burnham, C.A. Moulding techniques in lipstick manufacture: A comparative evaluation. Int. J. Cosmet. Sci. 1980, 2, 143–173. [Google Scholar] [CrossRef] [PubMed]
- El-Nokaly, M.; Vatter, M.L.; Walling, D.W.; Leatherbury, N.C.; Peterson, C.L. Non-Sweating Lipsticks. U.S. Patent, US5843407A, 18 October 1993. [Google Scholar]
- Fernandes, A.R.; Dario, M.F.; Pinto, C.A.S.d.O.; Kaneko, T.M.; Baby, A.R.; Velasco, M.V.R. Stability evaluation of organic lip balm. Braz. J. Pharm. Sci. 2013, 49, 293–299. [Google Scholar] [CrossRef]
- Wei, K.; Thomas, C.; Taylor, R.; Tanner, P.; Stella, Q.; Smith, E.; Clapp, M. Multi-Phase Personal Care Composition. U.S. Patent, US20050100570A1, 1 November 2005. [Google Scholar]
- Rheology School. Available online: http://www.brookfield.co.uk/education/rheology_papers_benchmark_products.asp#top (accessed on 17 May 2018).
- Using the Power Law Model to Quantify Shear Thinning Behavior on a Rotational Rheometer. Available online: https://www.azom.com/article.aspx?ArticleID=11624 (accessed on 17 May 2018).
- Brummer, R. Rheology Essentials of Cosmetic and Food Emulsions; Springer: Berlin, Germany, 2006. [Google Scholar]
- Alam, M.M.; Aramaki, K. Glycerol effects on the formation and rheology of hexagonal phase and related gel emulsion. J. Colloid Interface Sci. 2009, 336, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Mcgee, T.; Sgaramella, R.P.; Vedantam, V.K. Preparation of an Emulsion Comprising Lamellar Liquid Crystal (llc) Particles Containing Fragrance. Patent WO2008061384 A1, 29 May 2008. [Google Scholar]




| INCI Name | Lipstick 1 % (w/w) | Lipstick 2 % (w/w) | Lipstick 3 % (w/w) |
|---|---|---|---|
| Phase A | |||
| Castor oil | 25.8 | 25.8 | 25.8 |
| Triglyceride | 16.0 | 16.0 | 16.0 |
| Isoeicosane | 17.0 | 17.0 | 17.0 |
| Meadowfoam seed oil | 5.0 | 5.0 | 5.0 |
| Lanolin alcohol | 5.0 | 5.0 | 5.0 |
| Microcrystalline wax | 2.0 | 2.0 | - |
| Ozokerite wax | 5.0 | - | 5.0 |
| Alkenone wax | - | 5.0 | 2.0 |
| Candelilla wax | 7.0 | 7.0 | 7.0 |
| Carnauba wax | 3.0 | 3.0 | 3.0 |
| Phase B | |||
| Red 7 (and) castor oil | 2.0 | 2.0 | 2.0 |
| Mica pearl white | 11.0 | 11.0 | 11.0 |
| Phase C | |||
| Tocopherol | 0.2 | 0.2 | 0.2 |
| Propylene glycol (and) diazolidinyl urea (and) methylparaben (and) propylparaben | 1.0 | 1.0 | 1.0 |
| INCI Name | Lip Balm 1 % (w/w) | Lip Balm 2 % (w/w) | Lip Balm 3 % (w/w) |
|---|---|---|---|
| Phase A | |||
| Petrolatum | 45.5 | 45.5 | 45.5 |
| Jojoba oil | 22.0 | 22.0 | 22.0 |
| Almond oil | 13.0 | 13.0 | 13.0 |
| Microcrystalline wax | 8.0 | 8.0 | - |
| Ozokerite | 8.0 | - | 8.0 |
| Alkenones | - | 8.0 | 8.0 |
| Avocado butter | 3.0 | 3.0 | 3.0 |
| Phase B | |||
| Tocopherol | 0.5 | 0.5 | 0.5 |
| INCI Name | Cream 1 % (w/w) | Cream 2 % (w/w) | Cream 3 % (w/w) | Cream 4 % (w/w) |
|---|---|---|---|---|
| Phase A | ||||
| Water | 68.8 | 68.8 | 68.8 | 68.8 |
| Propanediol | 5 | 5 | 5 | 5 |
| Xanthan gum | 0.2 | 0.2 | 0.2 | 0.2 |
| Phase B | ||||
| Isopropyl isostearate | 15 | 15 | 15 | 15 |
| Alkenones | 5 | - | - | - |
| Cetyl alcohol | - | 5 | - | - |
| Stearic acid | - | - | 5 | - |
| Glyceryl monostearate | - | - | - | 5 |
| Sorbitan oleate | 1.5 | 1.5 | 1.5 | 1.5 |
| Polysorbate 80 | 3.5 | 3.5 | 3.5 | 3.5 |
| Phase C | ||||
| Propylene glycol (and) Diazolidinyl urea (and) Methyl paraben (and) Propyl paraben | 1 | 1 | 1 | 1 |
| Ingredient | δD | δP | δH | MVol | ISG | SolS | LogK |
|---|---|---|---|---|---|---|---|
| 37:3 methyl alkenone | 16.3 | 0.6 | 2.5 | 613 | 57.2 | 0.3 | 16.81 |
| 37:2 methyl alkenone | 16.2 | 0.7 | 2.2 | 621 | 58.8 | 0.3 | 15.83 |
| 38:2 ethyl alkenone | 16.1 | 0.8 | 2.2 | 638 | 60.1 | 0.3 | 16.27 |
| Mixture combined according to [9] | 16.2 | 0.7 | 2.3 | 622 | 58.5 | 0.3 | - |
| Sample | Viscosity at 1 s−1 (cP) | Viscosity at 100 s−1 (cP) | G’ at 10 rad/s (Pa) |
|---|---|---|---|
| Cream 1 | 1470 ± 10 | 54 ± 1 | 11 ± 1 |
| Cream 2 | 6950 ± 110 | 147 ± 1 | 45 ± 10 |
| Cream 3 | 8460 ± 570 | 169 ± 1 | 1560 ± 1100 |
| Cream 4 | 19,400 ± 2500 | 400 ± 10 | 4710 ± 4300 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntosh, K.; Smith, A.; Young, L.K.; Leitch, M.A.; Tiwari, A.K.; Reddy, C.M.; O’Neil, G.W.; Liberatore, M.W.; Chandler, M.; Baki, G. Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products. Cosmetics 2018, 5, 34. https://doi.org/10.3390/cosmetics5020034
McIntosh K, Smith A, Young LK, Leitch MA, Tiwari AK, Reddy CM, O’Neil GW, Liberatore MW, Chandler M, Baki G. Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products. Cosmetics. 2018; 5(2):34. https://doi.org/10.3390/cosmetics5020034
Chicago/Turabian StyleMcIntosh, Kyle, Amber Smith, Lisa K. Young, Michael A. Leitch, Amit K. Tiwari, Christopher M. Reddy, Gregory W. O’Neil, Matthew W. Liberatore, Mark Chandler, and Gabriella Baki. 2018. "Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products" Cosmetics 5, no. 2: 34. https://doi.org/10.3390/cosmetics5020034
APA StyleMcIntosh, K., Smith, A., Young, L. K., Leitch, M. A., Tiwari, A. K., Reddy, C. M., O’Neil, G. W., Liberatore, M. W., Chandler, M., & Baki, G. (2018). Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products. Cosmetics, 5(2), 34. https://doi.org/10.3390/cosmetics5020034