Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Clinical Evaluation at Baseline
2.3. Transonychial Water-Loss Evaluation
2.4. Skin Multispectral Imaging Evaluation
2.5. Hair Mineral Analysis
2.6. Subjective Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Transonychial Water-Loss Evaluation
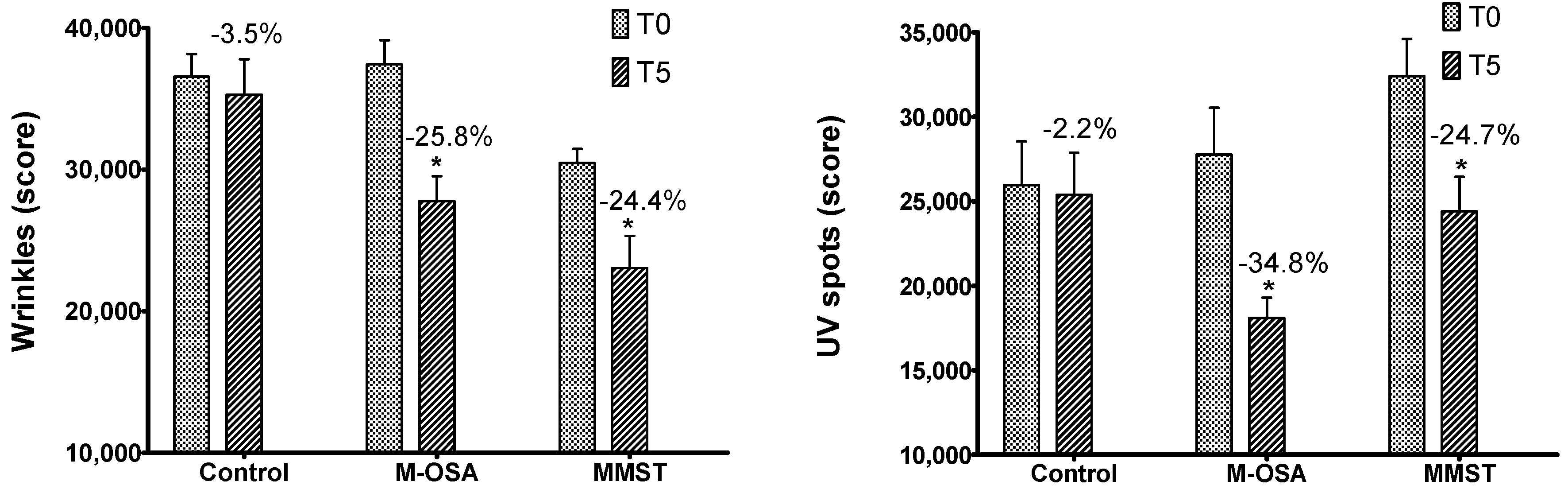
3.2. Skin Multispectral Imaging Evaluation
3.3. Hair Mineral Analysis
3.4. Subjective Evaluation
3.5. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jugdaohsingh, R.; Calomme, M.R.; Robinson, K.; Nielsen, F.; Anderson, S.H.; D’haese, P.; Geusens, P.; Loveridge, N.; Thompson, R.P.; Powell, J.J. Increased longitudinal growth in rats on a silicon-depleted diet. Bone 2008, 43, 596–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reffitt, D.M.; Jugdaohsingh, R.; Thompson, R.P.; Powell, J.J. Silicic acid: Its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion. J. Inorg. Biochem. 1999, 76, 141–147. [Google Scholar] [CrossRef]
- Martin, K.R. Silicon: The Health Benefits of a Metalloid. In Interrelations between Essential Metal Ions and Human Diseases; Siegel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 451–473. [Google Scholar]
- Jurkic, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and therapeutic effects of orthosilicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metabol. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Bissé, E.; Epting, T.; Beil, A.; Lindinger, G.; Lang, H.; Wieland, H. Reference values for serum silicon in adults. Anal. Biochem. 2005, 337, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.; Calomme, M.; Timchenko, A.; Paepe, K.D.; Demeester, N.; Rogiers, V.; Clarys, P.; Berghe, D.V. Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin. Arch. Dermatol. Res. 2005, 297, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Calomme, M.R.; Vanden Berghe, D.A. Supplementation of calves with stabilized orthosilicic acid. Effect on the Si, Ca, Mg, and P concentrations in serum and the collagen concentration in skin and cartilage. Biol. Trace Elem. Res. 1997, 56, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lassus, A. Colloidal silicic acid for oral and topical treatment of aged skin, fragile hair and brittle nails in females. J. Int. Med. Res. 1993, 21, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsingh, R.; Berghe, D.A.V.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to Calcium/Vitamin D3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Musculoskelet. Disord. 2008, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Wickett, R.R.; Kossmann, E.; Barel, A.; Demeester, N.; Clarys, P.; Berghe, D.V.; Calomme, M. Effect of oral intake of choline-stabilized orthosilicic acid on hair tensile strength and morphology in women with fine hair. Arch. Dermatol. Res. 2007, 299, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon. In Handbook of Nutritionally Essencial Mineral Elements; O’Dell, B.L., Sunde, R.A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 603–618. [Google Scholar]
- Jugdaohsingh, R.; Reffitt, D.M.; Oldham, C.; Day, J.P.; Fifield, L.K.; Thompson, R.P.; Powell, J.J. Oligomeric but not monomeric silica prevents aluminum absorption in humans. Am. J. Clin. Nutr. 2000, 71, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jugdaohsingh, R.; Anderson, S.H.; Tucker, K.L.; Elliott, H.; Kiel, D.P.; Thompson, R.P.; Powell, J.J. Dietary silicon intake and absorption. Am. J. Clin. Nutr. 2002, 75, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripanyakorn, S.; Jugdaohsingh, R.; Dissayabutr, W.; Anderson, S.H.; Thompson, R.P.; Powell, J.J. The comparative absorption of silicon from different foods and food supplements. Br. J. Nutr. 2009, 102, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Hui, M.; Anderson, S.H.; Kinrade, S.D.; Powell, J.J. The silicon supplement ‘Monomethylsilanetriol’ is safe and increases the body pool of silicon in healthy Pre-menopausal women. Nutr. Metabol. 2013, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen Vitello Kalil, C.L.; Campos, V.; Cignachi, S.; Favaro Izidoro, J.; Prieto Herman Reinehr, C.; Chaves, C. Evaluation of cutaneous rejuvenation associated with the use of ortho-silicic acid stabilized by hydrolyzed marine collagen. J. Cosmet. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon. In Biochemistry of the Essential Ultratrace Elements; Frieden, E., Ed.; Plenum Press: New York, NY, USA, 1984; pp. 257–291. [Google Scholar]
- Le Loch, R.S. El Silicio Orgánico; Editorial Sirio: Málaga, Spain, 2010. [Google Scholar]
- Seaborn, C.D.; Nielsen, F.H. Silicon: A nutritional beneficence for bones, brains and blood Vessels? Nutr. Today 1993, 28, 13–18. [Google Scholar] [CrossRef]
- Farrer, J.H.; Rajfer, J. Silicate Urolithiasis. J. Urol. 1994, 132, 739–740. [Google Scholar] [CrossRef]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the possible nutritional importance of silicon. J. Trace Elem. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Popplewell, J.F.; King, S.J.; Day, J.P.; Ackrill, P.; Fifield, L.K.; Cresswell, R.G.; Di Tada, M.L.; Liu, K. Kinectics of uptake and elimination of silicic acid by a human of silicic by a human subjects: A novel application of 32Si and accelerator mass spectrometry. J. Inorg. Biochem. 1998, 69, 177–180. [Google Scholar] [CrossRef]
- Pruksa, S.; Siripinyanond, A.; Powell, J.J.; Jugdaohsingh, R. A silicon study in human volunteers; a study to establish the variance in silicon excretion versus intake. Nutr. Metabol. 2014, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; Arola, L. Report of Results PR 303122014—Nutritional Bioavailability Study of Three Silica Rich Food Complements; Centre Tecnològic de Nutrición y Salut: Reus, Espanha, 2015. [Google Scholar]
- Pozebon, D.; Scheffler, G.L.; Dressler, V.L. Elemental hair analysis: A review of procedures and applications. Anal. Chim. Acta 2017, 992, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Hernández, A.F.; Márquez, C.; Femia, P.; Olmedo, P.; López-Guarnido, O.; Pla, A. Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci. Total Environ. 2011, 409, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Moreda-Piñeiro, J. Determination of major and trace elements in human scalp hair by pressurized-liquid extraction with acetic acid and inductively coupled plasma–optical-emission spectrometry. Anal. Bioanal. Chem. 2007, 388, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Davenward, S.; Bentham, P.; Wright, J.; Crome, P.; Job, D.; Polwart, A.; Exley, C. Silicon-Rich Mineral Water as a Non-Invasive Test of the ‘Aluminum Hypothesis’ in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, J.A.; Moore, P.B.; Ferrier, I.N.; Lilley, J.S.; Barker, J.; Templar, J.; Day, J.P. Effect of silicon on gastrointestinal absorption of aluminium. Lancet 1993, 342, 211–212. [Google Scholar] [CrossRef]
- Frisardi, V.; Solfrizzi, V.; Capurso, C.; Kehoe, P.G.; Imbimbo, B.P.; Santamato, A.; Dellegrazie, F.; Seripa, D.; Pilotto, A.; Capurso, A.; et al. Aluminum in the diet and Alzheimer’s disease: From current epidemiology to possible disease-modifying treatment. J. Alzheimer’s Dis. 2010, 20, 17–30. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, M.J.; Meseguer, I.; Sanchez-Reus, M.I.; Schultz, A.; Olivero, R.; Benedí, J.; Sánchez-Muniz, F.J. Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; Campbell, A. Aluminum and Neurodegenerative Diseases. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2017; pp. 131–156. [Google Scholar]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Aluminum should now be considered a primary etiological factor in Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef]
- Carlisle, E. The nutritional essentiality of silicon. Nutr. Rev. 1982, 40, 193–198. [Google Scholar] [CrossRef] [PubMed]





| Characteristic | G1 | G2 | G3 |
|---|---|---|---|
| Age (M ± SD) | 48.5 ± 5.1 | 52.8 ± 5.9 | 47.6 ± 7.1 |
| Phototype (n, %) | |||
| III | 8, 47.0 | 5, 29.4 | 7, 41.2 |
| IV | 8, 47.0 | 11, 64.7 | 8, 47.0 |
| V | 1, 6.0 | 1, 5.9 | 2, 11.8 |
| Photodamage (n, %) | |||
| Light | 9, 52.9 | 1, 5.9 | 7, 41.2 |
| Moderate | 7, 41.2 | 15, 88.2 | 9, 52.9 |
| Severe | 1, 5.9 | 1, 5.9 | 1, 5.9 |
| Skin oiliness (n, %) | |||
| Dry | 2, 11.8 | 1, 5.9 | 0, 0.0 |
| Normal | 9, 52.9 | 10, 58.8 | 10, 58.8 |
| Oily | 6, 35.3 | 6, 35.3 | 7, 41.2 |
| Wrinkles (n, %) | |||
| Fine | 4, 23.6 | 2, 11.8 | 6, 35.3 |
| Medium | 10, 58.8 | 7, 41.2 | 6, 35.3 |
| Deep | 3, 17.6 | 8, 47.0 | 5, 29.4 |
| Presence of spots (n, %) | 17, 100.0 | 17, 100.0 | 17, 100.0 |
| Group | Parameter | T3 (M ± SD) | T5 (M ± SD) | p |
|---|---|---|---|---|
| G1 (n = 17) | Nails | |||
| Yellowing | 0.88 ± 1.73 | 5.13 ± 3.68 | 0.03 | |
| G2 (n = 17) | Skin | |||
| Oiliness | 3.50 ± 3.07 | 6.00 ± 3.12 | 0.02 | |
| Darkened areas | 4.40 ± 2.84 | 7.30 ± 1.49 | 0.02 | |
| Whitening velocity | 3.90 ± 2.69 | 6.60 ± 2.63 | 0.02 | |
| Nails | ||||
| Stains | 3.30 ± 3.71 | 6.20 ± 3.05 | 0.03 | |
| G3 (n = 17) | Skin | |||
| Darkened areas | 3.88 ± 2.42 | 7.63 ± 2.20 | 0.04 | |
| Wrinkles/Expression lines (lips) | 4.00 ± 2.78 | 6.11 ± 2.03 | 0.03 | |
| Wrinkles/Expression lines (forehead) | 4.50 ± 3.27 | 6.90 ± 1.73 | 0.01 | |
| Hair | ||||
| Vitality | 6.67 ± 2.61 | 8.58 ± 1.00 | 0.03 | |
| Nails | ||||
| Yellowing | 3.33 ± 4.56 | 8.22 ± 2.68 | 0.05 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.O.; Freire, É.S.; Polonini, H.C.; Da Silva, P.J.L.C.; Brandão, M.A.F.; Raposo, N.R.B. Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair. Cosmetics 2018, 5, 41. https://doi.org/10.3390/cosmetics5030041
Ferreira AO, Freire ÉS, Polonini HC, Da Silva PJLC, Brandão MAF, Raposo NRB. Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair. Cosmetics. 2018; 5(3):41. https://doi.org/10.3390/cosmetics5030041
Chicago/Turabian StyleFerreira, Anderson Oliveira, Érika Santos Freire, Hudson Caetano Polonini, Paulo José Lopes Cândido Da Silva, Marcos Antônio Fernandes Brandão, and Nádia Rezende Barbosa Raposo. 2018. "Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair" Cosmetics 5, no. 3: 41. https://doi.org/10.3390/cosmetics5030041
APA StyleFerreira, A. O., Freire, É. S., Polonini, H. C., Da Silva, P. J. L. C., Brandão, M. A. F., & Raposo, N. R. B. (2018). Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair. Cosmetics, 5(3), 41. https://doi.org/10.3390/cosmetics5030041





