Cosmetic Formulation Based on an Açai Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Total Phenol Content Determination and Evaluation of Antioxidant Capacity
- (1)
- 50 mL 0.3 M acetate buffer pH 3.6 (1.23 g of sodium acetate in 50 mL of water acidifying with acetic acid);
- (2)
- 5 mL of stock solution of 5 mM TPTZ (2,4,6-tripyridyl-s-triazine) (15.6 mg) in 40 mM HCl;
- (3)
- 5 mL of 5 mM FeCl3·6 H2O (16.2 mg) in 40 mM HCl.
2.3. Preparation of the O/W Emulsion
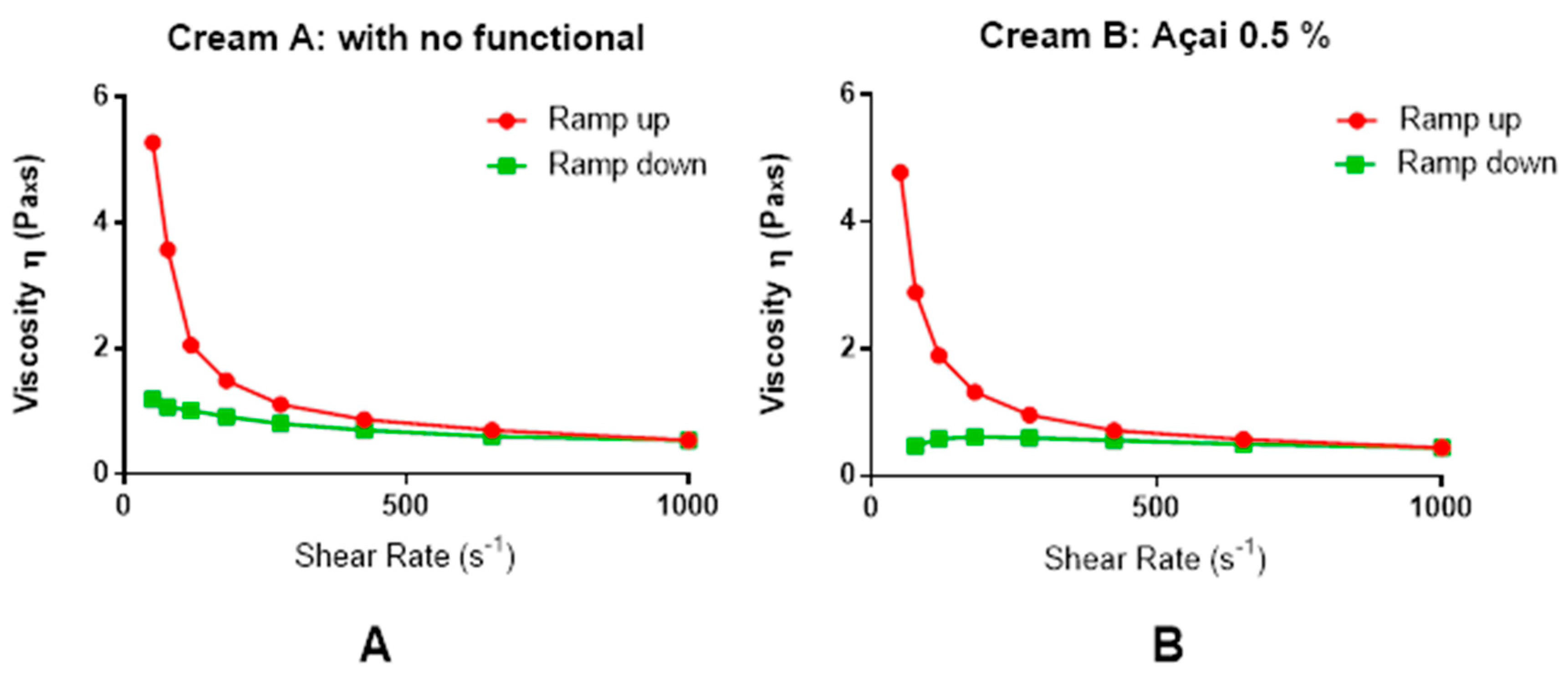
2.4. Physicochemical Characterization of O/W Cream
2.5. Challenge Test
2.6. In Vitro Experiments
2.7. Assessment of the Metabolic Activity of Viable Cells (MTS)
2.8. Total Antioxidant Capacity (TAC)
2.9. Local Compatibility Test with Human Skin (Irritant Potential)
2.10. Açai Formulations Sensory Analysis
2.11. Statistical Analysis
3. Results and Discussion
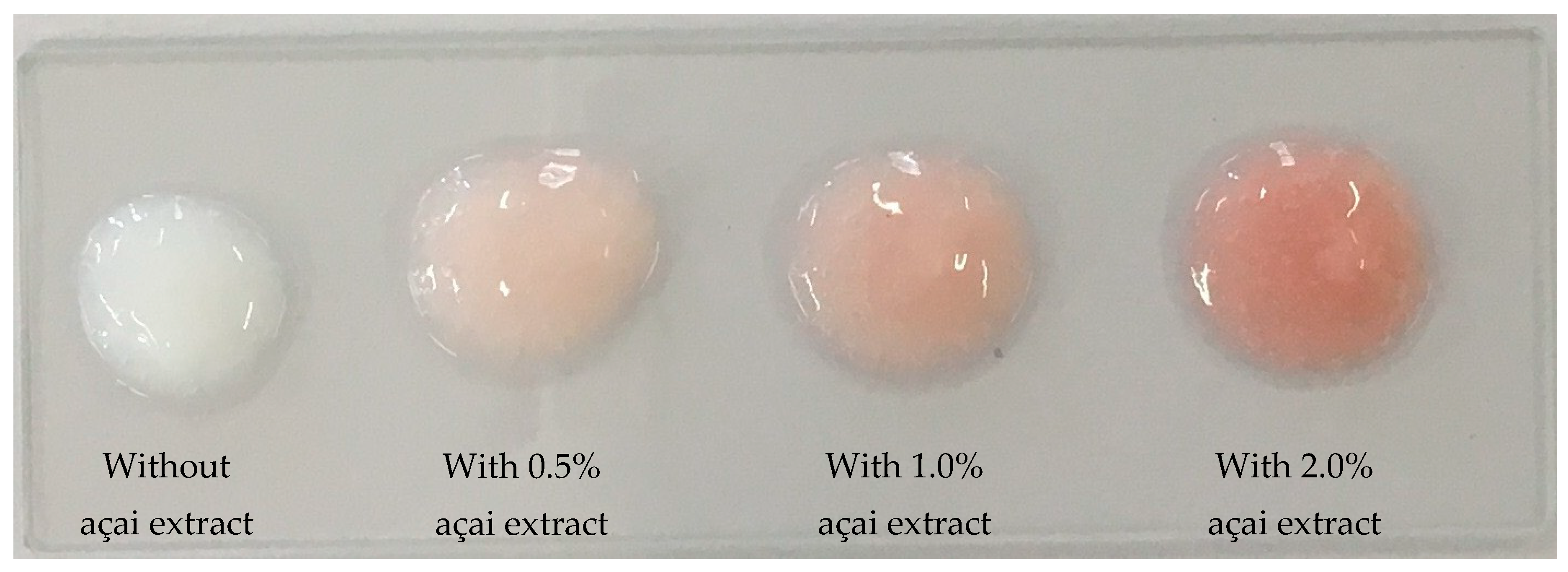
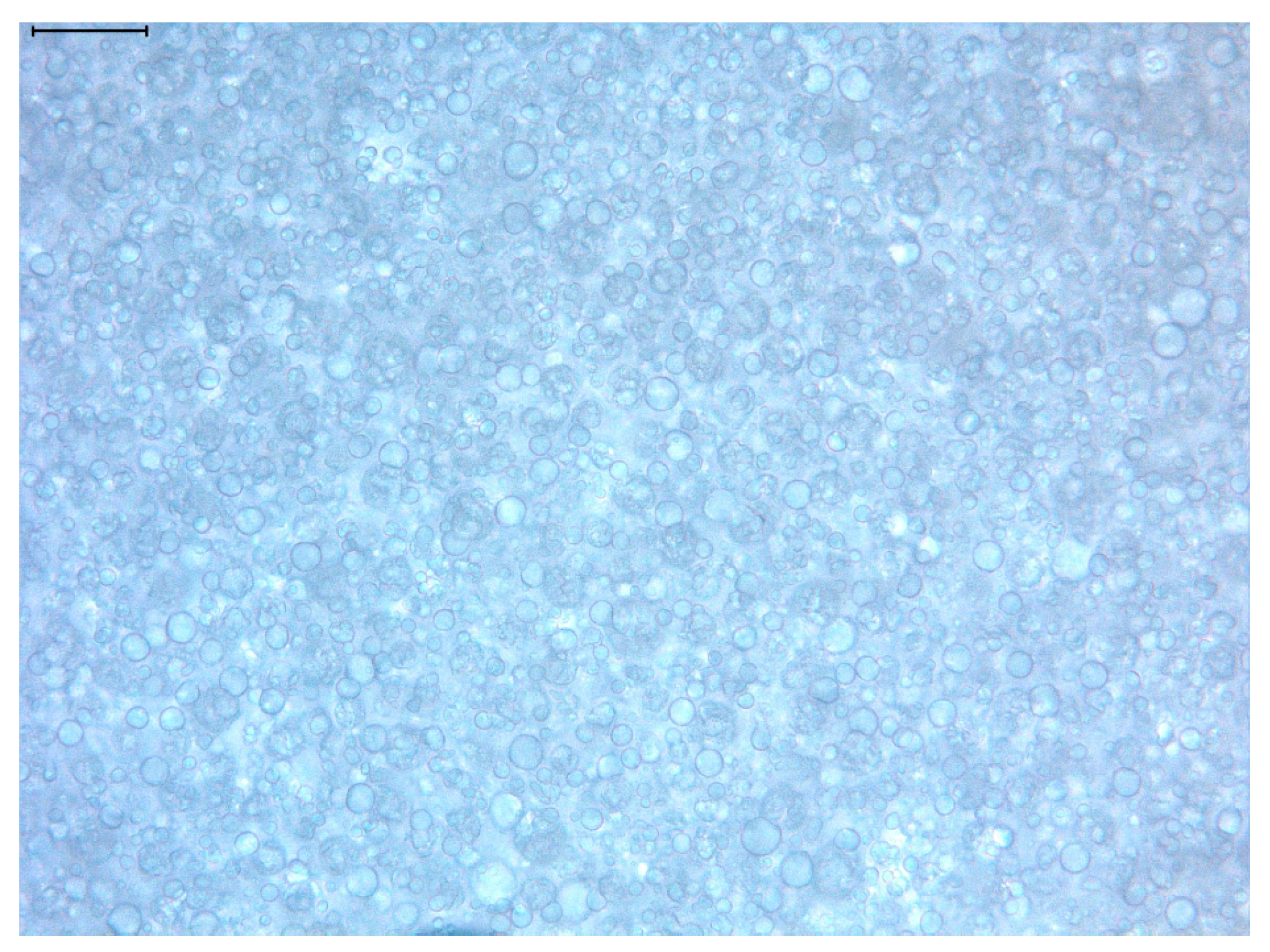
3.1. General Characteristics of the Açai Formulation
3.2. Total Phenol Content and Antioxidant Activity of O/W Emulsions
3.3. Effects of Açai on Metabolic Activity of Viable BJ-5TA Human Fibroblasts
3.4. Antioxidant Activity of Açai on BJ-5TA Human Fibroblasts
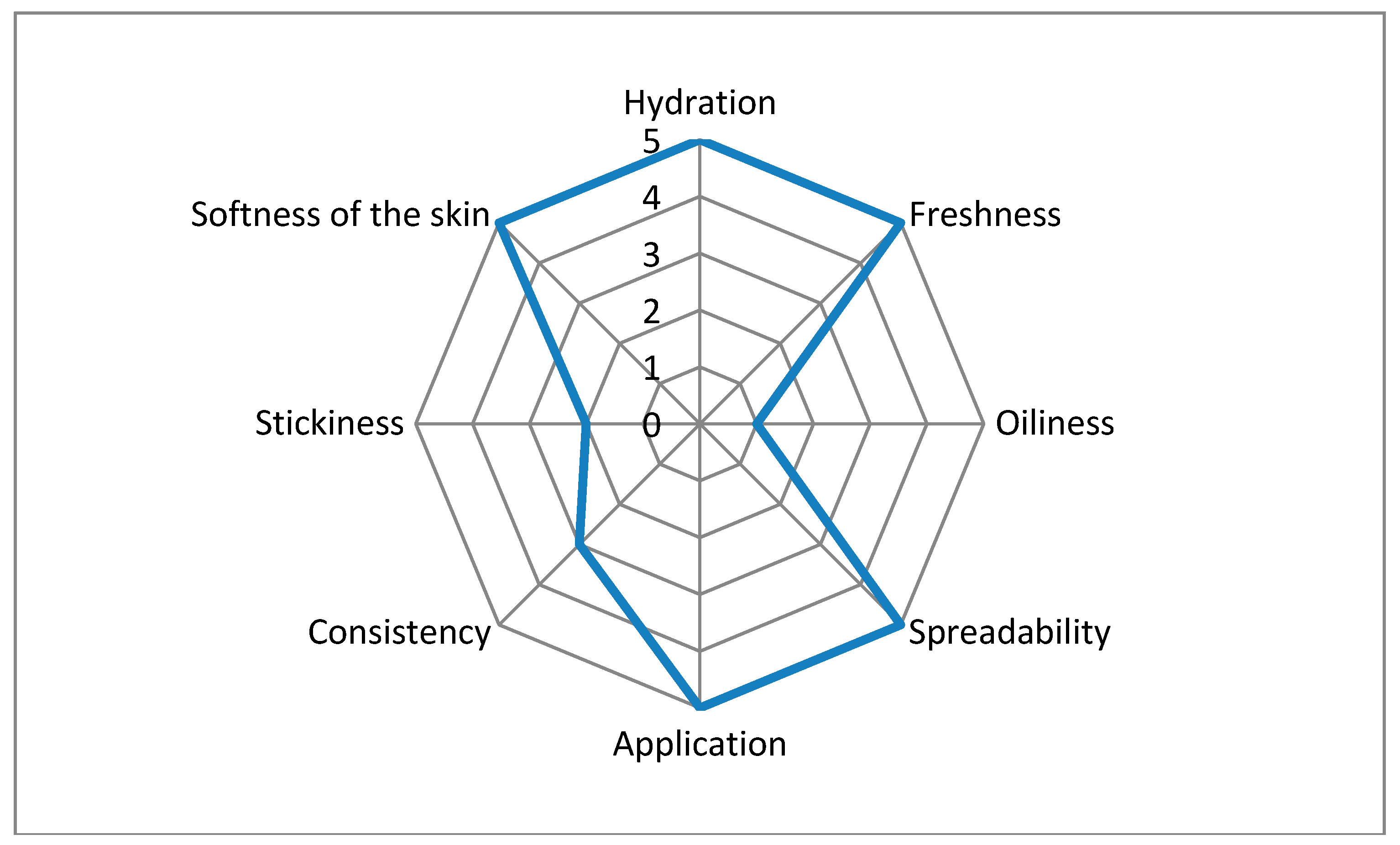
3.5. Sensory Analysis
4. Conclusions
Author Contribution
Funding
Acknowledgments
Conflicts of Interest
References
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical eriche oil from açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthäler, R.; Belandrino, R.; Maia, J.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total antioxidant scavenging capacities of Euterpe oleracea Mart. (açaí) fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Shauss, A.G.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and antiinflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatória. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Lichtenthäler, R.; Zimmermann, B.F.; Papagiannopoulos, M.; Fabriucius, H.; Marx, F. Total oxidant scavenging capacity of Euterpe oleracea Mart. (Açaí) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Gallori, S.; Bilia, A.R.; Bergonzi, M.C.; Barbosa, W.L.R.; Vincieri, F.F. Polyphenolic constituents of fruit pulp of Euterpe oleracea Mart. (açai palm). Chromatographia 2004, 59, 739–743. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Zhang, K.M.; Yu, H.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Xia, X.J. Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci. 2010, 179, 202–208. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Zorzetto, C.; Sanchez-Mateo, C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F.; et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum Hypericum canariense and Hypericum grandifolium. Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft Und-Technologie 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Maleci Bin, L.; Giuliani, C.; et al. Phytochemical Analysis Biological Activity and Secretory Structures of Stachys annua (L.) L. subsp annua (Lamiaceae) from Central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and Antioxidant Activity of the Chamazulene-Rich Essential Oil Obtained from Artemisia arborescens L Growing on the Isle of La Maddalena Sardinia Italy. Chem. Biodivers. 2013, 10, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.P.; Basketter, D.A.; Baverel, M.; Diembeck, W.; Matthies, W.; Mougin, D.; Röthlisberger, R.; Coroama, M. Test guidelines for the assessment of skin tolerance of potentially irritant cosmetic ingredients in man. Food Chem. Toxicol. 1997, 35, 1099–1106. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]


| Phase | Commercial Name and Supplier | INCI Name | Amount (%) | Function |
|---|---|---|---|---|
| Phase A Oil Phase | Emulium kappa 2 | Candelilla jojoba, ridebranpolyglycerul-3 esters, glyceryl stearate | 6.0 | Emulsifier |
| Alcool cetilstearilico TA 1618 | Cetearyl alcohol | 2.0 | Thickning agent | |
| Karité butter | Butyrospermum Parkii butter | 2.0 | Emollient | |
| Cetiol LC | Coco Caprilate/Caprate | 5.0 | Emollient | |
| Soja oil | Glycine Soja Oil | 5.0 | Emollient | |
| Phase B Acqueous phase | Water | Aqua | q.s. 100.0 | Solvent |
| Avicel PC 591 | Microcrystalline cellulose (and) cellulose gum | 1.5 | Thickening agent | |
| Phase C | Titrated açai extract | Acai Extract | 0.5, 1.0, 2.0 | Functional |
| Phase D | Euxyl K712 | sodium benzoate, potassium sorbate, water | 1.0 | Preservative |
| Natrlquest E30 | Trisodium Ethylenediamine Disuccinate | 0.5 | Chelant | |
| Citric acid | Citric acid | q.s. to pH 5.0 | Acidifier |
| Parameters | O/W Emulsion without Açai Extract | O/W Emulsion with 0.5% Açai Extract | O/W Emulsion with 1% Açai Extract | O/W Emulsion with 2% Açai Extract | |
|---|---|---|---|---|---|
| pH | 5.03 ± 0.12 | 5.02 ± 0.14 | 5.04 ± 0.08 | 5.05 ± 0.11 | |
| Density (g/L) | 0.98 ± 0.02 | 0.98 ± 0.01 | 0.98 ± 0.02 | 0.98 ± 0.03 | |
| Color | L* | 75.21 | 66.98 | 63.80 | 58.46 |
| a* | –1.23 | 5.49 | 7.84 | 11.30 | |
| b* | –2.98 | 1.75 | 3.27 | 4.51 | |
| Centrifugation | No separation phase | No separation phase | No separation phase | No separation phase | |
| Accelerated stability | Stable | Stable | Stable | Stable | |
| Long term stability | Stable | Stable | Stable | Stable | |
| Challenge test | Passed (Criterion A) | Passed (Criterion A) | Passed (Criterion A) | Passed (Criterion A) | |
| Mean Irritation Index (after 15 min) | 0 | 0 | 0 | 0 | |
| Mean Irritation Index (after 24 h) | 0 | 0 | 0 | 0 | |
| Product | FOLIN CIOCALTEAU (μg GAE/g) | DPPH (µmoli TE/g) | ABTS (µmoli TE/g) | FRAP (µmoli TE/g) |
|---|---|---|---|---|
| Açai extract | 35.50 ± 2.6 | 148.4 ± 10.9 | 57.8 ± 5.2 | 134.8 ± 8.2 |
| O/W Emulsion without açai extract | 2.3 ± 0.2 | 5.8 ± 1.2 | 0.70 ± 0.3 | 1.2 ± 0.1 |
| O/W Emulsion with 0.5% açai extract | 20. 3 ± 0.8 | 66.8 ± 1.5 | 21.02 ± 0.5 | 52.8 ± 1.2 |
| O/W Emulsion with 1.0% açai extract | 25.8 ± 0.7 | 70.6 ± 1.8 | 20.01 ± 0.5 | 55.2 ± 1.2 |
| O/W Emulsion with 2.0% açai extract | 29.2 ± 0.7 | 82.5 ± 2.5 | 13.30 ± 0.4 | 60.7 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Censi, R.; Vargas Peregrina, D.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.G.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. https://doi.org/10.3390/cosmetics5030048
Censi R, Vargas Peregrina D, Lacava G, Agas D, Lupidi G, Sabbieti MG, Di Martino P. Cosmetic Formulation Based on an Açai Extract. Cosmetics. 2018; 5(3):48. https://doi.org/10.3390/cosmetics5030048
Chicago/Turabian StyleCensi, Roberta, Dolores Vargas Peregrina, Giovanna Lacava, Dimitrios Agas, Giulio Lupidi, Maria Giovanna Sabbieti, and Piera Di Martino. 2018. "Cosmetic Formulation Based on an Açai Extract" Cosmetics 5, no. 3: 48. https://doi.org/10.3390/cosmetics5030048
APA StyleCensi, R., Vargas Peregrina, D., Lacava, G., Agas, D., Lupidi, G., Sabbieti, M. G., & Di Martino, P. (2018). Cosmetic Formulation Based on an Açai Extract. Cosmetics, 5(3), 48. https://doi.org/10.3390/cosmetics5030048








