Alkenones, a Renewably Sourced, Biobased Wax as an SPF Booster for Organic Sunscreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of Sunscreens
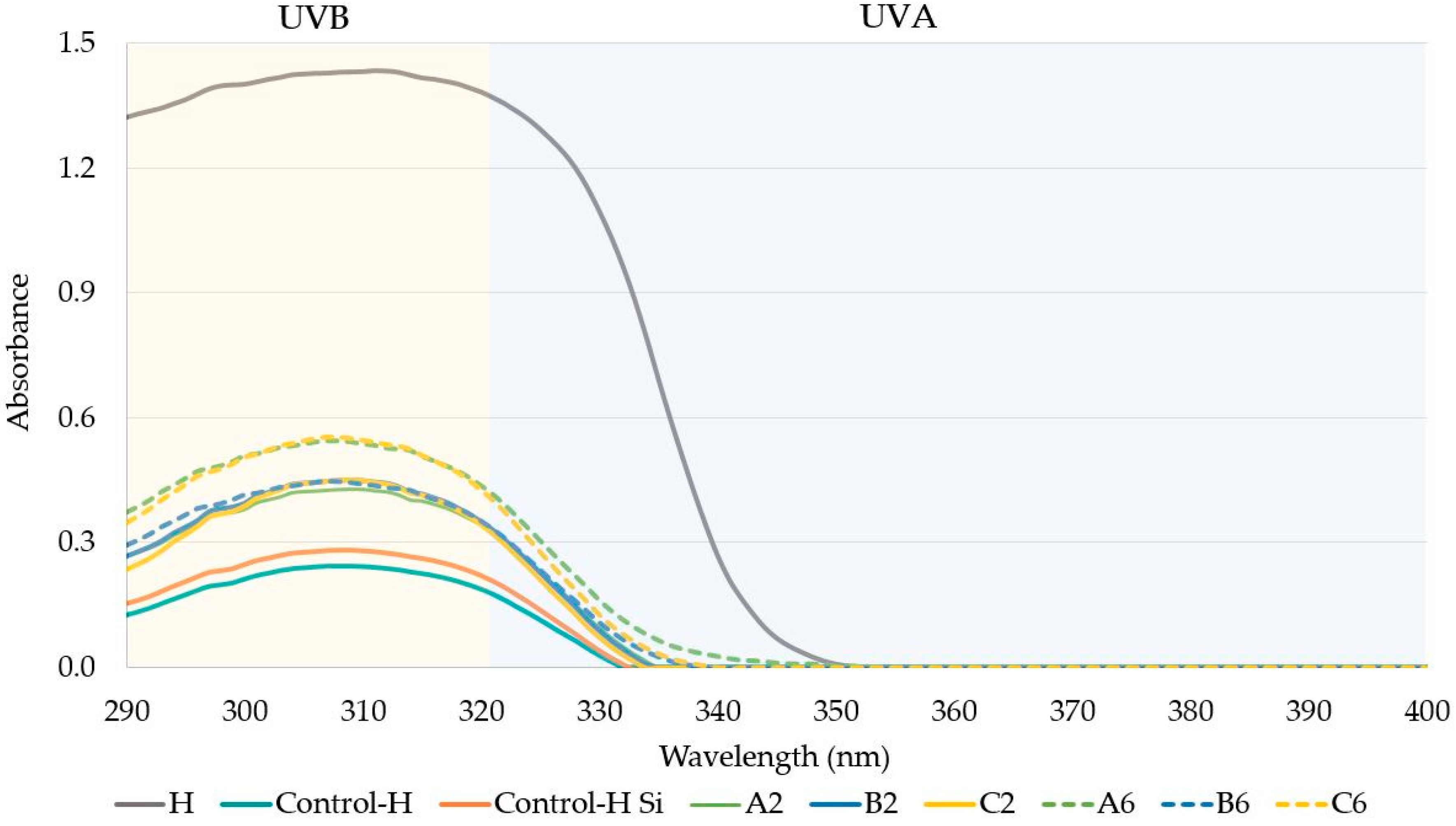
2.2.2. In Vitro SPF
2.2.3. Water-Resistance Testing
2.2.4. Viscosity
2.2.5. pH Testing
2.2.6. Spreadability Testing
2.2.7. Stability Testing
3. Results and Discussions
3.1. In Vitro SPF and Water Resistance
3.2. Viscosity
3.3. pH Testing
3.4. Spreadability Testing
3.5. Sunscreen Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pathak, M.A. Sunscreens: Topical and systemic approaches for protection of human skin against harmful effects of solar radiation. J. Am. Acad. Dermatol. 1982, 7, 285–312. [Google Scholar] [CrossRef]
- Green, A.; Williams, G.; Neale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistutta, D.; Frost, C.; et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomised controlled trial. Lancet 1999, 354, 723–729. [Google Scholar] [CrossRef]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Lai, W.; Yan, T.; Wu, Y.; Wan, M.; Yi, J.; Matsui, M.S. Sunburn protection as a function of sunscreen application thickness differs between high and low spfs. Photodermatol. Photoimmunol. Photomed. 2012, 28, 120–126. [Google Scholar] [CrossRef]
- Teramura, T.; Mizuno, M.; Asano, H.; Naito, N.; Arakane, K.; Miyachi, Y. Relationship between sun-protection factor and application thickness in high-performance sunscreen: Double application of sunscreen is recommended. Clin. Exp. Dermatol. 2012, 37, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Korn, V.; Surber, C.; Imanidis, G. Skin surface topography and texture analysis of sun-exposed body sites in view of sunscreen application. Skin Pharmacol. Physiol. 2016, 29, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J. New and emerging sunscreen technologies. In Clinical Guide to Sunscreens and Photoprotection; Lim, H.L., Draelos, Z.D., Eds.; Informa Healthcare: New York, NY, USA, 2009; pp. 155–168. [Google Scholar]
- Hunter, A.; Trevino, M. Film-formers enhance water resistance and spf in sun care products. Cosmet. Toilet. 2004, 119, 51–56. [Google Scholar]
- O’Lenick, A.J., Jr. Naturals and Organics in Cosmetics: Trends and Technology; Allured Business Media: Carol Stream, IL, USA, 2010. [Google Scholar]
- Pitman, S. The Rise and Rise of Vegan Claims for Cosmetics. Available online: https://www.cosmeticsdesign.com/Article/2018/09/19/The-rise-and-rise-of-vegan-claims-for-cosmetics (accessed on 27 November 2018).
- Wood, L. Global Vegan Cosmetics Market 2018–2023. Available online: https://www.businesswire.com/news/home/20180907005122/en/Global-Vegan-Cosmetics-Market-2018-2023-Analysis-Product (accessed on 29 November 2018).
- Lintner, K. Definitions, legal requirements, and an attempt to harmonize (global?) characterization. In Global Regulatory Issues for the Cosmetics Industry; Lintner, K., Ed.; William Andrew Inc.: Norwich, NY, USA, 2009; Volume 2, p. 45. [Google Scholar]
- Knowlton, J.L.; Pearce, S.E.M. Handbook of Cosmetic Science and Technology; Elsevier Advanced Technology: Oxford, UK, 1993. [Google Scholar]
- Volkman, J.K.; Eglinton, G.; Corner, E.D.S.; Forsberg, T.E.V. Long-chain alkenes and alkenones in the marine coccolithophorid emiliania huxleyi. Phytochemistry 1980, 19, 2619–2622. [Google Scholar] [CrossRef]
- Eltgroth, M.L.; Watwood, R.L.; Wolfe, G.V. Production and cellular localization of neutral long-chain lipids in the haptophyte algae isochrysis galbana and emiliana huxleyi. J. Phycol. 2005, 41, 1000–1009. [Google Scholar] [CrossRef]
- What Is Biopreferred? Available online: https://www.biopreferred.gov/BioPreferred/faces/pages/AboutBioPreferred.xhtml (accessed on 21 December 2018).
- Offredo, H. Marine ingredients for skin care: An ocean of resources. In Harry’s Cosmetology; Rosen, M.R., Ed.; Chemical Publishing Co., Inc.: Los Angeles, CA, USA, 2015; Volume 2. [Google Scholar]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, G.W.; Williams, J.R.; Craig, A.M.; Nelson, R.K.; Gosselin, K.M.; Reddy, C.M. Accessing monomers, surfactants, and the queen bee substance by acrylate cross-metathesis of long-chain alkenones. J. Am. Oil Chem. Soc. 2017, 94, 831–840. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Culler, A.R.; Williams, J.R.; Burlow, N.P.; Gilbert, G.J.; Carmichael, C.A.; Nelson, R.K.; Swarthout, R.F.; Reddy, C.M. Production of jet fuel range hydrocarbons as a coproduct of algal biodiesel by butenolysis of long-chain alkenones. Energy Fuels 2015, 29, 922–930. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Yen, T.Q.; Leitch, M.A.; Wilson, G.R.; Brown, E.A.; Rider, D.A.; Reddy, C.M. Alkenones as renewable phase change materials. Renew. Energy 2019, 134, 89–94. [Google Scholar] [CrossRef]
- McIntosh, K.; Smith, A.; Young, L.; Leitch, M.; Tiwari, A.; Reddy, C.; O’Neil, G.; Liberatore, M.; Chandler, M.; Baki, G. Alkenones as a promising green alternative for waxes in cosmetics and personal care products. Cosmetics 2018, 5, 34. [Google Scholar] [CrossRef]
- Sb2571. Available online: https://www.capitol.hawaii.gov/session2018/bills/SB2571_.HTM (accessed on 10 July 2018).
- O’Neil, G.W.; Williams, J.R.; Wilson-Peltier, J.; Knothe, G.; Reddy, C.M. Experimental protocol for biodiesel production with isolation of alkenones as coproducts from commercial isochrysis algal biomass. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- WACKER. Tehnical Data Sheet for Belsil(r) REG 1102. Available online: https://www.brenntag.com/media/documents/bsi/product_data_sheets/life_science/wacker_silicones_pc/belsil_reg_1102_pds.pdf (accessed on 10 September 2018).
- Title 21. Part 201. Subpart g. §201.327 Over-the-Counter Sunscreen Drug Products; Required Labeling Based on Effectiveness Testing. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=b7ff601aa9ccc10f7671b082628fcb53&mc=true&node=se21.4.201_1327&rgn=div8 (accessed on 1 August 2018).
- Food and Drug Administration; HHS. Labeling and Effectiveness Testing; Sunscreen Drug Products for Over-the-Counter Human Use; Federal Register; Government Publishing Office: Washington, DC, USA, 2011; Volume 76, pp. 35620–35665. [Google Scholar]
- O’Lenick, T.; Lott, D. The effects of solvents on sunscreens: A new ester to improve efficiency. Cosmet. Toilet. 2013, 126, 431–436. [Google Scholar]
- Sohn, M. Uv booster and photoprotection. In Principles and Practice of Photoprotection; Wang, S.Q., Lim, H.L., Eds.; Adis: Berlin, Germany, 2016; pp. 227–245. [Google Scholar]
- Agrapidis-Paloympis, L.E.; Nash, R.A.; Shaath, N.A. The effect of solvents on the ultraviolet absorbance of sunscreens. J. Soc. Cosmet. Chem. 1987, 38, 209–221. [Google Scholar]
- Moisturizing Cream Spreadability. Available online: https://www.brookfieldengineering.com/applications/texture-applications/personal-care-products/moisturizing-cream-spreadability (accessed on 11 December 2018).
- Hewitt, J. Factors influencing efficacy of oil-dispersed physical sunscreens. IFSCC Mag. 2000, 3, 18–23. [Google Scholar]
- Silva, D.G.; Sarruf, F.D.; Oliveira, L.C.D.; Areas, E.P.G.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V.R.; Baby, A.R. Influence of particle size on appearance and in vitro efficacy of sunscreens. Braz. J. Pharm. Sci. 2013, 49, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, J.P.; Dahms, G.H. The Influence of Rheology on Efficacy of Physical Sunscreens; IFSCC: Montreux, Switzerland, 1995; pp. 313–323. [Google Scholar]
- Xu, S.; Kwa, M.; Agarwal, A.; Rademaker, A.; Kundu, R.V. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016, 152, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Dimitrovska Cvetkovska, A.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting spf in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.; Klette, E.; Ruppert, S.; Bimzcok, R.; Klebon, B.; Heinrich, U.; Tronnier, H.; Johncock, W.; Peters, S.; Pflucker, F.; et al. In vitro sun protection factor: Still a challenge with no final answer. Skin Pharmacol. Physiol. 2010, 23, 201–212. [Google Scholar] [CrossRef] [PubMed]




| INCI Name | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (w/w) | ||||||||||||
| Phase A | ||||||||||||
| Ethylhexyl methoxycinnamate | 5 | - | - | - | 5 | - | - | - | 5 | - | - | - |
| Homosalate | - | 5 | - | - | - | 5 | - | - | - | 5 | - | - |
| Octocrylene | - | - | 5 | - | - | - | 5 | - | - | - | 5 | - |
| Octyl salicylate | - | - | - | 5 | - | - | - | 5 | - | - | - | 5 |
| Heptyl undecylenate | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Polyglyceryl-10-stearate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Alkenones * | 3 | 3 | 3 | 3 | - | - | - | - | - | - | - | - |
| Beeswax | - | - | - | - | 3 | 3 | 3 | 3 | - | - | - | - |
| Cetyl alcohol | - | - | - | - | - | - | - | - | 3 | 3 | 3 | 3 |
| Phase B | ||||||||||||
| Water | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 | 72 |
| Propanediol | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Phase C | ||||||||||||
| Propylene glycol (and) Diazolidinyl urea (and) Methyl paraben (and) Propyl paraben | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| INCI Name | Control-OMC | Control-OMC Si | Control-H | Control-H Si | Control-O | Control-O Si | Control-OS | Control-OS Si | Control-A | Control-B | Control-C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % (w/w) | |||||||||||
| Phase A | |||||||||||
| Ethylhexyl methoxycinnamate | 5 | 5 | - | - | - | - | - | - | - | - | - |
| Homosalate | - | - | 5 | 5 | - | - | - | - | - | - | - |
| Octocrylene | - | - | - | - | 5 | 5 | - | - | - | - | - |
| Octyl salicylate | - | - | - | - | - | - | 5 | 5 | - | - | - |
| Heptyl undecylenate | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Polyglyceryl-10-stearate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dimethicone (and) dimethicone/vinyltrimethylsiloxysilicate crosspolymer | - | 1 | - | 1 | - | 1 | - | 1 | - | - | - |
| Phase B | |||||||||||
| Water | 75 | 74 | 75 | 74 | 75 | 74 | 75 | 74 | 80 | 80 | 80 |
| Propanediol | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Phase C | |||||||||||
| Propylene glycol (and) Diazolidinyl urea (and) Methyl paraben (and) Propyl paraben | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sunscreen | Compared to 5% UV Filter | Compared to Control |
|---|---|---|
| A1 | 335 * | 7 |
| B1 | 468 * | 39 * |
| C1 | 389 * | 20 |
| A2 | 215 * | 39 * |
| B2 | 241 * | 51 * |
| C2 | 251 * | 55 * |
| A3 | −27 | 44 * |
| B3 | −12 | 75 * |
| C3 | −5 | 89 * |
| A4 | 328 * | 80 * |
| B4 | 351 * | 90 * |
| C4 | 326 * | 79 * |
| Sample | Absorption Peak (nm) | Sample | Absorption Peak (nm) |
|---|---|---|---|
| A1 | 310 | A3 | 304 |
| B1 | 310 | B3 | 304 |
| C1 | 310 | C3 | 304 |
| A5 | 308 | A7 | 300 |
| B5 | 307 | B7 | 300 |
| C5 | 308 | C7 | 301 |
| OMC | 311 | O | 311 |
| Control-OMC | 297 | Control-O | 297 |
| Control-OMC Si | 297 | Control-O Si | 297 |
| A2 | 309 | A4 | 308 |
| B2 | 309 | B4 | 309 |
| C2 | 309 | C4 | 309 |
| A6 | 307 | A8 | 307 |
| B6 | 307 | B8 | 307 |
| C6 | 307 | C8 | 308 |
| H | 311 | OS | 311 |
| Control-H | 308 | Control-OS | 309 |
| Control -H Si | 309 | Control-OS Si | 309 |
| Sample | Viscosity at 25 s−1 (cP) | Sample | Viscosity at 25 s−1 (cP) |
|---|---|---|---|
| A1 | 430 ± 10 | A3 | 410 ± 20 |
| B1 | 950 ± 30 | B3 | 950 ± 60 |
| C1 | 8050 ± 700 | C3 | 8300 ± 300 |
| A5 | 1760 ± 50 | A7 | 1870 ± 130 |
| B5 | 2690 ± 30 | B7 | 2160 ± 20 |
| C5 | 8200 ± 1500 | C7 | 8520 ± 460 |
| Control-OMC | 1030 ± 20 | Control-O | 320 ± 10 |
| Control-OMC Si | 1210 ± 20 | Control-O Si | 570 ± 10 |
| A2 | 990 ± 60 | A4 | 730 ± 20 |
| B2 | 2140 ± 230 | B4 | 1940 ± 60 |
| C2 | 7900 ± 900 | C4 | 6360 ± 230 |
| A6 | 2140 ± 140 | A8 | 2350 ± 110 |
| B6 | 2750 ± 40 | B8 | 2700 ± 80 |
| C6 | 9500 ± 1560 | C8 | 7400 ± 60 |
| Control-H | 960 ± 30 | Control-OS | 880 ± 20 |
| Control -H Si | 990 ± 20 | Control-OS Si | 850 ± 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, A.; Abou-Dahech, M.S.; Reddy, C.M.; O’Neil, G.W.; Chandler, M.; Baki, G. Alkenones, a Renewably Sourced, Biobased Wax as an SPF Booster for Organic Sunscreens. Cosmetics 2019, 6, 11. https://doi.org/10.3390/cosmetics6010011
Huynh A, Abou-Dahech MS, Reddy CM, O’Neil GW, Chandler M, Baki G. Alkenones, a Renewably Sourced, Biobased Wax as an SPF Booster for Organic Sunscreens. Cosmetics. 2019; 6(1):11. https://doi.org/10.3390/cosmetics6010011
Chicago/Turabian StyleHuynh, An, Mariam S. Abou-Dahech, Christopher M. Reddy, Gregory W. O’Neil, Mark Chandler, and Gabriella Baki. 2019. "Alkenones, a Renewably Sourced, Biobased Wax as an SPF Booster for Organic Sunscreens" Cosmetics 6, no. 1: 11. https://doi.org/10.3390/cosmetics6010011






