Epidermal Endocannabinoid System (EES) and its Cosmetic Application
Abstract
:1. Endocannabinoid and Epidermal Endocannabinoid System (EES)
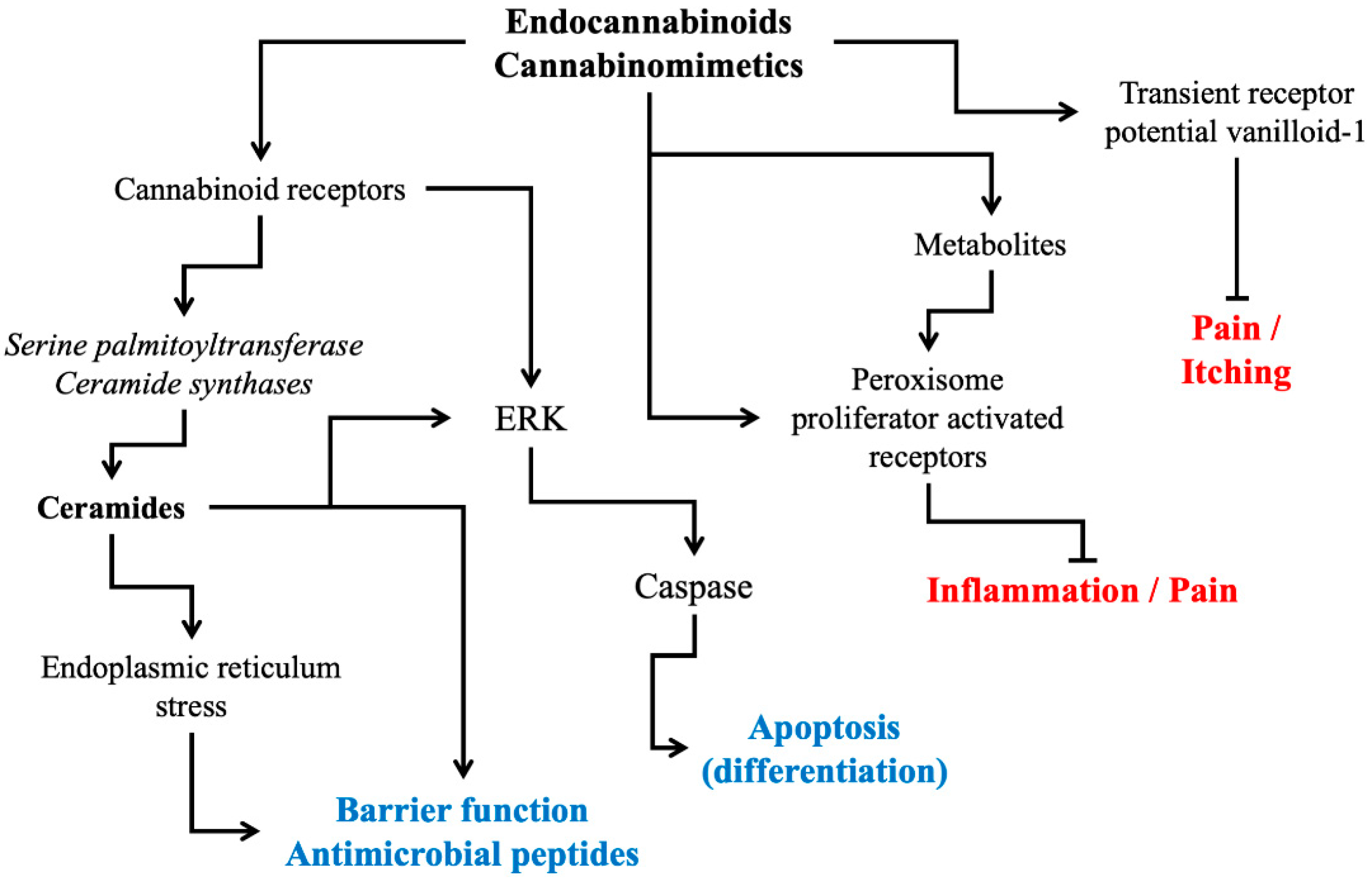
2. Cannabinoid Receptor Modulation: Direct and Indirect Signaling Pathways
3. Physiological and Pathophysiological Roles of Cannabinoid Receptors in Skin
4. Development of Endocannabinomimetics and its Cosmetic Application
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gaoni, Y.; Mechoulam, R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Arena, C.; Manera, C. Beyond the direct activation of cannabinoid receptors: New strategies to modulate the endocannabinoid system in CNS-related diseases. Recent Pat. CNS Drug Discov. 2016, 10, 122–141. [Google Scholar] [CrossRef]
- Piscitelli, F.; Bradshaw, H.B. Endocannabinoid analytical methodologies: Techniques that drive discoveries that drive techniques. Adv. Pharmacol. 2017, 80, 1–30. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 receptor pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Svízenská, I.; Dubový, P.; Sulcová, A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—A short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Río, C.D.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gómez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sánchez, C. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2013, 32, 2534–2542. [Google Scholar] [CrossRef]
- Adinolfi, B.; Romanini, A.; Vanni, A.; Martinotti, E.; Chicca, A.; Fogli, S.; Nieri, P. Anticancer activity of anandamide in human cutaneous melanoma cells. Eur. J. Pharmacol. 2013, 718, 154–159. [Google Scholar] [CrossRef]
- Cohen, L.J.; Kang, H.S.; Chu, J.; Huang, Y.H.; Gordon, E.A.; Reddy, B.V.; Ternei, M.A.; Craig, J.W.; Brady, S.F. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4825–E4834. [Google Scholar] [CrossRef] [Green Version]
- Mallipeddi, S.; Janero, D.R.; Zvonok, N.; Makriyannis, A. Functional selectivity at G-protein coupled receptors: Advancing cannabinoid receptors as drug targets. Biochem. Pharmacol. 2017, 128, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Gómez del Pulgar, T.; Velasco, G.; Sánchez, C.; Haro, A.; Guzmán, M. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem. J. 2002, 363, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.; Sander, B.; Bielawski, J.; Hannun, Y.A.; Flygare, J. Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol. Cancer Res. 2009, 7, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Raduner, S.; Majewska, A.; Chen, J.Z.; Xie, X.Q.; Hamon, J.; Faller, B.; Altmann, K.H.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef]
- Moore, D.J.; Rawlings, A.V. The chemistry, function and (patho)physiology of stratum corneum barrier ceramides. Int. J. Cosmet. Sci. 2017, 39, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Elias, P.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Shin, K.O.; Lee, Y.M.; Holleran, W.M.; Uchida, Y. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J. Investig. Dermatol. 2013, 133, 1942–1949. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Fowler, C.J. Plant-derived, synthetic and endogenous cannabinoids as neuroprotective agents. Non-psychoactive cannabinoids, ‘entourage’ compounds and inhibitors of N-acyl ethanolamine breakdown as therapeutic strategies to avoid pyschotropic effects. Brain Res. Brain Res. Rev. 2003, 41, 26–43. [Google Scholar] [CrossRef]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Haruna, T.; Soga, M.; Morioka, Y.; Imura, K.; Furue, Y.; Yamamoto, M.; Hayakawa, J.; Deguchi, M.; Arimura, A.; Yasui, K. The inhibitory effect of S-777469, a cannabinoid type 2 receptor agonist, on skin inflammation in mice. Pharmacology 2017, 99, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gaffal, E.; Cron, M.; Glodde, N.; Bald, T.; Kuner, R.; Zimmer, A.; Lutz, B.; Tüting, T. Cannabinoid 1 receptors in keratinocytes modulate proinflammatory chemokine secretion and attenuate contact allergic inflammation. J. Immunol. 2013, 190, 4929–4936. [Google Scholar] [CrossRef]
- Marks, D.H.; Friedman, A. The Therapeutic Potential of Cannabinoids in Dermatology. Skin Ther. Lett. 2018, 23, 1–5. [Google Scholar]
- Oddi, S.; Maccarrone, M. Endocannabinoids and Skin Barrier Function: Molecular Pathways and Therapeutic Opportunities. In Skin Stress Response Pathways; Wondrak, G.T., Ed.; Springer International Publishing: Berlin, Germany, 2016; pp. 301–323. [Google Scholar] [CrossRef]
- Roelandt, T.; Heughebaert, C.; Bredif, S.; Giddelo, C.; Baudouin, C.; Msika, P.; Roseeuw, D.; Uchida, Y.; Elias, P.M.; Hachem, J.P. Cannabinoid receptors 1 and 2 oppositely regulate epidermal permeability barrier status and differentiation. Exp. Dermatol. 2012, 21, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Gaffal, E.; Glodde, N.; Jakobs, M.; Bald, T.; Tüting, T. Cannabinoid 1 receptors in keratinocytes attenuate fluorescein isothiocyanate-induced mouse atopic-like dermatitis. Exp. Dermatol. 2014, 23, 401–406. [Google Scholar] [CrossRef]
- Sugawara, K.; Bíró, T.; Tsuruta, D.; Tóth, B.I.; Kromminga, A.; Zákány, N.; Zimmer, A.; Funk, W.; Gibbs, B.F.; Zimmer, A.; et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J. Allergy Clin. Immunol. 2012, 129, 726–738. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef]
- Bort, A.; Alvarado-Vazquez, P.A.; Moracho-Vilrriales, C.; Virga, K.G.; Gumina, G.; Romero-Sandoval, A.; Asbill, S. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol. Pain 2017, 13, 174480691668822. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.-P.; Kim, B.; Kim, B.-W. Selective Cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann. Dermatol. 2018, 28, 22–29. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Park, B.M.; Jeon, J.E.; Lee, S.H.; Mann, S.; Ahn, S.K.; Hong, S.P.; Jeong, S.K. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int. J. Dermatol. 2015, 54, e401–e408. [Google Scholar] [CrossRef] [PubMed]
- Gaffal, E.; Cron, M.; Glodde, N.; Tüting, T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Guida, F.; Moriello, A.S.; De Chiaro, M.; Piscitelli, F.; de Novellis, V.; Maione, S.; Di Marzo, V. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the TRPV1 receptor and anti-hyperalgesic activity. Pharmacol. Res. 2011, 63, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Sosa, S.; Laezza, C.; De Bortoli, M.; Tubaro, A.; Bifulco, M. Rimonabant reduces keratinocyte viability by induction of apoptosis and exerts topical anti-inflammatory activity in mice. Br. J. Pharmacol. 2011, 162, 84–93. [Google Scholar] [CrossRef]
- Mecs, L.; Tuboly, G.; Toth, K.; Nagy, E.; Nyari, T.; Benedek, G.; Horvath, G. Peripheral antinociceptive effect of 2-arachidonoyl-glycerol and its interaction with endomorphin-1 in arthritic rat ankle joints. Clin. Exp. Pharmacol. Physiol. 2010, 37, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, N.; Nasca, M.R.; Micali, G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta Dermatovenerol. Croat. 2007, 15, 80–83. [Google Scholar] [PubMed]
- Petrosino, S.; Puigdemont, A.; Della Valle, M.F.; Fusco, M.; Verde, R.; Allarà, M.; Aveta, T.; Orlando, P.; Di Marzo, V. Adelmidrol increases the endogenous concentrations of palmitoylethanolamide in canine keratinocytes and down-regulates an inflammatory reaction in an in vitro model of contact allergic dermatitis. Vet. J. 2016, 207, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Wakui, J.; Gokoh, M.; Kishimoto, S.; Sugiura, T. Suppression by WIN55212-2, a cannabinoid receptor agonist, of inflammatory reactions in mouse ear: Interference with the actions of an endogenous ligand, 2-arachidonoylglycerol. Eur. J. Pharmacol. 2006, 538, 154–162. [Google Scholar] [CrossRef]
- Bieberich, E.; Kawaguchi, T.; Yu, R.K. N-Acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J. Biol. Chem. 2000, 275, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Buchanan, B.; Zuccolo, J.; Poulin, M.M.; Gabriele, J.; Baranowski, D.C. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS ONE 2018, 13, e0196396. [Google Scholar] [CrossRef]


| Compounds | (Proposed) Mode of Action | Study Model | Efficacy Observed | Reference |
|---|---|---|---|---|
| Echinacea species-derived alkylamide | CB2R agonist Partial inhibition of FAAH | In vitro study | Reduction of pro-inflammatory cytokines (IL-6 and IL-8) | [37] |
| Clinical study on AE | Reduction of local SCORADindex | |||
| Increases in SC intercellular lipids | ||||
| JWH015 | Selective CB2R agonist | In vitro study | Reduction of pro-inflammatory cytokines (IL-6 and MCP-1) | [38] |
| AEA mimic compound | Selective CB1R agonist | In vitro study | Reduction of mast cell activation | [39] |
| Oxazolone induced AE model | Suppression of mast cells infiltration into skin | |||
| AEA mimic compound | Selective CB1R agonist | Skin barrier recovery after acute disruption | Accelerated recovery of skin barrier function | [40] |
| TPA-induced acute inflammation model | Reduction of oedema | |||
| Oxazolone induced AE model | Improvement in skin barrier function | |||
| THC | CBR agonist | DNFB-mediated allergic contact dermatitis model | Reduction of keratinocyte-derived pro-inflammatory mediators | [41] |
| N-palmitoyl-vanillamide (palvanil) | Potential CBR agonist Potential FAAH inhibitor | In vitro study | Lower pungency and stronger anti-hyperalgesic activity | [42] |
| Eye-wiping assay | anti-hyperalgesic activity | |||
| Rimonabant (SR141716) | CB1R antagonist | Croton oil-induced ear dermatitis model | Reduction of oedema and leukocyte infiltration | [43] |
| 2-AG | CBR agonist | Carrageenan-induced joint inflammation model | Antinociceptive effects | [44] |
| Adelmidrol | CBR agonist | Clinical study on AE | Improvement in IGA score | [45] |
| Adelmidrol | Increasing intracellular PEA concentration (Entourage effect) | In vitro study | Inhibition of the release of the pro-inflammatory chemokine MCP-2 | [46] |
| WIN55212-2 | CBR agonist | TPA-induced acute inflammation model | Reduction of oederma and leukocyte infiltration | [47] |
| SR144528 | CB2R antagonist | TPA-induced acute inflammation model | Reduction of oederma and leukocyte infiltration |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, M.S.; Lee, S.H.; Park, B.D. Epidermal Endocannabinoid System (EES) and its Cosmetic Application. Cosmetics 2019, 6, 33. https://doi.org/10.3390/cosmetics6020033
Jeong S, Kim MS, Lee SH, Park BD. Epidermal Endocannabinoid System (EES) and its Cosmetic Application. Cosmetics. 2019; 6(2):33. https://doi.org/10.3390/cosmetics6020033
Chicago/Turabian StyleJeong, Sekyoo, Min Seok Kim, Sin Hee Lee, and Byeong Deog Park. 2019. "Epidermal Endocannabinoid System (EES) and its Cosmetic Application" Cosmetics 6, no. 2: 33. https://doi.org/10.3390/cosmetics6020033
APA StyleJeong, S., Kim, M. S., Lee, S. H., & Park, B. D. (2019). Epidermal Endocannabinoid System (EES) and its Cosmetic Application. Cosmetics, 6(2), 33. https://doi.org/10.3390/cosmetics6020033






