Development and Characterization of New Topical Hydrogels Based on Alpha Lipoic Acid—Hydrotalcite Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrotalcites
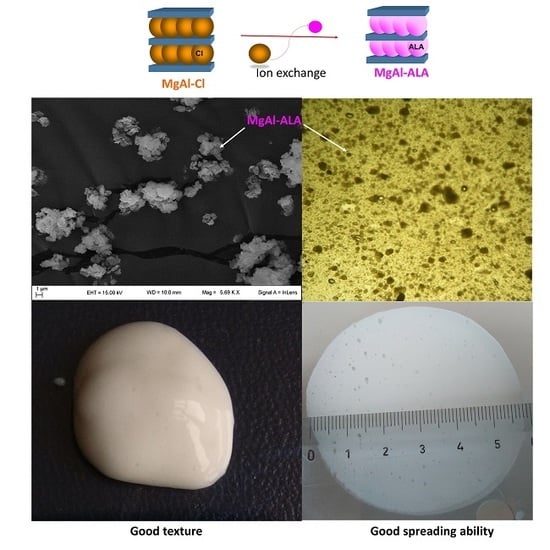
2.3. Preparation of Hydrotalcite-ALA Hybrids
2.4. X-ray Analysis
2.5. Metal Composition Determination
2.6. Thermogravimetric Analysis (TGA)
2.7. Single Particle Optical Sensing (SPOS) Analysis
2.8. Differential Scanning Calorimetry (DSC)
2.9. Morphological Characterization by Scanning Electron Microscopy Analysis (SEM)
2.10. Flow Properties Determination
2.11. Cell Culture
2.12. Samples Preparation
2.13. MTT Assay
2.14. Thermal Stability of ALA Samples using DSC
2.15. Preparation of Hydrogels
2.16. Rheological Measurements
2.17. Spreadability Measurements
2.18. Optical Microscope Observation
2.19. Stability Studies of Hydrogels
- Accelerated stability studies: The samples were submitted to eight 4 °C/45 °C cycles. Each cycle consisted in the storage of each sample alternatively at 4 °C (for 48 h) and 45 °C (for 48 h).
- Centrifugation test: the samples were centrifuged at 25 °C for 20 min at 3000 rpm (Hettich zentrifugen, Universal 32 R).
2.20. In Vitro Release Studies
2.21. Quantitative Determination of ALA by HPLC Method
3. Results and Discussion
3.1. Hybrid Preparation and Characterization
3.2. Study of Thermal Stability of ALA Samples by DSC Analysis
3.3. Hydrogel Composition Optimization
3.4. Rheological Characterization
- -
- η = viscosity
- -
- m = is a constant represented by the log η value when log γ = 0
- -
- γ = shear rate
- -
- n = power-law index.
3.5. Spreadability Measurement
3.6. Stability Studies of Hydrogels to Temperature
3.7. MTT Test
3.8. In Vitro Release Capability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kramer, K.; Hoppe, P.P.; Packer, L. R α Lipoic acid. In Nutraceuticals in Health and Disease Prevention; Dekker, M., Ed.; CRC Press: New York, NY, USA, 2001; pp. 273–275. [Google Scholar]
- Solmonson, A.; De Berardinis, R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitner, H. Randomized, placebo-controlled, double blind study on the clinical efficacy of a cream containing 5% α-lipoic acid related to photoageing of facial skin. Br. J. Dermatol. 2003, 149, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the skin: Understanding formulation and efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Papadopoulou, M.; Dubois, J. Effect of α-lipoic acid on proteasomal induction: Protection against oxidative damage in human skin fibroblasts cell line NHDF. Pharmacol. Pharm. 2017, 8, 292–305. [Google Scholar] [CrossRef]
- Matsugo, S.; Yan, L.J.; Han, D.; Trischler, H.J.; Packer, L. Elucidation of antioxidant activity of α-lipoic acid toward hydroxyl radical. Biochem. Biophys. Res. Commun. 1995, 208, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 5, 809696. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.D.; Masilamoni, J.G.; Arul, V.; Kumar, M.S.; Baraneedharan, U.; Paul, S.F.; Sakthivelu, I.V.; Jesudason, E.P.; Jayakumar, R. Radioprotective effect of DL-α-lipoic acid on mice skin fibroblasts. Cell Biol. Toxicol. 2009, 25, 331–340. [Google Scholar] [CrossRef]
- Li, G.; Fu, J.; Zhao, Y.; Ji, K.; Luan, T.; Zang, B. Alpha-lipoic acid exerts anti-inflammatory effects on lipopolysaccharide-stimulated rat mesangial cells via inhibition of nuclear factor kappa B (NF-κB) signaling pathway. Inflammation 2015, 38, 510–519. [Google Scholar] [CrossRef]
- Moura, F.A.; De Andrade, K.Q.; Dos Santos, J.C.F.; Goulart, M.O.F. Lipoic acid: Its antioxidant and anti-inflammatory role and clinical applications. Curr. Top. Med. Chem. 2015, 15, 458–483. [Google Scholar] [CrossRef]
- Yıldırım Baş, F.; Bayram, D.; Arslan, B.; Armağan, I.; Yeşilot, S.; Çiçek, E.; Yorgancıgil, E. Effect of alpha lipoic acid on smoking-induced skin damage. Cutan. Ocul. Toxicol. 2017, 36, 67–73. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishiura, H.; Yamaguchi, Y.; Hirata, K.; Kubota, Y. α-Lipoic Acid Nanoparticles and Methods for Preparing Thereof. U.S. Patent 9,079,874, 14 July 2015. [Google Scholar]
- Li, Y.X.; Park, E.Y.; Lima, S.T. Stabilization of alpha-lipoic acid by complex formation with octenylsuccinylated high amylose starch. Food Chem. 2018, 242, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Liu, T.; Wang, Q.; Xia, Q.; Bian, X. In vitro evaluation of alpha-lipoic acid loaded lipid nanocapsules for topical delivery. J. Microencapsul. 2017, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Musashi, M.; Nagasawa, T.; Shimura, N.; Igarashi, R.; Yamaguchi, Y. Novel nanocapsule of α-lipoic acid reveals pigmentation improvement: α-Lipoic acid stimulates the proliferation and differentiation of keratinocyte in murine skin by topical application. Exp. Dermatol. 2019, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kulkamp-Guerreiro, I.C.; Terroso, T.F.; Assumpção, E.R.; Berlitz, S.J.; Contri, R.V.; Pohlmann, A.R.; Guterres, S.S. Development and stability of innovative semisolid formulations containing nanoencapsulated lipoic acid for topical use. J. Nanosci. Nanotechnol. 2012, 12, 7723–7732. [Google Scholar] [CrossRef] [PubMed]
- Segall, A.; Sosa, M.; Alami, A.; Enero, C.; Hormaechea, F.; Pizzorno, M.T.; Bregni, C.; Serrao, R. Stability study of lipoic acid in the presence of vitamins A and E in o/w emulsions for cosmetic application. J. Cosmet. Sci. 2004, 55, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zou, A.; Yang, X.; Liu, F.; Xia, Q.; Ye, R.; Mu, B. The effect of polymer-surfactant emulsifying agent on the formation and stability of α-lipoic acid loaded nanostructured lipid carriers (NLC). Food Hydrocoll. 2013, 32, 72–78. [Google Scholar] [CrossRef]
- Takahashi, H.; Bungo, Y.; Mikuni, K. The Aqueous solubility and thermal stability of α-lipoic acid are enhanced by cyclodextrin. Biosci. Biotechnol. Biochem. 2011, 75, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Nakamura, M.; Isobe, T.; Murata, Y.; Kawashima, S. Stabilization of α-lipoic acid by complex formation with chitosan. Food Chem. 2008, 109, 167–171. [Google Scholar] [CrossRef]
- Kofuji, K.; Isobe, T.; Murata, Y. Controlled release of alpha-lipoic acid through incorporation into natural polysaccharide-based gel beads. Food Chem. 2009, 115, 483–487. [Google Scholar] [CrossRef]
- Li, Y.X.; Kim, Y.J.; Reddy, C.K.; Lee, S.J.; Lim, S.T. Enhanced bioavailability of alpha-lipoic acid by complex formation with octenylsuccinylated high-amylose starch. Carbohydr. Polym. 2019, 219, 39–45. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Nocchetti, M.; Sisani, M.; Pagano, C. Preformulation studies on host-guest composites for oral administration of BCS class IV drugs: HTlc and furosemide. Appl. Clay Sci. 2011, 53, 696–703. [Google Scholar] [CrossRef]
- Pagano, C.; Tiralti, M.C.; Perioli, L. Nanostructured hybrids for the improvement of folic acid biopharmaceutical properties. J. Pharm. Pharmacol. 2016, 68, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Pagano, C. Inorganic matrices: An answer to low drug solubility problem. Exp. Opin. Drug Deliv. 2012, 9, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Conterosito, E.; Croce, G.; Palin, L.; Pagano, C.; Perioli, L.; Viterbo, D.; Boccaleri, E.; Paul, G.; Milanesio, M. Structural characterization and thermal and chemical stability of bioactive molecule-hydrotalcite (LDH) nanocomposites. Chem. Phys. 2013, 15, 13418–13433. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimization of wine phenol extraction by layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Perioli, L.; Pagano, C.; Nocchetti, M.; Latterini, L. Development of smart semisolid formulations to enhance retinoic acid topical application. J. Pharm. Sci. 2015, 104, 3904–3912. [Google Scholar] [CrossRef]
- Nae, H. Rheological properties of topical formulations (Chapter 11). In Handbook of Formulating Dermal Applications: A Definitive Practical Guide, 1st ed.; Dayan, N., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2016. [Google Scholar]
- Focke, W.W.; Molefe, D.; Labuschagne, F.J.W.; Ramjee, S. The influence of stearic acid coating on the properties of magnesium hydroxide, hydromagnesite, and hydrotalcite powders. J. Mater. Sci. 2009, 44, 6100–6109. [Google Scholar] [CrossRef] [Green Version]
- Albiston, L.; Franklin, K.R.; Lee, E.; Smeulders, J.B. Rheology and microstructure of aqueous layered double hydroxide dispersions. J. Mater. Chem. 1996, 6, 871–877. [Google Scholar] [CrossRef]
- Nakayama, H.; Wade, N.; Tsuhako, M. Intercalation of aminoacids and peptides into MgAl layered double hydroxide by recostruction method. Int. J. Pharm. 2004, 269, 469–478. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New synthetic routes to hydrotalcite-like compounds—Characterisation and properties of the obtained materials. Eur. J. Inorg. Chem. 1998, 10, 1439–1446. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Hausner, H.H. Friction conditions in a mass of metal powder. Int. J. Powder Metall. 1967, 3, 7–13. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM) Council of Europe. European Pharmacopoeia, 9th ed.; Density of Powders, Bulk Density and Tapped (Volume 1, Paragraph 2.9.34); EDQM Council of Europe: Strasburg, France, 2017. [Google Scholar]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Vannini, S.; Lazzarini, A.; Floridi, A.; Moretti, M.; Villarini, M.; Fioretti, B.; Beccari, T.; et al. Acid sphingomyelinase as target of Lycium Chinense: Promising new action for cell health. Lipids Health Dis. 2016, 15, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In vitro protective effects of lycium barbarum berries cultivated in Umbria (Italy) on human hepatocellular carcinoma cells. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Italian Health Ministry. Commissione permanente per la revisione e la pubblicazione della farmacopea ufficiale. Monograph “Gel base per preparazione semisolida per applicazione cutanea”. In Italian Pharmacopoeia, 12th ed.; Istituto Poligrafico e Zecca Dello Stato: Roma, Italy, 2008; p. 1171. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Lejeune, B.; Pourrat, A. Formulation of Propolis Extract Emulsions. I. O/W creams based on non-ionic surfactants and various consistency agents pages. Drug Dev. Ind. Pharm. 1995, 21, 1907–1915. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A.K. Spreading of semisolid formulations an update. Pharm. Technol. N. Am. 2002, 26, 84–105. [Google Scholar]
- The European Cosmetic. Toiletry and Perfumery Association (Colipa), Cosmetics Europe: Guidelines on Stability Testing of Cosmetic Products; The European Cosmetic: Lentilly, France, 2004. [Google Scholar]
- Racz, C.P.; Santa, S.; Tomoaia-Cotisel, M.; Borodi, G.; Kacso, I.; Pirnau, A.; Bratu, I. Inclusion of a-lipoic acid in β-cyclodextrin. Physical–chemical and structural characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 193–199. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare (EDQM) Council of Europe. European Pharmacopoeia, 9th ed.; Fineness, Powder (Volume 1, Paragraph 2.9.35); EDQM Council of Europe: Strasburg, France, 2017. [Google Scholar]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-alpha lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef]
- Kozlowska, J.; Pauter, K.; Sionkowska, A. Carrageenan-based hydrogels: Effect of sorbitol and glycerin on the stability, swelling and mechanical properties. Polym. Test. 2018, 67, 7–11. [Google Scholar] [CrossRef]
- Li, Y.; Bi, H.-Y.; Shen, S.-L.; Mao, X.-M.; Wang, C.-R.; Jin, Y.-S. Thixotropic behavior of hydrotalcite-like compound/cationic starch suspensions: Effects of mass ratio of hydrotalcite-like compound to cationic starch, electrolyte, and temperature. J. Disp. Sci. Technol. 2011, 32, 1239–1246. [Google Scholar] [CrossRef]
- Perioli, L.; Pagano, C. Inorganic-organic hybrids to improve the performances of anti-inflammatory topical formulations. Int. J. Pharm. Res. Rev. 2016, 5, 1–18. [Google Scholar]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chhabra, R.P.; Irvine, T.F.; Capobianchi, M. Non-newtonian flows. In Handbook of Thermal Engineering, 2nd ed.; Chhafra, R.P., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 229–230. [Google Scholar]
- Young, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide onto hydrotalcite-like compounds (HTlcs) at high temperatures. Ind. Eng. Chem. Res. 2001, 40, 204–209. [Google Scholar] [CrossRef]
- Tsuji-Naito, K.; Ishikura, S.; Akagawa, M.; Saeki, H. α-Lipoic acid induces collagen biosynthesis involving prolyl hydroxylase expression via activation of TGF-β-Smad signaling in human dermal fibroblasts. Connect. Tissue Res. 2010, 51, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dai, L.; Wang, J.; Jian, Y.; Mei, Z.; Pei, X.; Xiong, X.; Yuan, W.; Wu, F. Beneficial effects of lipoic acid on post-burn hypertrophic scarring model. Int. J. Pharmacol. 2018, 14, 733–739. [Google Scholar]
- Wang, X.; Wu, J. Modulating effect of fatty acids and sterols on skin aging. J. Funct. Foods 2019, 57, 135–140. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Latterini, L.; Nocchetti, M.; Ceccarini, M.R.; Marani, M.; Ramella, D.; Ricci, M. Folic acid-layered double hydroxides hybrids in skin formulations: Technological, photochemical and in vitro cytotoxicity on human keratinocytes and fibroblasts. Appl. Clay Sci. 2019, 168, 382–395. [Google Scholar] [CrossRef]
- Choi, S.J.; Choy, J.H. Layered double hydroxide nanoparticles as target-specific delivery carriers: Uptake mechanism and toxicity. Nanomedicine 2011, 6, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, M.; Kim, D.H. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanopart. Res. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Nishiura, H.; Sugimoto, K.; Akiyama, K.; Musashi, M.; Kubota, Y.; Yokoyama, T.; Yamashita, Y.; Kuriki, T.; Yamaguchi, Y. A novel nano-capsule of α-lipoic acid as a template of core-shell structure constructed by self-assembly. J. Nanomed. Nanotechol. 2013, 4, 2. [Google Scholar] [CrossRef]







| Hydrogel | Composition | ||||
|---|---|---|---|---|---|
| NaCMC (%) | HEC (%) | Sorbitol (%) | HTlc-Cl (%) | Water (%) | |
| P1 | 5.00 | - | 10.00 | 17.48 | 67.52 |
| P2 | 3.50 | - | 10.00 | 17.48 | 69.02 |
| P3 | 3.00 | - | 10.00 | 17.48 | 69.52 |
| P4 | 2.50 | - | 10.00 | 17.48 | 70.02 |
| P5 | - | 2.50 | 10.00 | 17.48 | 70.02 |
| P6 | - | 2.00 | 10.00 | 17.48 | 70.52 |
| P7 | - | 1.75 | 10.00 | 17.48 | 70.77 |
| P8 | - | 1.25 | 10.00 | 17.48 | 71.27 |
| Hydrogel | Composition | ||||||
|---|---|---|---|---|---|---|---|
| Free ALA (%) | MgAl-ALA (%) | ZnAl-ALA (%) | HTlc-Cl (%) | HEC (%) | Sorbitol (%) | Water (%) | |
| ALA-H | 5 | - | - | 17.48 | 1.75 | 10.00 | 70.77 |
| MgAl-ALA-H | - | 11.93 | - | 5.55 | 1.75 | 10.00 | 70.77 |
| ZnAl-ALA-H | - | - | 17.48 | - | 1.75 | 10.00 | 70.77 |
| Formulation | Appearance | Sulfurous Odor * | Color |
|---|---|---|---|
| Tiobec® |  Separation | ++ | White (no colour changes were observed comparing the cream before and after the experiment) |
| ALA-H |  Presence of crystals on the surface | ++ | Yellow (no colour changes were observed comparing the cream before and after the experiment) |
| MgAl-ALA-H |  | - | White (no colour changes were observed comparing the cream before and after the experiment) |
| ZnAl-ALA-H |  | - | White (no colour changes were observed comparing the cream before and after the experiment) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.; Calarco, P.; Ceccarini, M.R.; Beccari, T.; Ricci, M.; Perioli, L. Development and Characterization of New Topical Hydrogels Based on Alpha Lipoic Acid—Hydrotalcite Hybrids. Cosmetics 2019, 6, 35. https://doi.org/10.3390/cosmetics6020035
Pagano C, Calarco P, Ceccarini MR, Beccari T, Ricci M, Perioli L. Development and Characterization of New Topical Hydrogels Based on Alpha Lipoic Acid—Hydrotalcite Hybrids. Cosmetics. 2019; 6(2):35. https://doi.org/10.3390/cosmetics6020035
Chicago/Turabian StylePagano, Cinzia, Paola Calarco, Maria Rachele Ceccarini, Tommaso Beccari, Maurizio Ricci, and Luana Perioli. 2019. "Development and Characterization of New Topical Hydrogels Based on Alpha Lipoic Acid—Hydrotalcite Hybrids" Cosmetics 6, no. 2: 35. https://doi.org/10.3390/cosmetics6020035
APA StylePagano, C., Calarco, P., Ceccarini, M. R., Beccari, T., Ricci, M., & Perioli, L. (2019). Development and Characterization of New Topical Hydrogels Based on Alpha Lipoic Acid—Hydrotalcite Hybrids. Cosmetics, 6(2), 35. https://doi.org/10.3390/cosmetics6020035