Development of an Innovative and Eco-Friendly UV Radiation Absorber, Based on Furan Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Preliminary Screening
2.3. Molecular Design
2.3.1. Synthesis of 2-ethylhexyl 2-cyanoacetate
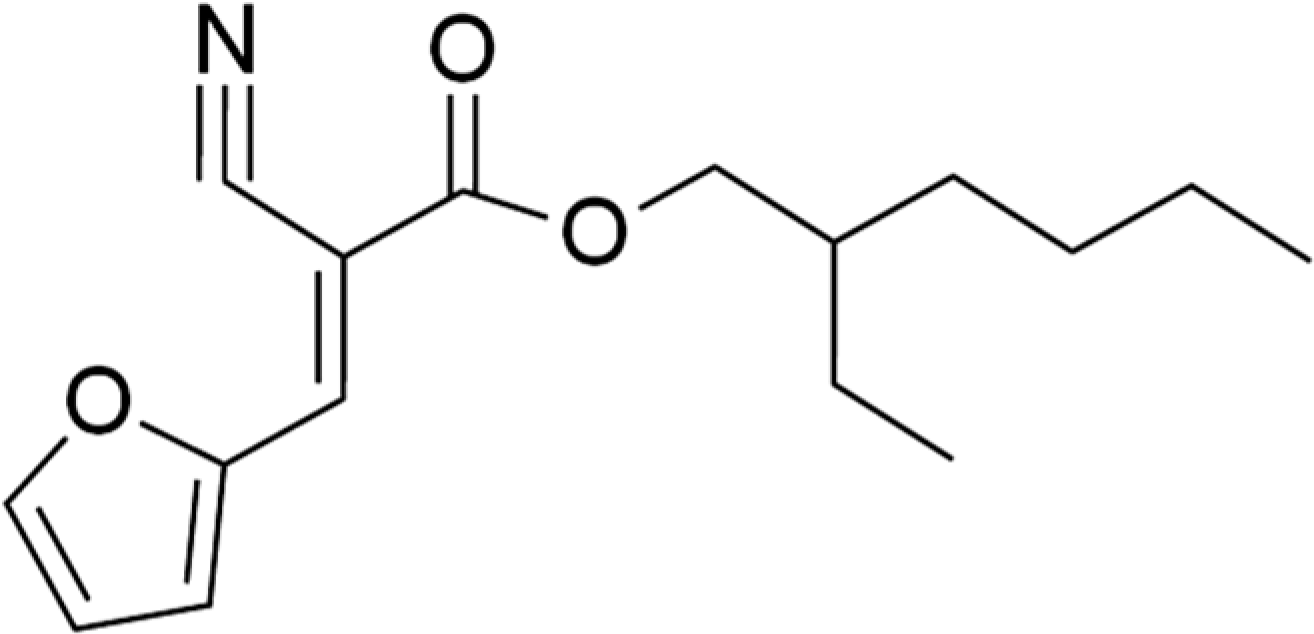
2.3.2. Synthesis of (E,Z)-2-Ethylhexyl 2-Cyano-3-(furan-2-yl)acrylate
2.4. Evaluation of Properties
2.4.1. UV Spectrum and Critical Lambda (λMAX)
- 1-
- Butyl methoxydibenzoylmethane 10−5 M solution
- 2-
- Diethylamino hydroxybenzoyl hexyl benzoate 10−5 M solution.
2.4.2. Solubility
2.4.3. In Vivo Determination of the Sun Protection Factor (SPF)
2.4.4. In Vitro Determination of the UVA Protection Factor (UVA)
3. Results
3.1. Chemistry—Analysis of Characterization
- 1H-NMR: (CDCl3, 400 MHz): δ 8.01 (1H,s, H-4), 7.75 (1H, d, J = 1.7 Hz, H-1), 7.40 (1H, d, J = 3.7 Hz, H-3), 6.66 (1H, dd, J = 3.7, 1.7 Hz, H-2), 4.23–4.20 (2H,m, H-5), 1.73–1.67 (1H, m, H-6), 1.48–1.29 (8H, m, H-7, H-8, H-9, H-11), 0.96–0.88 (6H,m, H-10, H-12).
- 13C NMR (CDCl3, 100 MHz): δ 162.73 (COO), 148.79 (C-γ), 148.14 (C-β), 139.35 (C-1), 121.51 (C-2), 115.16 (CN), 113.80 (C-3), 98.81 (C-α), 68.90 (C-5), 38.77 (C-6), 30.29 (C-7), 28.89 (C-8), 23.74 (C-9), 22.90 (C-11), 13.98 (C-10), 10.97 (C-12)
- GC-MS: 10.35 min
3.2. Chemistry—Determination of Melting Point
3.3. Evaluation of Sun Protection Properties
3.3.1. UV Spectrum and Critical Lambda (λMAX)
3.3.2. Solubility
- 1-
- Di-Ethyl-Hexyl Adipate
- 2-
- Octocrylene
- 3-
- Octyl Methoxy Cinnamate
- 4-
- Ethyl Hexyl Salicylate
- 5-
- Ethanol.
3.3.3. In Vivo Determination of the Sun Protection Factor (SPF)
3.3.4. In Vitro Determination of UVA Protection Factor (UVA)
4. Discussion
4.1. Chemistry
4.2. Evaluation Properties
4.2.1. UV Spectrum and Critical Lambda (λMAX)
4.2.2. Solubility
4.2.3. In Vivo Determination of the Sun Protection Factor (SPF)
4.2.4. In Vitro Determination of UVA Protection Factor (UVA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Metzger, J.O.; Eissen, M.C.R. Chimie, Concepts on the contribution of chemistry to a sustainable development: Renewable raw materials. C. R. Chim. 2004, 7, 569–581. [Google Scholar] [CrossRef]
- Beerling, J.; Sahota, A. Sustainability: How the Cosmetics Industry Is Greening Up; John Wiley & Sons: London, UK, 2014. [Google Scholar]
- Schlumpf, M.; Durrer, S.; Faass, O.; Ehnes, C.; Fuetsch, M.; Gaille, C.; Timms, B. Developmental toxicity of UV filters and environmental exposure: A review. Int. J. Androl. 2008, 31, 144–151. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Tsui, M.M.; Leung, H.W.; Wai, T.C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countrie. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hitce, J.; Xu, J.; Brossat, M.; Frantz, M.C.; Dublanchet, A.C.; Philippe, M.; Dalko-Csiba, M. UN-sustainable development goals: How can sustainable/green chemistry contribute? Green chemistry as a source of sustainable innovations in the cosmetic industry. Curr. Opin. Green Sustain. Chem. 2018, 13, 164–169. [Google Scholar] [CrossRef]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Osterwalder, U.; Herzog, B. Chemistry and Properties of Organic and Inorganic UV Filters. In Clinical Guide to Sunscreens and Photoprotection; CRC Press: Boca Raton, FL, USA, 2008; pp. 27–54. [Google Scholar]
- Mturi, G.J.; Martincigh, B.S. Photostability of the sunscreening agent 4-tert-butyl-4-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A Chem. 2008, 200, 410–420. [Google Scholar] [CrossRef]
- Gasparro, F.P. Sunscreen Photobiology: Molecular, Cellular and Physiological Aspects; Springer: Cham, Switzerland, 1997; pp. 11–42. [Google Scholar]
- Zhu, Y.; Li, W.; Lu, Y.; Zhang, T.; Jameel, H.; Chang, H.M.; Ma, L. Production of furfural from xylose and corn stover catalyzed by a novel porous carbon solid acid in γ-valerolactone. RSC Adv. 2017, 7, 29916–29924. [Google Scholar] [CrossRef] [Green Version]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- COSMETICS–Sun Protection Test Methods-In Vivo Determination of the Sun Protection Factorn (SPF). ISO 24444:2010. Available online: https://www.iso.org/standard/46523.html (accessed on 30 November 2019).
- Moyal, D.; Chardon, A.; Kollias, N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. (Part 1) Calibration of the method. Photodermatol. Photoimmunol. Photomed. 2000, 16, 245–249. [Google Scholar] [CrossRef]
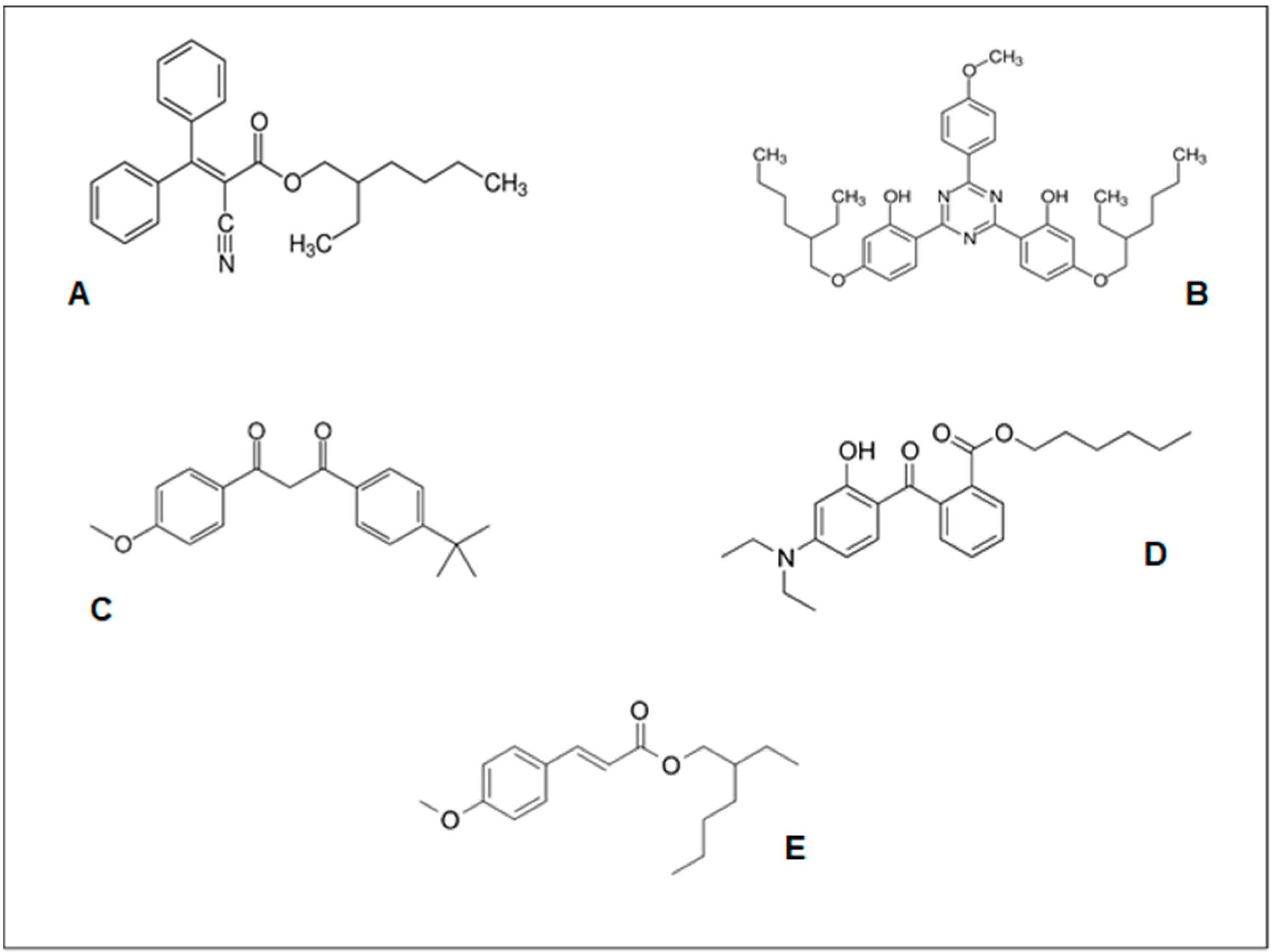






| Compound | Raw Formula | Molar Mass (g/mol) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|
| Octocrylene | C24H27NO2 | 361.48 | 14.0 | 1.05 |
| Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine | C38H49N3O5 | 627.81 | 83.0 | 1.10 |
| Butyl Methoxydibenzoylmethane | C20H22O3 | 310.39 | 83.5 | 1.08 |
| Diethylamino Hydroxybenzoyl Hexyl Benzonate | C24H31NO4 | 397.51 | 54.0 | 1.16 |
| Ethyl Hexyl Methoxycinnamate | C18H26O3 | 290.40 | −25.0 | 1.01 |
| Phase | INCI Name | % w/w |
|---|---|---|
| A | PEG-30 DIPOLYHYXROXYSTEARATE | 3.00–6.00 |
| POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE | 3.00–6.00 | |
| DIBUTYL ADIPATE | 8.00–12.00 | |
| BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE | 7.00–10.00 | |
| BUTYLOCTYL SALICYLATE | 1.00–3.00 | |
| ISONYL ISONONANOATE | 1.00–3.00 | |
| DIETHYLHEXYL CARBONATE | 10.00–15.00 | |
| A1 | TITANIUM DIOXIDE, SILICA | 6.00 |
| A2 | ZINC OXIDE, TRIETHOXYCAPRYLYLSILANE | 4.10 |
| B | AQUA | q.s. |
| GLYCERIN | 1.00–3.00 | |
| ALLANTOIN | 0.02–0.05 | |
| MAGNESIUM SULFATE | 0.50–0.10 | |
| DISODIUM EDTA | 0.10–0.30 | |
| C | PARFUM | 0.20–0.50 |
| PRESERVATIVES | q.s. | |
| 100.00 |
| Phase | INCI Name | % w/w |
|---|---|---|
| A | PEG-30 DIPOLYHYXROXYSTEARATE | 3.00–6.00 |
| POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE | 3.00–6.00 | |
| DIBUTYL ADIPATE | 8.00–12.00 | |
| BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE | 7.00–10.00 | |
| BUTYLOCTYL SALICYLATE | 1.00–3.00 | |
| (E,Z)-2-ETHYLHEXYL-2-CYANO-3-(FURAN-2-YL)ACRYLATE | 5.00 | |
| ISONYL ISONONANOATE | 1.00–3.00 | |
| DIETHYLHEXYL CARBONATE | 10.00–15.00 | |
| A1 | TITANIUM DIOXIDE, SILICA | 6.00 |
| A2 | ZINC OXIDE, TRIETHOXYCAPRYLYLSILANE | 4.10 |
| B | AQUA | q.s. |
| GLYCERIN | 1.00–3.00 | |
| ALLANTOIN | 0.02–0.05 | |
| MAGNESIUM SULFATE | 0.50–0.10 | |
| DISODIUM EDTA | 0.10–0.30 | |
| C | PARFUM | 0.20–0.50 |
| PRESERVATIVES | q.s. | |
| 100.00 |
| Instrument | Model of the Instrument |
|---|---|
| SpectroAnalyser | Labsphere UV2000S |
| Applied amount of product per area | 1.3 mg/cm2 |
| Plate manufacturer/Lot number | Helioscreen HD6 Lot 335 |
| Solar simulator for UV exposure | ATLAS SUNTEST CPS+ |
| Raw UVA irradiance | 1.78 |
| Irradiance correction factor | 3.72 |
| UVA calibrated irradiance (mW/cm2) | 6.6216 mW/cm2 |
| Phase | INCI | % w/w |
|---|---|---|
| A | WATER | q.s. |
| PROPYLENE GLYCOL | 1.00–2.00 | |
| XANTHAN GUM | 0.20–0.40 | |
| CARBOMER | 0.10–0.30 | |
| DISODIUM EDTA | 0.05–0.10 | |
| B | OCTOCRYLENE | 3.00 |
| BUTYL METHOXYDIBENZOYLMETHANE | 5.00 | |
| ETHYLHEXYL METHOXYCINNAMATE | 3.00 | |
| BIS-ETHYLHEXYLOXYPHENOL-METHOXYPHENYL TRIAZINE | 2.00 | |
| CETYL ALCOHOL | 0.50–1.5 | |
| STEARETH-21 | 2.00–3.00 | |
| STEARETH-2 | 3.00–5.00 | |
| DICAPRYLYL CARBONATE | 5.00–7.00 | |
| DECYL COCOATE | 5.00–7.00 | |
| PHENOXYETHANOL | 0.30–0.60 | |
| METHYLPARABEN | 0.10–0.20 | |
| ETHYLPARABEN | 0.10–0.20 | |
| C | CYCLOPENTASILOXANE | 1.00–2.00 |
| D | TRIETHANOLAMINE | 0.10–0.30 |
| 100.00 |
| Phase | INCI | % w/w |
|---|---|---|
| A | WATER | q.s. |
| PROPYLENE GLYCOL | 1.00–2.00 | |
| XANTHAN GUM | 0.20–0.40 | |
| CARBOMER | 0.10–0.30 | |
| DISODIUM EDTA | 0.05–0.10 | |
| B | OCTOCRYLENE | 3.00 |
| (E,Z)-2-ETHYLHEXYL-2-CYANO-3-(FURAN-2-YL)ACRYLATE | 5.00 | |
| ETHYLHEXYL METHOXYCINNAMATE | 3.00 | |
| BIS-ETHYLHEXYLOXYPHENOL-METHOXYPHENYL TRIAZINE | 2.00 | |
| CETYL ALCOHOL | 0.50–1.5 | |
| STEARETH-21 | 2.00–3.00 | |
| STEARETH-2 | 3.00–5.00 | |
| DICAPRYLYL CARBONATE | 5.00–7.00 | |
| DECYL COCOATE | 5.00–7.00 | |
| PHENOXYETHANOL | 0.30–0.60 | |
| METHYLPARABEN | 0.10–0.20 | |
| ETHYLPARABEN | 0.10–0.20 | |
| C | CYCLOPENTASILOXANE | 1.00–2.00 |
| D | TRIETHANOLAMINE | 0.10–0.30 |
| 100.00 |
| Parameters | Average SPF | Standard Deviation |
|---|---|---|
| Standard Formulation | 15.66 | 1.20 |
| Standard Formulation + 5% (E,Z)-2-ethylhexyl 2-cyano-3-(furan-2-yl)acrylate | 17.30 | 2.70 |
| Formulation | UVAPF0 | Dv. Std UVAPF0 | ISO in Vitro UVAPF | Dv. Std (ISO in Vitro UVAPF) |
|---|---|---|---|---|
| Standard Sunscreen Formulation | 14.98 | 0.41 | 12.9 | 0.64 |
| Standard Sunscreen Formulation + (E,Z)-2-ethylhexyl 2-cyano-3-(furan-2-yl)acrylate | 10.20 | 0.26 | 8.40 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomo, B.; Luca, B.; Nicola, L.; Luigi, R. Development of an Innovative and Eco-Friendly UV Radiation Absorber, Based on Furan Moieties. Cosmetics 2020, 7, 6. https://doi.org/10.3390/cosmetics7010006
Giacomo B, Luca B, Nicola L, Luigi R. Development of an Innovative and Eco-Friendly UV Radiation Absorber, Based on Furan Moieties. Cosmetics. 2020; 7(1):6. https://doi.org/10.3390/cosmetics7010006
Chicago/Turabian StyleGiacomo, Busalacchi, Beverina Luca, Lionetti Nicola, and Rigano Luigi. 2020. "Development of an Innovative and Eco-Friendly UV Radiation Absorber, Based on Furan Moieties" Cosmetics 7, no. 1: 6. https://doi.org/10.3390/cosmetics7010006
APA StyleGiacomo, B., Luca, B., Nicola, L., & Luigi, R. (2020). Development of an Innovative and Eco-Friendly UV Radiation Absorber, Based on Furan Moieties. Cosmetics, 7(1), 6. https://doi.org/10.3390/cosmetics7010006






