Simil-Microfluidic Nanotechnology in Manufacturing of Liposomes as Hydrophobic Antioxidants Skin Release Systems
Abstract
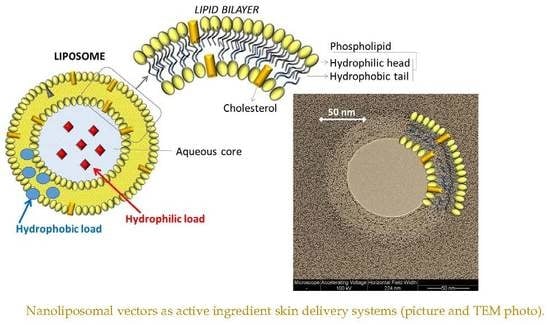
:1. Introduction
2. Materials and Methods
2.1. Antioxidant Nanoliposomes Production through the Simil-Microfluidic Apparatus
2.1.1. Materials
2.1.2. Manufacturing Technique
2.2. Vesicles Characterization
2.2.1. Morphology
2.2.2. Size and Zeta Potential
2.2.3. Encapsulation Efficiency (e.e.) and Effective Load
2.2.4. Stability
2.2.5. Statistical Evaluation
3. Results and Discussions
3.1. Manufacturing Issues
3.2. Liposomes Characterization
3.2.1. Morphology
3.2.2. Size and Zeta Potential
3.2.3. Encapsulation Efficiency and Effective Load
3.2.4. Stability
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhravar, Z.; Ebrahimnejad, P.; Daraee, H.; Akbarzadeh, A. Nanoliposomes: Synthesis methods and applications in cosmetics. J. Cosmet. Laser Ther. 2016, 18, 174–181. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-based cosmetics for hair care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Lin, L.L.; Nufer, K.L.; Tomihara, S.; Prow, T.W. Non-invasive nanoparticle imaging technologies for cosmetic and skin care products. Cosmetics 2015, 2, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Nanotechnology, inflammation and the skin barrier: Innovative approaches for skin health and cosmesis. Cosmetics 2015, 2, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef] [Green Version]
- UNION, P. Regulation (EC) No 1223/2009 of the european parliament and of the council. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Preud’homme, L.; Depues, A.; Noiset, S. Cosmetic regulatory writing. Med. Writ. 2014, 23, 186–189. [Google Scholar] [CrossRef]
- SCCS. Guidance on the Safety Assessment of Nanomaterials in Cosmetics. 2012. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_233.pdf (accessed on 2 April 2020).
- Ansell, J.; Rauscher, H. Report of the Joint Regulator-Industry Ad Hoc Working Group: Currently Available Methods for Characterization of Nanomaterials; International Cooperation on Cosmetics Regulation (ICCR-5): Paris, France, 2011; Available online: http://ec.europa.eu/consumers/sectors/cosmetics/files/pdf/iccr5_char_nano_en.pdf (accessed on 2 April 2020).
- Zoghi, A.; Khosravi-Darani, K.; Omri, A. Process variables and design of experiments in liposome and nanoliposome research. Mini Rev. Med. Chem. 2018, 18, 324–344. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, H.R.A.; Bishe, P.; Lashgari, N.A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in cosmetics. J. Skin Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef] [Green Version]
- Salvetová, E.; Muthný, T. Delivery systems in cosmetics. Chemistry 2014, 9, 3. [Google Scholar]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal Vitamin D3 as an Anti-Aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D. Effect of lipid composition on in vitro release and skin deposition of curcumin encapsulated liposomes. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.; Setoguchi, S.; Yamakawa, H.; Watase, D.; Terada, K.; Matsunaga, K.; Karube, Y.; Takata, J. Prodrugs for Skin Delivery of Menahydroquinone-4, an Active Form of Vitamin K2 (20), Could Overcome the Photoinstability and Phototoxicity of Vitamin K2 (20). Int. J. Mol. Sci. 2019, 20, 2548. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for delivery of antioxidants on the skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Dimitrova, J. Therapeutic efficacy of local choleclaciferol in facial seborrheic dermatitis. Proc. ARSA-Adv. Res. Sci. Areas 2016. [Google Scholar] [CrossRef]
- Philips, N.; Ding, X.; Kandalai, P.; Marte, I.; Krawczyk, H.; Richardson, R. The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts. Cosmetics 2019, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, A.A.; Houshmand, G.; Ghorbanzadeh, B.; Nemati, M.; Behmanesh, M.A. Topical vitamin K1 promotes repair of full thickness wound in rat. Indian J. Pharmacol. 2014, 46, 409. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S.A. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.A.; Taher, Z.M.; Muda, R.; Aziz, R. Cosmeceuticals and Natural Cosmetics; Recent Trends in Research into Malaysian Medicinal Plants Research; Penerbit UTM Press: Johor, Malaysia, 2017; pp. 126–175. [Google Scholar]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell. Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Gyamera, B.; Kim, Y.-H. Preparation and Characterization of Liposomes Containing Green Tea and Roselle Extracts to be Used in Cosmetics. J. Int. Dev. Coop. 2019, 14, 131–160. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; Santos, L. Delivery systems for cosmetics-From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Heinrich, H.; Ursula, F. Improved Liposomal Formulations of Lipophilic Compounds; European Patent Office: Munich, Germany, 2010. [Google Scholar]
- Imanaka, H.; Ando, H.; Makino, T. Skin-Whitening Cosmetic. Google Patents US006669932B2, 30 December 2003. [Google Scholar]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Caccavo, D.; Cascone, S.; Lamberti, G. Polymeric and Lipid-Based Systems for Controlled Drug Release: An Engineering Point of View. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–304. [Google Scholar]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [Green Version]
- Bochicchio, S.; Dalmoro, A.; Recupido, F.; Lamberti, G.; Barba, A.A. Nanoliposomes production by a protocol based on a simil-microfluidic approach. In Advances in Bionanomaterials; Springer: Berlin, Germany, 2018; pp. 3–10. [Google Scholar]
- Bochicchio, S.; Dalmoro, A.; Bertoncin, P.; Lamberti, G.; Moustafine, R.I.; Barba, A.A. Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: Conventional and next-generation approaches. RSC Adv. 2018, 8, 34614–34624. [Google Scholar] [CrossRef] [Green Version]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.; Amadeu, M.S.; Lancellotti, M.; Hollanda, L.M.D.; Machado, D. The role of nanomaterials in cosmetics: National and international legislative aspects. Quím. Nova 2015, 38, 599–603. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, S.; Kanojia, N.; Grewal, A.S.; Arora, S. Nanotechnology: A Modern Contraption in Cosmetics and Dermatology. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Niemiec, S.; Nystrand, G.; Wang, J. Method of Manufacturing Liposomes. Google Patents 462,218,8, 11 November 1986. [Google Scholar]
- Hood, R.R.; Kendall, E.L.; DeVoe, D.L.; Quezado, Z.; Junqueira, M.; Finkel, J.C.; Vreeland, W.N. Microfluidic formation of nanoscale liposomes for passive transdermal drug delivery. In Proceedings of the 2013 Microsystems for Measurement and Instrumentation: Fulfilling the Promise (MAMNA), Gaithersburg, MD, USA, 14 May 2013. [Google Scholar]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. CCID 2010, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Jalili, S.; Saeedi, M. Study of curcumin behavior in two different lipid bilayer models of liposomal curcumin using molecular dynamics simulation. J. Biomol. Struct. Dyn. 2016, 34, 327–340. [Google Scholar] [CrossRef]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behaviour. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Duman, G.; Aslan, İ.; Yekta Özer, A.; İnanç, İ.; Taralp, A. Liposome, gel and lipogelosome formulations containing sodium hyaluronate. J. Liposome Res. 2014, 24, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Kaszás, N.; Gróf, P.; Lenti, K.; Maghami, K.; Antal, I.; Klebovich, I.; Petrikovics, I.; Budai, M. Liposomes for topical use: A physico-chemical comparison of vesicles prepared from egg or soy lecithin. Sci. Pharm. 2013, 81, 1151–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausili, A.; Clemente, J.; Pons-Belda, Ó.D.; deGodos, A.M.; Corbalan-Garcia, S.; Torrecillas, A.; Teruel, J.A.; Gomez-Fernandez, J.C. Interaction of vitamin K1 and vitamin K2 with DMPC and their location in the membrane. Langmuir 2020, 36, 1062–1073. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 569. [Google Scholar]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.; Chen, S.; Gao, Y. α-Tocopherol liposome loaded chitosan hydrogel to suppress oxidative stress injury in cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef]


| Numerical Size (nm) ± SD | Z Average, (nm) ± SD | PDI ± SD | Zeta Potential (mV) ± SD | |
|---|---|---|---|---|
| Unloaded nanoliposomes | 90.00 ± 17.0 | 246.3 ± 1.10 | 0.37 ± 0.04 | −35.2 ± 0.83 |
| Vit. D3–nanolip. | 87.42 ± 17.3 | 343.9 ± 61.9 | 0.40 ± 0.07 | −38.5 ± 1.6 |
| Aged samples | 117.2 ± 42.6 | 502.7 ± 10.9 | 0.50 ± 0.03 | −37.2 ± 2.1 |
| Vit. K2–nanolip. | 144.8 ± 32.7 | 289.1 ± 61.9 | 0.31 ± 0.01 | −36.2 ± 0.34 |
| Aged samples | 95.62 ± 33.0 | 322.9 ± 6.85 | 0.37 ± 0.03 | −36.6 ± 3.0 |
| Vit. E–nanolip. | 118.2 ± 51.9 | 294.0 ± 35.3 | 0.38 ± 0.05 | −26.51 ± 2.0 |
| Aged samples | 101.6 ± 18.0 | 303.9 ± 6.72 | 0.45 ± 0.06 | −38.8 ± 3.3 |
| Cur–nanolip. | 83.70 ± 17.5 | 525.3 ± 18.9 | 0.67 ± 0.02 | −17.9 ± 3.0 |
| Aged samples | 96.74 ± 15.7 | 378.4 ± 47.0 | 0.72 ± 0.1 | −19.9 ± 3.2 |
| Theoretical Load % | Effective Load % | e.e. % ± SD | e.e. % ± SD after 1 Month (Aged Samples) | |
|---|---|---|---|---|
| Vit. D3–nanolip. | 10.4 | 9.20 | 88.4 ± 2.5 | 87.3 ± 0.71 |
| Vit. K2–nanolip. | 10.4 | 9.80 | 94.7 ± 0.77 | 93.8 ± 0.43 |
| Vit. E–nanolip. | 16.2 | 15.10 | 93.2 ± 0.10 | 94.2 ± 0.60 |
| Cur.–nanolip. | 11.2 | 11.0 | 98.4 ± 0.20 | 97.6 ± 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochicchio, S.; Dalmoro, A.; De Simone, V.; Bertoncin, P.; Lamberti, G.; Barba, A.A. Simil-Microfluidic Nanotechnology in Manufacturing of Liposomes as Hydrophobic Antioxidants Skin Release Systems. Cosmetics 2020, 7, 22. https://doi.org/10.3390/cosmetics7020022
Bochicchio S, Dalmoro A, De Simone V, Bertoncin P, Lamberti G, Barba AA. Simil-Microfluidic Nanotechnology in Manufacturing of Liposomes as Hydrophobic Antioxidants Skin Release Systems. Cosmetics. 2020; 7(2):22. https://doi.org/10.3390/cosmetics7020022
Chicago/Turabian StyleBochicchio, Sabrina, Annalisa Dalmoro, Veronica De Simone, Paolo Bertoncin, Gaetano Lamberti, and Anna Angela Barba. 2020. "Simil-Microfluidic Nanotechnology in Manufacturing of Liposomes as Hydrophobic Antioxidants Skin Release Systems" Cosmetics 7, no. 2: 22. https://doi.org/10.3390/cosmetics7020022
APA StyleBochicchio, S., Dalmoro, A., De Simone, V., Bertoncin, P., Lamberti, G., & Barba, A. A. (2020). Simil-Microfluidic Nanotechnology in Manufacturing of Liposomes as Hydrophobic Antioxidants Skin Release Systems. Cosmetics, 7(2), 22. https://doi.org/10.3390/cosmetics7020022