Physicochemical Aspects of the Performance of Hair-Conditioning Formulations
Abstract
:1. Introduction
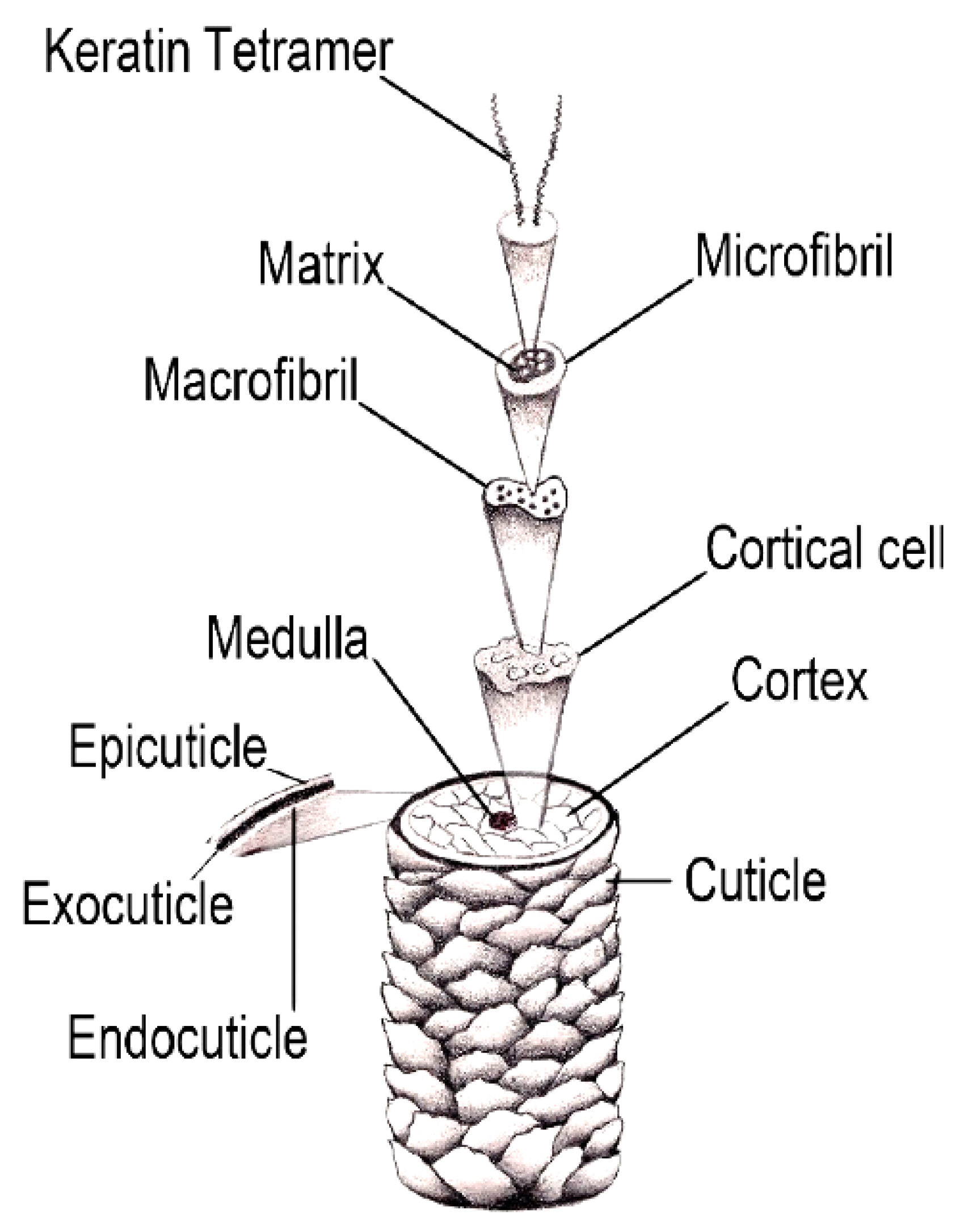
2. Fundamental Aspects of the Hair Structure
3. Physicochemical Bases of the Hair-Conditioning Process
4. Composition of Formulations for Hair Conditioning
4.1. Surfactants
- Anionic surfactants. These surfactants are characterized by the presence of a negatively charged polar group (carboxylates, sulfates, sulfonates or phosphates) bound to a hydrophobic chain, in most cases an alkyl one. These surfactants are considered to be the most efficient in the cleaning of sebum fats and dirt in general. However, an excessive deposition of this surfactant onto the surface of the hair fibers during the washing process may increase the number of negative charges, which would be detrimental for the conditioning process as result of the increase in the friction between fibers. Some examples of anionic surfactants found in commercial shampoos are sodium laureth sulfate (SLES), sodium lauroyl methyl isethionate (SLMI) or sodium methyl lauroyl taurate (SLMT) [7,41,42,43,44,45,46].
- Cationic surfactants. These surfactants are characterized by their positively charged polar group (generally quaternary ammonium), playing a central role in the neutralization of the negative charge existing on the damaged hair surface and minimizing frizz. They may be also used as softeners. Examples of cosmetically acceptable cationic surfactants for hair-conditioning products are benzalkonium chloride, trimetrhylalkylammonium chloride or cetylpyridinium cetrimonium chloride [3,47].
- Nonionic surfactants. These surfactants do not exhibit a net electrical charge in aqueous solutions due to the absence of hydrophilic dissociable groups. These surfactants are less aggressive than other surfactants, and they are widely used as emulsifiers and solubilizers in cosmetic formulations. The most common among this type of surfactants are those based on ethylene oxides, the so-called ethoxylated surfactants. Another important class of nonionic surfactants are “multihydroxy” molecules, such as glycol esters, glycerol and polyglycerol esters, glycosides and polyglycosides and sucrose esters [7,46].
- Zwitterionic surfactants. These surfactants contain both cationic and anionic groups in their hydrophilic heads and are characterized by the possibility of controlling their net charge by tuning the pH. They have good foaming, detergent and wetting properties and are very mild, presenting excellent dermatological properties; therefore, they are used to reduce the aggressiveness of anionic surfactants. The most common are N-alkyl betaines, derived from trimethylglycine (betaine) [45,46,48].
4.2. Polymers
5. Model Surfaces
6. Quantitative Evaluation of the Adsorption of Cosmetic Ingredients
7. Physicochemical Aspects Involved in the Conditioning Process
7.1. Mixtures of Oppositely Charged Polyelectrolytes and Surfactants in Solution
7.2. Adsorption of Polyelectrolyte–Surfactant Mixtures onto Solid Surfaces
7.3. Deposition Enhanced by Dilution
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robbins, C.R. Chemical and Physical Behavior of Human Hair; Science & Business Media: New York, NY, USA, 1988. [Google Scholar]
- Sinclair, R.D. Healthy Hair: What is it? J. Investig. Dermatol. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo, A Review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yang, W.; Wang, B.; Meyers, M.A. Structure and mechanical behavior of human hair. Mat. Sci. Eng. C 2017, 73, 152–163. [Google Scholar] [CrossRef]
- Mitsui, T. New Cosmetic Science; Elsevier Science: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution; John Wiley&Sons: Chichester, UK, 2002. [Google Scholar]
- Fernández-Peña, L.; Guzmán, E.; Leonforte, F.; Serrano-Pueyo, A.; Regulski, K.; Tournier-Couturier, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of molecular structure of eco-friendly glycolipid biosurfactants on the adsorption of hair-care conditioning polymers. Colloids Surf. B Biointerfaces 2020, 185, 110578. [Google Scholar] [CrossRef]
- Baghdadli, N.; Luengo, G.S. A Closer Look at the Complex Hydrophilic/Hydrophobic Interactions Forces at the Human Hair Surface. J. Phys. Conf. Ser. 2008, 100, 052034. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Ortega, F.; Bagdhalli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G. Adsorption of Polyelectrolytes and Polyelectrolytes-Surfactant Mixtures at Surfaces: A Physico-chemical Approach to a Cosmetic Challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and charged polymers at interfaces. Phys. Rep. 2003, 380, 1–95. [Google Scholar] [CrossRef] [Green Version]
- Linse, P.; Källrot, N. Polymer Adsorption from Bulk Solution onto Planar Surface: Effect of Polymer Flexibility and Surface Attraction in Good Solvent. Macromolecules 2010, 43, 2054–2068. [Google Scholar] [CrossRef]
- Cohen-Stuart, M.A. Kinetics of polyelectrolyte adsorption. J. Phys. Cond. Matt. 1997, 9, 7767–7783. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E.; Chuliá, R.; Ortega, F.; Rubio, R.G.; Miller, R. Fluid to soft-glass transition in a quasi-2D system: Thermodynamic and rheological evidences for a Langmuir monolayer. Phys. Chem. Chem. Phys. 2011, 13, 9534–9539. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirrell, M. Polyelectrolyte Complexes: Fluid or Solid? ACS Cent. Sci. 2018, 4, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Schlenoff, J.B. The Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Site-specific perspective on interactions in polyelectrolyte complexes: Toward quantitative understanding. J. Chem. Phys. 2018, 149, 163314. [Google Scholar] [CrossRef]
- Fares, H.M.; Ghoussoub, Y.E.; Delgado, J.D.; Fu, J.; Urban, V.S.; Schlenoff, J.B. Scattering Neutrons along the Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2018, 51, 4945–4955. [Google Scholar] [CrossRef]
- Hössel, P.; Dieing, R.; Nörenberg, R.; Pfau, A.; Sander, R. Conditioning polymers in today’s shampoo formulations - efficacy, mechanism and test methods. Int. J. Cosmetic Sci. 2000, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luengo, G.; Galliano, A.; Dubief, C. Aqueous Lubrication in Cosmetics. In Aqueous Lubrication: Natural and Biomimetic Approaches; Spencer, N., Ed.; World Scientific Publishing Ltd.: London, UK, 2014. [Google Scholar] [CrossRef]
- Miyake, M. Recent progress of the characterization of oppositely charged polymer/surfactant complex in dilution deposition system. Adv. Colloid Interface Sci. 2017, 239, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Faucher, J.A.; Goddard, E.D.; Hannan, R.B. Sorption and Desorption of a Cationic Polymer by Humans Hair: Effects of Salt Solutions. Textile Res. J. 1977, 47, 616–620. [Google Scholar] [CrossRef]
- Goddard, E.D.; Schmitt, R.L. Atomic force microscopy investigations into the adsorption of cationic polymers. Cosmet. Toilet. 1994, 109, 55–61. [Google Scholar]
- Bhushan, B. Nanoscale characterization of human hair and hair conditioners. Progr. Mat. Sci. 2008, 53, 585–710. [Google Scholar] [CrossRef]
- Hordinsky, M.; Avancini Caramori, A.P.; Donovan, J.C. Hair Physiology and Grooming. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; Wiley-Blackwell: Chichester, UK, 2016; pp. 222–226. [Google Scholar]
- Zhang, Y.; Alsop, R.J.; Soomro, A.; Yang, F.-C.; Rheinstädter, M.C. Effect of shampoo, conditioner and permanent waving on the molecular structure of human hair. PeerJ 2015, 3, e1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halal, J.; Schoon, D.D. Hair Structure and Chemistry Simplified; Milady/Thomson Learning: Boston, MA, USA, 2002. [Google Scholar]
- Velasco, M.; Dias, T.; Freitas, A.; Vieira, N.; Pinto, C.; Kaneko, T.; Baby, A. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Braz. J. Pharm. Sci. 2009, 45, 153–162. [Google Scholar] [CrossRef]
- Naizet, S. Dess de Cosmetologie, Monographie; Les c Heveux Gras; Diplôme D’études Supérieures Spécialisées en Cosmétologie, Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2016. [Google Scholar]
- Swift, J.A.; Smith, J.R. Microscopical investigations on the epicuticle of mammalian keratin fibres. J. Microsc. 2001, 204, 203–211. [Google Scholar] [CrossRef]
- Breakspear, S.; Smith, J.R.; Luengo, G. Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface. J. Struct. Biol. 2005, 149, 235–242. [Google Scholar] [CrossRef]
- Huson, M.; Evans, D.; Church, J.; Hutchinson, S.; Maxwell, J.; Corino, G. New insights into the nature of the wool fibre surface. J. Struct. Biol. 2008, 163, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.N.; Rivett, D.E. The role of 18-methyleicosanoic acid in the structure and formation of mammalian hair fibres. Micron 1997, 28, 469–485. [Google Scholar] [CrossRef]
- Andre, V.; Norenberg, R.; Hossel, P.; Pfau, A. The role of polymer-surfactant interactions in the adsorption process of hair-conditioning polymers. Macromol. Symp. 1999, 145, 169–179. [Google Scholar] [CrossRef]
- Pfau, A.; Hössel, P.; Vogt, S.; Sander, R.; Schrepp, W. The interaction of cationic polymers with human hair. Macromol. Symp. 1998, 126, 241–252. [Google Scholar] [CrossRef]
- Gavazzoni Dias, M.F.R. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Grote, M.B.; Russell, G.D. Shampoo Compositions. U.S. Patent 4,741,855, 3 May 1988. [Google Scholar]
- Savary, G.; Grisel, M.; Picard, C. Cosmetics and personal care products. In Natural Polymers: Industry Techniques and Applications; Olatunji, O., Ed.; Springer Intl. Pub.: Basel, Switzerland, 2016; pp. 219–261. [Google Scholar]
- Fisk, P. Chemical Risk Assessment: A Manual for REACH; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Tadros, T.F. Applied Surfactants: Principles and Applications; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Llamas, S.; Guzmán, E.; Baghdadli, N.; Ortega, F.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of poly(diallyldimethylammonium chloride)—sodium methyl-cocoyl-taurate complexes onto solid surfaces. Colloids Surf. A 2016, 505, 150–157. [Google Scholar] [CrossRef]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Llamas, S.; Guzman, E.; Akanno, A.; Fernandez-Pena, L.; Ortega, F.; Campbell, R.A.; Miller, R.; Rubio, R.G. Study of the Liquid/Vapor Interfacial Properties of Concentrated Polyelectrolyte-Surfactant Mixtures Using Surface Tensiometry and Neutron Reflectometry: Equilibrium, Adsorption Kinetics, and Dilational Rheology. J. Phys. Chem. C 2018, 122, 4419–4427. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Llamas, S.; Ortega, F.; G Rubio, R. Two Different Scenarios for the Equilibration of Polycation—Anionic Solutions at Water–Vapor Interfaces. Coatings 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Llamas, S.; Fernández-Peña, L.; Léonforte, F.; Baghdadli, N.; Cazeneuve, C.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of a natural amphoteric surfactant in the bulk and adsorption behavior of polyelectrolyte-surfactant mixtures. Colloids Surf. A 2020, 585, 124178. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; S Luengo, G.; Rubio, A.M.; Rey, A.; Léonforte, F. Self-Consistent Mean Field Calculations of Polyelectrolyte-Surfactant Mixtures in Solution and upon Adsorption onto Negatively Charged Surfaces. Polymers 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Tyagi, R. Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries. Cosmetics 2013, 1, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Surfactant-Like Behavior for the Adsorption of Mixtures of a Polycation and Two Different Zwitterionic Surfactants at the Water/Vapor Interface. Molecules 2019, 24, 3442. [Google Scholar] [CrossRef] [Green Version]
- Im, S.H. Shampoo compositions. In Handbook of Hair in Health and Disease; Preedy, V.R., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 4334–4376. [Google Scholar]
- Guzmán, E.; Ortega, F.; Baghdadli, N.; Luengo, G.S.; Rubio, R.G. Effect of the molecular structure on the adsorption of conditioning polyelectrolytes on solid substrates. Colloids Surf. A 2011, 375, 209–218. [Google Scholar] [CrossRef]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care; Marcel Dekker, Inc.: Basel, Switzerland, 1999. [Google Scholar]
- Kleinschmidt, F.; Stutbenrauch, C.; Delacotte, J.; von Klitzing, R.; Langevin, D. Stratification of foam films containing polyelectrolytes. Influence of the polymer backbone’s rigidity. J. Phys. Chem. B 2009, 113, 3972–3980. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Luengo, G.S.; Rubio, R.G. Adsorption of Conditioning Polymers on Solid Substrates with Different Charge Density. ACS Appl. Mat. Interfaces 2011, 3, 3181–3188. [Google Scholar] [CrossRef]
- Flick, E.W. Cosmetic and Toiletry Formulations; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 2001. [Google Scholar]
- Gorbovitskiĭ, S.E.; Golovanov, E.D. The origin, biochemical composition and physiology of skin tissue fluid. Vestn. Dermatol. Venerol. 1972, 46, 3–8. [Google Scholar] [PubMed]
- Clay, R.C.; Cook, K.; Routh, J.I. Studies in the Composition of Human Hair. J. Am. Chem. Soc. 1940, 60, 2709–2710. [Google Scholar] [CrossRef]
- Dupres, V.; Langevin, D.; Guenoun, P.; Checco, A.; Luengo, G.; Leroy, F. Wetting and Electrical Properties of the Human Hair Surface: Delipidation observed at the Nanoscale. J. Colloid Interface Sci. 2007, 306, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Méndez, S.; Barba, C.; Pons, R.; Martí, M.; Parra, J.L. Lamellar rearrangement of internal lipids from human hair. Chem. Phys. Lipids 2008, 155, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Swift, J. Maple syrup urine disease hair reveals the importance of 18-methyleicosanoic acir in cuticular delamination. Micron 2005, 36, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, E.; Ortega, F.; Prolongo, M.G.; Starov, V.M.; Rubio, R.G. Influence of the molecular architecture on the adsorption onto solid surfaces: Comb-like polymers. Phys. Chem. Chem. Phys. 2011, 13, 16416–16423. [Google Scholar] [CrossRef] [Green Version]
- Naderi, A.; Iruthayaraj, J.; Pettersson, T.; Makuska, R.; Claesson, P.M. Effect of Polymer Architecture on the Adsorption Properties of a Nonionic Polymer. Langmuir 2008, 24, 6676–6682. [Google Scholar] [CrossRef]
- Olanya, G.; Iruthayaraj, J.; Poptoshev, E.; Makuska, R.; Vareikis, A.; Claesson, P.M. Adsorption Characteristics of Bottle-Brush Polymers on Silica: Effect of Side Chain and Charge Density. Langmuir 2008, 24, 5341–5349. [Google Scholar] [CrossRef]
- Vörös, J. The Density and Refractive Index of Adsorbing Protein Layers. Biophys. J. 2004, 87, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Pena, L. Estudio físico-químico de fluidos complejos de uso cosmético. PhD Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2019. [Google Scholar]
- Halthur, T.J.; Elofsson, U.M. Multilayers of Charged Polypeptides As Studied by in Situ Ellipsometry and Quartz Crystal Microbalance with Dissipation. Langmuir 2004, 20, 1739–1745. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyl-dimethylammonium chloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Lee, S.; Zürcher, S.; Dorcier, A.; Luengo, G.S.; Spencer, N.D. Adsorption and Lubricating Properties of Poly(L-lysine)-graft-poly(ethylene glycol) on Human-Hair Surfaces. ACS Appl. Mat. Interface 2009, 1, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, A. Analysis of structural changes in bleached keratin fibers (black and white human hair) using Raman Spectroscopy. Biopolymers 2006, 81, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Luengo, G.S.; Rutland, M.W. Interactions between crossed hair fibers at the nanoscale. Langmuir 2010, 26, 18909–18915. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.R.d.C.; Machado, L.D.B.; Velasco, M.V.R.; Matos, J.d.R. DSC measurements applied to hair studies. J. Therm. Anal Calorim. 2018, 132, 1429–1437. [Google Scholar] [CrossRef]
- Källrot, N.; Dahlqvist, M.; Linse, P. Dynamics of Polymer Adsorption from Bulk Solution onto Planar Surfaces. Macromolecules 2009, 42, 3641–3649. [Google Scholar] [CrossRef]
- Scheujtens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 1. Partition function, segment density distribution, and adsorption isotherms. J. Phys. Chem. 1979, 83, 1619–1635. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Leermakers, F.A.M.; Luengo, G.S. Surfactant-polymer interactions: Molecular architecture does matter. Soft Matter 2015, 11, 2504–2511. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Lénforte, F.; Leermakers, F.A.M.; Luengo, G.S. Modeling of Polyelectrolyte Adsorption from Micellar Solutions onto Biomimetic Substrates. J. Phys. Chem. B 2017, 121, 8638–8651. [Google Scholar] [CrossRef] [Green Version]
- Goddard, E.D.; Hannan, R.B. Polymer/Surfactant Interactions. J. Am. Oil Chem. Soc 1977, 54, 5615–5666. [Google Scholar] [CrossRef]
- Campbell, R.A.; Arteta, M.Y.; Angus-Smyth, A.; Nylander, T.; Varga, I. Effects of Bulk Colloidal Stability on Adsorption Layers of Poly(diallyldimethylammonium Chloride)/Sodium Dodecyl Sulfate at the Air/Water Interface Studied by Neutron Reflectometry. J. Phys. Chem. B 2011, 115, 15202–15213. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.J.; Neuman, R.D.; Claesson, P.M. Desorption of Low-Charge-Density Polyelectrolyte Adlayers in Aqueous Sodium n-Dodecyl Sulfate Solution. J. Colloid Interface Sci. 2001, 237, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, O.J.; Ernstsson, M.; Neuman, R.D.; Claesson, P.M. Effect of Polyelectrolyte Charge Density on the Adsorption and Desorption Behavior on Mica. Langmuir 2002, 18, 1604–1612. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer–surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef]
- Khan, N.; Brettmann, B. Intermolecular Interactions in Polyelectrolyte and Surfactant Complexes in Solution. Polymers 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Kabanov, V.A.; Zezin, A.B. Soluble interpolymeric complexes as a new class of synthetic polyelectrolytes. Pure Appl. Chem. 1984, 56, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Thalberg, K.; Lindman, B. Polymer-surfactant interactions recent developments. In Nteractions of Surfactants with Polymers and Proteins; Goddard, E.D., Ananthapadmanabhan, K.P., Eds.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; Klitzing, R.V. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef]
- Leung, P.S.; Goddard, E.D.; Han, C.; Glinka, C.J. A study of polycationic/anionic systems. Colloids Surf. 1985, 13, 47–62. [Google Scholar] [CrossRef]
- Goldraich, M.; Schwartz, J.R.; Burns, J.L.; Talmon, Y. Microstructures formed in a mixed system of a cationic polymer and an anionic surfactant. Colloids Surf. A 1997, 125, 231–244. [Google Scholar] [CrossRef]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Antunes, F.; Aidarova, S.; Miguel, M.; Nylander, T. Polyelectrolyte-surfactant association—from fundamentals to applications. Colloid J. 2014, 76, 585–594. [Google Scholar] [CrossRef]
- Bergfeldt, K.; Piculell, L.; Linse, P. Segregation and Association in Mixed Polymer Solutions from Flory−Huggins Model Calculations. J. Phys. Chem. 1996, 100, 3680–3687. [Google Scholar] [CrossRef]
- Hansson, P.; Lindman, B. Surfactant–polymer interactions. Curr. Opin. Colloid Interface Sci. 1996, 1, 604–613. [Google Scholar] [CrossRef]
- Goddard, E.D.; Phillips, T.S.; Hannan, R.B. Water soluble polymer-surfactant interaction I. J. Soc. Cosmet. Chem. 1975, 26, 461–475. [Google Scholar]
- Goddard, E.D.; Hannan, R.B. Cationic polymer/anionic surfactant interactions. J. Colloid Interface Sci. 1976, 55, 73–79. [Google Scholar] [CrossRef]
- Nizri, G.; Magdassi, S.; Schmidt, J.; Cohen, Y.; Talmon, Y. Microstructural Characterization of Micro- and Nanoparticles Formed by Polymer−Surfactant Interactions. Langmuir 2004, 2011, 4380–4385. [Google Scholar] [CrossRef]
- Chiappisi, L.; Hoffmann, I.; Gradzielski, M. Complexes of oppositely charged polyelectrolytes and surfactants—Recent developments in the field of biologically derived polyelectrolytes. Soft Matter 2013, 9, 3896–3909. [Google Scholar] [CrossRef] [Green Version]
- Korte, M.; Akari, S.; Kühn, H.; Baghdadli, N.; Möhwald, H.; Luengo, G.S. Distribution and Localization of Hydrophobic and Ionic Chemical Groups at the Surface of Bleached Human Hair Fibers. Langmuir 2014, 30, 12124–12129. [Google Scholar] [CrossRef]
- Nylander, T.; Samoshina, Y.; Lindman, B. Formation of polyelectrolyte–surfactant complexes on surfaces. Adv. Colloid Interface Sci. 2006, 123, 105–123. [Google Scholar] [CrossRef]
- Penfold, J.; Thomas, R.K.; Taylor, D.J.F. Polyelectrolyte/surfactant mixtures at the air–solution interface. Curr. Opin. Colloid Interface Sci. 2006, 11, 337–344. [Google Scholar] [CrossRef]
- Deo, P.; Deo, N.; Somasundaran, P.; Moscatelli, A.; Jockusch, S.; Turro, N.J.; Ananthapadmanabhan, K.P.; Ottaviani, M.F. Interactions of a Hydrophobically Modified Polymer with Oppositely Charged Surfactants. Langmuir 2007, 23, 5906–5913. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.D. Polymer—Surfactant interaction part II. Polymer and surfactant of opposite charge. Colloids Surf. 1986, 19, 301–329. [Google Scholar] [CrossRef]
- Mohr, A.; Nylander, T.; Piculell, L.; Lindman, B.; Boyko, V.; Bartels, F.W.; Liu, Y.; Kurkal-Siebert, V. Mixtures of Cationic Copolymers and Oppositely Charged Surfactants: Effect of Polymer Charge Density and Ionic Strength on the Adsorption Behavior at the Silica–Aqueous Interface. ACS Appl. Mater. Interfaces 2012, 4, 1500–1511. [Google Scholar] [CrossRef]
- Dedinaite, A.; Claesson, P.M. Interfacial Properties of Aggregates Formed by Cationic Polyelectrolyte and Anionic Surfactant. Langmuir 2000, 16, 1951–1959. [Google Scholar] [CrossRef]
- Piculell, L. Understanding and exploiting the phase behavior of mixtures of oppositely charged polymers and surfactants in water. Langmuir 2013, 29, 10313–10329. [Google Scholar] [CrossRef] [PubMed]
- Dhopatkar, N.; Park, J.H.; Chari, K.; Dhinojwala, A. Adsorption and Viscoelastic Analysis of Polyelectrolyte–Surfactant Complexes on Charged Hydrophilic Surfaces. Langmuir 2015, 31, 1026–1037. [Google Scholar] [CrossRef]
- Svensson, A.V.; Huang, L.; Johnson, E.S.; Nylander, T.; Piculell, L. Surface Deposition and Phase Behavior of Oppositely Charged Polyion/Surfactant Ion Complexes. 1. Cationic Guar versus Cationic Hydroxyethylcellulose in Mixtures with Anionic Surfactants. ACS Appl. Mat. Interfaces 2009, 1, 2431–2442. [Google Scholar] [CrossRef]
- Miyake, M.; Kakizawa, Y. Morphological study of cationic polymer-anionic surfactant complex precipitated in solution during the dilution process. J. Cosmet. Sci. 2010, 61, 298–301. [Google Scholar] [CrossRef]
- Jordan, S.L.; Zhang, X.; Amos, J.; Frank, D.; Menon, R.; Galley, R.; Davis, C.; Kalantar, T.; Ladika, M. Evaluation of novel synthetic conditioning polymers for shampoos. J. Cosmet. Sci. 2009, 60, 239–250. [Google Scholar] [CrossRef]








| Phase 1 | INCI Name | wt% |
|---|---|---|
| A | Water | q.s |
| B | Hydroxyethyl cellulose | 0.30 |
| C | Quaternium-82 cetyltrimethylammonium chloride | 1.00 3.33 |
| D | Cetyl alcohol Stearyl alcohol Pentaerythritol tetyracaprylate/tetracaprate Phenyl trimethicone | 1.50 2.00 2.50 0.50 |
| E | Wheat amino acids Hydrolyzed wheat protein PG-propyl silanetriol | 1.00 1.00 |
| F | Sodium hydroxide | q.s |
| G | Citric acid | q.s |
| H | Preservative, color, fragrance | q.s |
| Name | Composition |
|---|---|
| polyquaternium 2 | poly[bis(2-chloroethyl) ether-alt-1,3-bis [3-(dimethylamino)propyl]urea] quaternized |
| polyquaternium 6 | homopolymer of poly(diallyl-dimethyl-ammonium chloride) |
| polyquaternium 7 | copolymer containing 50 wt% diallyl-dimethylammonium chloride and 50 wt% acrylamide |
| polyquaternium 10 | hydroxyethyl cellulose quaternized with 2,3-epoxypropyl-trimethyl-ammonium chloride |
| polyquaternium 17 | poly[oxy-1,2-ethanediyl(dimethyliminio)-1,3-propanediylimino-(1,6-dioxo-1,6-hexanediyl)imino-1,3-propanediyl(dimethyliminio)-1,2-ethanediyl dichloride] |
| polyquaternium 18 | poly[oxy-1,2-ethanediyl(dimethyliminio)-1,3-propanediylimino-(1,6-dioxo-1,6-heptanediyl)imino-1,3-propanediyl(dimethyliminio)-1,2-ethanediyl dichloride] |
| polyquaternium 22 | copolymer containing 50 wt% diallyl-dimethylammonium chloride and 50 wt% acrylic acid |
| polyquaternium 39 | copolymer containing 50 wt% diallyl-dimethylammonium chloride, 25 wt% acrylamide and 25 wt% acrylic acid |
| polyquaternium 53 | copolymer containing 40 wt% methacrylamidopropyltrimonium chloride, 50 wt% acrylamide and 10 wt% acrylic acid |
| polyquaternium 67 | hydroxyethyl cellulose quaternized and polymerized with n-propyl-2-hydroxy-3-trimethyl ammonium and n-propyl-2-hydroxy-3-dimethyl dodecyl ammonium monomers |
| polyquaternium 86 | copolymer of vinyl pyrolidone, vinyl imidazole, vinyl imidazole quaternized with methyl chloride and methacrylic acid (25 wt% of each type of monomer) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. https://doi.org/10.3390/cosmetics7020026
Fernández-Peña L, Guzmán E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics. 2020; 7(2):26. https://doi.org/10.3390/cosmetics7020026
Chicago/Turabian StyleFernández-Peña, Laura, and Eduardo Guzmán. 2020. "Physicochemical Aspects of the Performance of Hair-Conditioning Formulations" Cosmetics 7, no. 2: 26. https://doi.org/10.3390/cosmetics7020026
APA StyleFernández-Peña, L., & Guzmán, E. (2020). Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics, 7(2), 26. https://doi.org/10.3390/cosmetics7020026






