Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety †
Abstract
:1. Introduction
2. Materials and Methods
2.1. In-Vitro Studies
2.1.1. Bacterial Strains
2.1.2. Bacterial Medium
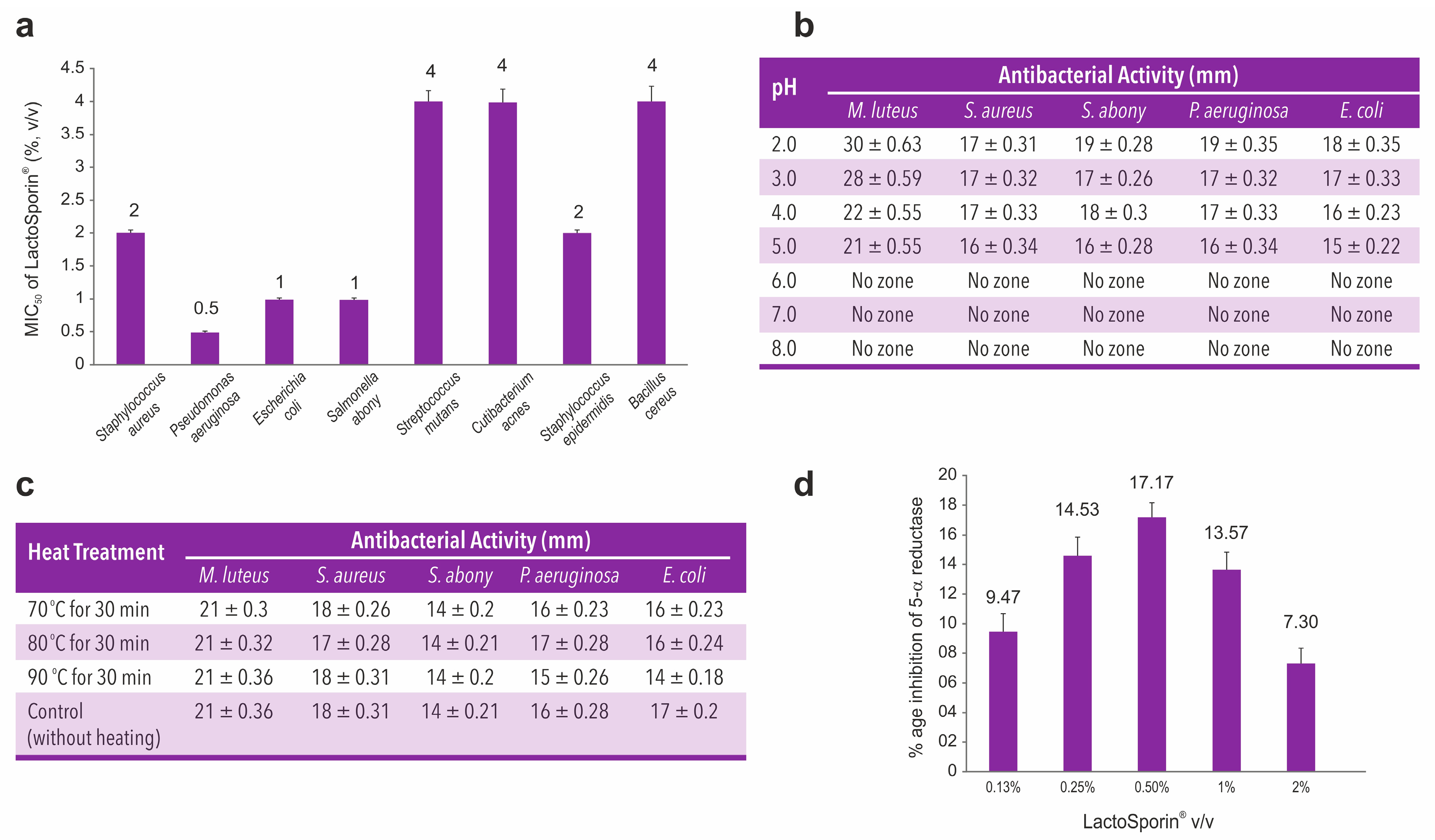
2.1.3. Antimicrobial Activity of LactoSporin (Minimum Inhibitory Concentration)
2.1.4. Effect of pH on Antimicrobial Activity of LactoSporin (pH Stability Study)
2.1.5. Effect of Temperature on Antimicrobial Activity of LactoSporin (Thermostability Study)
2.1.6. 5-Alpha Reductase Inhibitory Activity of LactoSporin
2.2. Clinical Studies
2.2.1. Test Product
2.2.2. Primary Skin Irritation Patch Test of LactoSporin
2.2.3. The Antiacne Potential of LactoSporin
Study Design
Study Population
Study Outcome Assessments
2.2.4. Ethics and Informed Consent Form
2.3. Statistical Analysis
3. Results
3.1. In-Vitro Antimicrobial, pH Stability, Thermostability and 5-Alpha Reductase Inhibition Studies
3.2. Primary Skin Irritation Patch Test
3.3. Antiacne Clinical Study
3.3.1. Study Population
3.3.2. Assessment Based on the Instrumental Counting of Acne Lesions
Antera 3D™ Assessment
Sebumeter® MPA 580 Assessment—Sebum Secretion
3.3.3. Dermatological Assessment
3.3.4. Assessment Based on Subject Self-Assessment Questionnaire:
3.3.5. Safety Outcomes:
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunliffe, W.J.; Gould, D.J. Prevalence of facial acne vulgaris in late adolescence and in adults. Br. Med. J. 1979, 1, 1109–1110. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.K.; Shalita, A.R. Acne vulgaris. Lancet 1998, 351, 1871–1876. [Google Scholar] [CrossRef]
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172 (Suppl. 1), 3–12. [Google Scholar] [CrossRef]
- Bagatin, E.; Timpano, D.L.; Guadanhim, L.R.; Nogueira, V.M.; Terzian, L.R.; Steiner, D.; Florez, M. Acne vulgaris: Prevalence and clinical forms in adolescents from Sao Paulo, Brazil. An. Bras. Dermatol. 2014, 89, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef]
- Sutaria, A.H.; Schlessinger, J. Acne vulgaris. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Cooper, A.J.; Harris, V.R. Modern management of acne. Med. J. Aust. 2017, 206, 41–45. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27. [Google Scholar]
- Jappe, U. Pathological mechanisms of acne with special emphasis on Propionibacterium acnes and related therapy. Acta Derm. Venereol. 2003, 83, 241–248. [Google Scholar] [CrossRef] [Green Version]
- See, J.-A.; Goh, C.L.; Hayashi, N.; Suh, D.H.; Casintahan, F.A. Optimizing the use of topical retinoids in Asian acne patients. J. Dermatol. 2018, 45, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Krowchuk, D.P.; Leyden, J.J.; Lucky, A.W.; Shalita, A.R.; Siegfried, E.C.; Thiboutot, D.M.; Van Voorhees, A.S.; Beutner, K.A.; Sieck, C.K.; et al. Guidelines of care for acne vulgaris management. J. Am. Acad. Dermatol. 2007, 56, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Dreno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; Lopez-Estebaranz, J.L.; et al. European evidence-based (S3) guidelines for the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. 1), 1–29. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Degitz, K. Antibiotics, azelaic acid and benzoyl peroxide in topical acne therapy. J. Dtsch. Dermatol. Ges. 2010, 8 (Suppl. 1), S24–S30. [Google Scholar] [CrossRef]
- Decker, L.C.; Deuel, D.M.; Sedlock, D.M. Role of lipids in augmenting the antibacterial activity of benzoyl peroxide against Propionibacterium acnes. Antimicrob. Agents Chemother. 1989, 33, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Fox, L.; Csongradi, C.; Aucamp, M.; du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef] [Green Version]
- Tester, R.; Al-Ghazzewi, F. The role of pre-and probiotics in skin care. Inside Cosmeceuticals 2012, 1, 5–9. [Google Scholar]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.M.; Denning, P.W. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: What is the current evidence? Clin. Perinatol. 2013, 40, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int. J. Food Microbiol. 1995, 28, 169–185. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Adler, B.L.; Kornmehl, H.; Armstrong, A.W. Antibiotic Resistance in Acne Treatment. JAMA Dermatol. 2017, 153, 810–811. [Google Scholar] [CrossRef]
- Leyden, J.J. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis 2004, 73, 6–10. [Google Scholar]
- Messi, P.; Guerrieri, E.; Bondi, M. Bacteriocin-like substance (BLS) production in Aeromonas hydrophila water isolates. FEMS Microbiol. Lett. 2003, 220, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Jun, K.D.; Kim, W.S.; Paik, H.D. Partial characterization of polyfermenticin SCD, a newly identified bacteriocin of Bacillus polyfermenticus. Lett. Appl. Microbiol. 2001, 32, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Ali, F. Method of Producing Partially Purified Extracellular Metabolite Products from Bacillus coagulans and Biological Applications Thereof. U.S. Patent 9596861B2, 21 March 2017. [Google Scholar]
- Da Cunha, N.B.; Cobacho, N.B.; Viana, J.F.; Lima, L.A.; Sampaio, K.B.; Dohms, S.S.; Ferreira, A.C.; de la Fuente-Núñez, C.; Costa, F.F.; Franco, O.L. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov. Today 2017, 22, 234–248. [Google Scholar] [CrossRef]
- Wormser, G.P.; Tang, Y.-W. Antibiotics in Laboratory Medicine, Edited by Victor Lorain Philadelphia: Lippincott Williams & Wilkins, 2005 832 pp., illustrated. $199.00 (cloth). Clin. Infect. Dis. 2005, 41, 577. [Google Scholar]
- Wayne, P. Performance Standards for Antimicrobial Disc Susceptibility Testing; National Committee for Clinical Laboratory Standards: Albany, NY, USA, 2002; Volume 12, pp. 1–53. [Google Scholar]
- Fried, R.G.; Wechsler, A. Psychological problems in the acne patient. Dermatol. Ther. 2006, 19, 237–240. [Google Scholar] [CrossRef]
- Tripathi, S.V.; Gustafson, C.J.; Huang, K.E.; Feldman, S.R. Side effects of common acne treatments. Expert Opin. Drug Saf. 2013, 12, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Therapeutic agents and herbs in topical application for acne treatment. Int. J. Cosmet. Sci. 2011, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Alexandre Rocha, M.; Sousa Costa, C.; Bagatin, E. Acne vulgaris: An inflammatory disease even before the onset of clinical lesions. Inflamm. Allergy-Drug Targets (Former. Curr. Drug Targets-Inflamm. Allergy) 2014, 13, 162–167. [Google Scholar]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [Green Version]
- Webster, G.F. The pathophysiology of acne. Cutis 2005, 76, 4–7. [Google Scholar]
- Kober, M.M.; Bowe, W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Women Dermatol. 2015, 1, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Palm, N.W.; de Zoete, M.R.; Flavell, R.A. Immune-microbiota interactions in health and disease. Clin. Immunol. 2015, 159, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, C.C.; Swink, S.M.; Horwinski, J.; Sfyroera, G.; Bugayev, J.; Grice, E.A.; Yan, A.C. The preadolescent acne microbiome: A prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatr. Dermatol. 2017, 34, 661–664. [Google Scholar] [CrossRef]
- Mankoci, S.; Ewing, J.; Dalai, P.; Sahai, N.; Barton, H.A.; Joy, A. Bacterial Membrane Selective Antimicrobial Peptide-Mimetic Polyurethanes: Structure–Property Correlations and Mechanisms of Action. Biomacromolecules 2019, 20, 4096–4106. [Google Scholar] [CrossRef]
- Cinque, B.; La Torre, C.; Melchiorre, E.; Marchesani, G.; Zoccali, G.; Palumbo, P.; Di Marzio, L.; Masci, A.; Mosca, L.; Mastromarino, P. Use of probiotics for dermal applications. In Probiotics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–241. [Google Scholar]
- Vermeer, L.S.; Lan, Y.; Abbate, V.; Ruh, E.; Bui, T.T.; Wilkinson, L.J.; Kanno, T.; Jumagulova, E.; Kozlowska, J.; Patel, J.; et al. Conformational flexibility determines selectivity and antibacterial, antiplasmodial, and anticancer potency of cationic alpha-helical peptides. J. Biol. Chem. 2012, 287, 34120–34133. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.F.; Leech, J.M.; Rogers, T.R.; McLoughlin, R.M. Staphylococcus aureus Colonization: Modulation of Host Immune Response and Impact on Human Vaccine Design. Front. Immunol. 2014, 4, 507. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Deplewski, D.; Rosenfield, R.L. Role of hormones in pilosebaceous unit development. Endocr. Rev. 2000, 21, 363–392. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinol 2011, 3, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, I.A.; de Canha, M.N.; Lall, N. Exploiting medicinal plants as possible treatments for acne vulgaris. In Medicinal Plants for Holistic Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–143. [Google Scholar]
- Sakuma, T.H.; Maibach, H.I. Oily skin: An overview. Skin Pharmacol. Physiol. 2012, 25, 227–235. [Google Scholar] [CrossRef]
- Kim, B.; Choi, J.; Park, K.; Youn, S.W. Sebum, acne, skin elasticity, and gender difference–which is the major influencing factor for facial pores? Skin Res. Technol. 2013, 19, e45–e53. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot. Perspect. 2020, 10, 3–4. [Google Scholar] [CrossRef] [Green Version]





| SN | Time | LactoSporin Cream | SLS (Sodium Lauryl Sulphate, 1% w/w) | ||
|---|---|---|---|---|---|
| Mean Irritation Score | Irritancy Assessment | Mean Irritation Score | Irritancy Assessment | ||
| 1 | 0 h | 0.17 | Nonirritant | 2.13 | Irritant |
| 2 | 24 h | 0.04 | Nonirritant | 1 | Irritant |
| 3 | Day 7 | 0.00 | Nonirritant | 0.79 | Nonirritant |
| Particulars | LactoSporin Cream | Benzoyl Peroxide | |
|---|---|---|---|
| Age Mean ± SD Years | 23.70 ± 2.07 | 24.88 ± 2.08 | |
| Sex, n (%) | Male | 18 (53) | 15 (44) |
| Female | 16 (47) | 19 (56) | |
| Lesion counts, mean ± SD | Open comedones | 1.03 ± 1.82 | 1.00 ± 1.74 |
| Closed comedones | 33.19 ± 16.76 | 30.25 ± 15.19 | |
| Papules | 7.19 ± 4.72 | 6.81 ± 4.58 | |
| Pustules | 0.53 ± 1.41 | 0.06 ± 0.25 | |
| Total | 41.94 ± 24.71 | 38.12 ± 21.76 | |
| Subjects’ skin types, n | Normal | 12 | 11 |
| Oily | 22 | 23 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety. Cosmetics 2020, 7, 70. https://doi.org/10.3390/cosmetics7030070
Majeed M, Majeed S, Nagabhushanam K, Mundkur L, Rajalakshmi HR, Shah K, Beede K. Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety. Cosmetics. 2020; 7(3):70. https://doi.org/10.3390/cosmetics7030070
Chicago/Turabian StyleMajeed, Muhammed, Shaheen Majeed, Kalyanam Nagabhushanam, Lakshmi Mundkur, H. R. Rajalakshmi, Kalpesh Shah, and Kirankumar Beede. 2020. "Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety" Cosmetics 7, no. 3: 70. https://doi.org/10.3390/cosmetics7030070
APA StyleMajeed, M., Majeed, S., Nagabhushanam, K., Mundkur, L., Rajalakshmi, H. R., Shah, K., & Beede, K. (2020). Novel Topical Application of a Postbiotic, LactoSporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate its Efficacy, Tolerability and Safety. Cosmetics, 7(3), 70. https://doi.org/10.3390/cosmetics7030070






