1. Introduction
The demand for effective skin care products has grown exponentially over the past few decades, mainly due to an overall increase in the age population along with a serious increase in air pollution [
1]. The consumer has not only become more self-conscious of their own health, but also of their environment, since concerns about pollution have been made evident across the population with the world’s current plastic waste crisis and the consequent circular economy strategies [
2]. For this reason, it is not uncommon to find consumers choosing what they are buying by looking carefully at the ingredients of the products. This is particularly recurrent in the field of food, but also in cosmetics, where claims such as “bio”, “green”, or “natural” are popularly used [
3,
4].
To meet consumer’s demands, the cosmetic sector is searching alternatives to current materials by developing innovative formulations, in which effective skin products are based on natural and sustainable ingredients [
5,
6]. Generating functional biobased beauty masks has become one of the key aspects to investigate in the cosmetic industry, being the use of bio-based hydrophilic polymers to produce beauty masks a good alternative to conventional masks [
1]. Among the many bio-based hydrophilic polymers, the use of polysaccharides, such as pullulan, may be a good option to generate efficient biobased beauty masks. Pullulan is a non-ionic water-soluble biopolymer produced using various substrates such as starch, distilled by-products or other residues [
7,
8]. The highly hydrophilic nature of this odorless material allows to instantaneously retain water when humidified and solubilizes very fast, thus appearing ideal to be used in contact with the skin. Consequently, the active ingredients are released immediately and waste generation is avoided [
9]. Additionally, pullulan has been declared safe with a generally recognized as safe (GRAS) status by the Food and Drug Administration in the USA, as well as certified harmless for food usage by regulations in other countries so it can deem as acceptable for their applications in the cosmetic field [
7,
10].
Electrospinning is an emerging technique which has been used in several fields such as pharma, biomedicine, food packaging, air filtration and cosmetics, with relatively high success [
9,
11,
12]. When compared to conventional methods to generate non-woven structures (i.e., melt blown), electrospinning has various advantages such as its nano-scale fiber diameter, which enables a high-surface/volume ratio and the versatility of the technology, which enables the processing of a vast number of different materials [
13]. The fibrous materials obtained from this technique appear as interconnected meshes of fibers with size approaching the nanoscale, which closely resembles the three-dimensional (3D) structure of the fibrous extracellular matrix (ECM). Such a structure can be very beneficial for skin care products, since a high skin contact and compatibility can be achieved due to the high surface area offered by the nanofibers and its 3D configuration [
14]. In the cosmetic field, the use of electrospinning can be also used to overcome current issues related to the preservation of active ingredients by efficiently trapping them inside the fibers or within their mesh pores. Conventional beauty facial masks are made of non-woven structures that have previously been soaked with active ingredients. If preservatives are not used, these ingredients are not efficiently protected by a wet formulation approach. As such, sensitive ingredients like antioxidants can undergo degradation even before the mask is used, thus reducing their effectiveness. A dry state beauty mask would certainly increase the stability of the nutrients, minimizing their oxidation or degradation and avoiding the use of emulsifiers, preservatives, and other chemicals, further enhancing the sustainability of the mask [
7,
14,
15]. However, certain active ingredients may poorly interact with the carrier mixture during electrospinning, hence negatively affecting the stability of the process, and ultimately leading to an inhomogeneous spatial distribution of the molecules in the tissue area. When this is the case, the availability of novel technologies able to impregnate porous materials with powders from the solid state, for example via high intensity alternating electric field, open the possibility of producing dry beauty masks based on nonwovens impregnated with bioactive fillers. Dry powder impregnation via electric field is a novel technology patented by Fibroline S.L. that allows the impregnation of powder form materials into porous structures (textiles, nonwovens, papers, foams, etc.) by applying an alternative electric field [
16]. The specific concentration of an electrical field in different areas leads to a very homogenous distribution of the particles inside the substrate. In this case, compared to customarily used wet processes, dry impregnation technologies help to create new biofriendly products by avoiding the use of solvents, reducing pollution, and also reducing considerably energy consumption.
Complexes of chitin nanofibrils-nanolignin-glycyrrethinic acid (CLA) have resulted promising for skin contact applications [
17,
18]. Chitin nanofibril (CN) is a non-toxic crystalline polymer derived from crustacean waste which has shown excellent skin compatibility due to its high moisturizing effectiveness. Nanolignin (NL), a negatively charged by-product derived from forestry and agri-food waste, is reported to show beneficial properties to human health, for example, antioxidant and photoprotective activities due to its high phenolic content. The positively charged CN can interact with the negatively charged LN to form nanocomplexes which in turn are able to incorporate biomolecules in their structure, as it is the case of glycyrrhetinic acid (GA) [
17,
18]. This acid is extracted from the licorice plant and characterized by their antioxidative, anti-inflammatory and antimicrobial properties. The strong anti-inflammatory efficacy of this acid is particularly important, since inflammation processes are the start point of many skin issues, such as acne or atopic skin [
9,
19,
20,
21].
In this work, the dry impregnation technology has allowed to impregnate this type of complexes into electrospun non-woven pullulan fibers that perfectly embedded the microparticles without affecting the integrity of the electrospun structure. Therefore, this study reports the production and validation of a pullulan electrospun beauty mask impregnated with CLA micro complexes with the dry powder impregnation technology. The effectiveness of the beauty mask was demonstrated by in vitro and in vivo tests in healthy human volunteers. This study suggests that the combination of these two innovative technologies may open new alternatives to conventional beauty masks by generating effective cosmetic products which can in turn provide sustainable and biofriendly solutions.
2. Materials and Methods
2.1. Materials
The powder complex consisting of CN-NL-GA (also named as CLA) was supplied by Texol s.r.l. (Pescara, Italy). Pullulan (cosmetic grade) was supplied by Hayashibara Co., Ltd. (Okayama, Japan). Deionized water was employed for pullulan dissolution. Immortalized human keratinocytes, HaCaT cell line, were obtained from ATCC-LGC Standards (Milan, Italy). Human mesenchymal stromal cells (HMSCs) were supplied from Merck Millipore S.A.S. (Burlington, MA, USA). MgCl2, Dulbecco’s Modified Essential Medium (D-MEM), L-glutamine, penicillin, streptomycin, and fetal calf serum were purchased from Invitrogen (Carlsbad, CA, USA). AlamarBlue and Calcein AM were bought from ThermoFisher Scientific (Waltham, MA, USA). LC Fast Start DNA Master SYBR Green kit was obtained from Roche Applied Science (Euroclone S.p.A., Pero, Italy). Dulbecco’s phosphate-buffered saline (DPBS) was provided by Sigma-Aldrich (Milan, Italy). StemMACS™ Adipodiff medium was obtained from Miltenyi Biotech s.r.l. (Bologna, Italy). Sodium Lauryl Sulphate (SLS) was supplied by Sigma Aldrich (Madrid, Spain).
2.2. Solution Preparation and Characterization
Pullulan solution was prepared in an aqueous solution with a 21 wt.% pullulan, heated at 37 °C and stirred at 450 rpm overnight. The physicochemical properties of the solution were analyzed in terms of viscosity, conductimetry and surface tension.
The apparent viscosity (ηa) was determined using a rotational viscosity meter Visco BasicPlus L from Fungilab S.A. at 50 rpm with a L3 spindle (San Feliu de Llobregat, Spain). The surface tension was measured using a DynoTester tensiometer from Krüss GmbH (Hamburg, Germany). The conductivity was evaluated using a conductivity meter Seven2Go™ from Metler Toledo 742-ISM (Schwerzenbach, Switzerland). Measurements were carried out at room temperature.
2.3. Electrospinning Pullulan Fiber Meshes
The pullulan fiber meshes were prepared using a Fluidnatek
TM LE-500 production equipment with an industrial 50 cm wide roll-to-roll system from Bioinicia S.L. (Valencia, Spain), shown in
Figure 1a. The solutions were pumped through a linear multi needle injector at a feed rate of 0.77 mL/h per needle, applying a voltage of +40 kV on the emitter and a voltage of −30 kV on the collector. The fibers were collected at a distance of 32.5 cm over black polyethylene (PE) substrate at a speed rate of 10 mm/s, under controlled environmental conditions of 20 °C and 30% relative humidity (RH). The deposition time was optimized and adjusted accordingly to obtain samples with a surface density of approximately 20 g/m
2 (
Figure 1b).
2.4. Impregnation of Electrospun Mesh
Electrospun pullulan meshes were impregnated with CLA using the dry powder impregnation technology (Fibroline S.A., Lyon, France) as illustrated in
Figure 2. Briefly, the samples were inserted in the impregnation chamber. Then, the engraved sleeve containing the powder was applied onto the pullulan mesh and the alternating electric field allowed the impregnation of the powder across the porosities of the non-woven pullulan material. The voltage applied to transfer the particles from the sleeve to the pullulan substrate was 40 kV and the rolls of material contained 3.5 wt% of CLA complexes. The process of impregnating the pullulan carrier sample has been illustrated throughout
Figure 2a–c.
2.5. Beauty Mask Production and Characterization
The fiber morphology was analyzed by Field Emission Scanning Electron Microscopy (FE-SEM) using a Hitachi S-4800 (Hitachi High Technologies Corp., Tokyo, Japan), with an electron beam acceleration of 10 kV. The samples for SEM analysis were previously sputtered with a gold-palladium mixture for 2 min. Fiber diameter was determined using ImageJ Software (version 1.52, National Institutes of Health, Bethesda, MD, USA). Average fiber diameter was assessed on at least 100 fibers randomly selected from the software.
The particle size distribution of the CLA complexes was measured with a Mastersizer 2000, (Malvern Instruments, Ltd., Worcestershire, UK) and the impregnated samples were observed via FE-SEM (FEI FEG-Quanta 450 instrument Field Electron and Ion Company, Hillsboro, OR, USA).
Chemical composition of the beauty masks was evaluated by Fourier transform infrared spectroscopy (FT-IR) using a Nicolet T380 instrument (ThermoFisher Scientific, Waltham, MA, USA) equipped with a Smart ITX Attenuated Total Refraction (ATR) accessory with diamond plate (ThermoFisher Scientific, Waltham, MA, USA).
Beauty mask samples prototypes used for in vivo studies were cut employing a Laser pro Spirit LS laser cut equipment. The size of the mask was approximately 22 cm length × 19 cm width with weighs of approximately 0.5–0.6 g.
2.6. In Vitro Skin Compatibility
Skin compatibility of the beauty masks was investigated in vitro using human dermal keratinocytes (HaCaT cells) to represent epidermal and human mesenchymal stromal cells (hMSCs) to represent connective (i.e., dermal/hypodermal) layers. Each material sample was sterilized overnight via UV irradiation. HaCaT cells were cultured in 75 cm2 tissue culture flasks using D-MEM supplemented with 1% penicillin-streptomycin, 1% L-glutamine and 10% fetal calf serum in a humidified incubator set at 37 °C in 95% air and 5% CO2. When 80% confluence was reached, the cells were replated in 12-well plates at a seeding density of 105 cells/well. The materials (1 cm2 each) were inserted in the wells containing HaCaT cells for 6 h and 24 h.
The immunomodulatory properties of pullulan and pullulan/CLA fiber were assayed via real time reverse transcriptase polymer chain reaction (qRT-PCR). At the timepoints, the mRNA was extracted from the cells and the levels of expression of a panel of cytokines involved in inflammatory response was evaluated. Specifically, the pro-inflammatory interleukins (ILs) IL-1 α, IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), as well as the anti-inflammatory cytokine transforming growth factor beta (TGF-β) and the antimicrobial peptide human beta defensin 2 (HBD-2) were evaluated. Briefly, the total RNA was isolated with TRizol, and 1 µm of RNA was reverse-transcribed into complementary DNA (cDNA) using random hexamer primers at 42 °C for 45 min, according to the manufacturer’s instructions. PCR was carried out with the LC Fast Start DNA Master SYBR Green kit using 2 µL of cDNA, corresponding to 10 ng of total RNA in a 20 µL final volume, 3 mM MgCl
2, and 0.5 µM sense and antisense primers (
Table 1). The results were normalized by the expression of the same cytokine in untreated cells, as a control.
Non-differentiated hMSCs were cultured in growth medium in presence of beauty mask samples, which solubilized inside for 3 days. Adipogenic differentiation of hMSCs was tested in vitro using pullulan and pullulan/CLA pre-treated cell samples, which were cultured in Adipodiff medium for 5 days. At the endpoint, the adipogenic pre-differentiated hMSCs were incubated with Calcein AM/DPBS solution, as from the manufacturer’s instructions, for 20 min at 37 °C in the dark, and observed under a FITC filter equipped inverted microscope (Nikon Eclipse CI; Nikon Instruments, Amsterdam, The Netherlands) with a digital camera.
At the abovementioned timepoints, the AlamarBlue test was performed on HaCaT cells, undifferentiated hMSCs and adipogenic pre-differentiated hMSCs. The test was pursued following the manufacturer’s protocol after 4 h incubation with the dye solution. AlamarBlue incorporates a REDOX indicator that changes color according to cell metabolic activity. The supernatants were read with a spectrophotometer (Victor3; PerkinElmer, Waltham, MA, USA) in a 96 well plate, using a double wavelength reading at 570 nm and 600 nm. Finally, the reduced percentage of the dye (%ABred) was calculated by correlating the absorbance values and the molar extinction coefficients of the dye at the selected wavelengths, as from the equation provided by the manufacturer. The equation applied is shown below, in which: λ = absorbance, s = sample, and c = control:
2.7. In Vivo Skin Compatibility
2.7.1. Inclusion and Exclusion Criteria
The study was carried out according to the Declaration of Helsinki as revised successively in Seoul (Ethical Principles for Medical Research Involving Human Subjects) [
22], after the approval of the local ethics Committee (Comité de ética de la investigación con medicamentos, La Fe, Spain; approval number FPNT-CEIB-04(B)). A total of 30 healthy female volunteers were recruited according to the following general inclusion and exclusion criteria described in
Table 2.
2.7.2. In Vivo Effectiveness Assessment
In order to board the in vivo assessment, the beauty mask (PBSK-1; referred as the electrospun pullulan carrier mask impregnated with CLA complexes) was compared with placebo mask (PBSK-2; referred as the electrospun pullulan carrier mask without active complexes impregnation). Two groups of 15 volunteers were recruited and underwent topical treatment during 4 weeks with mask PBSK-1 or mask PBSK-2. The masks were applied in the wet facial area for 10–15 min of application, once per week.
During the time course of the experiment, this is before (on day D
0), after 15 days (D
15) and 30 days of treatment (D
30), images were taken with a Bio3D Structured-light Scanner (Bionos Biotech, Valencia, Spain) from the areas of the forehead (glabellar lines) and crow’s feet (periocular wrinkles). Images were processed through specific software by generating 3D reconstructions from 2D images of the volunteers. Examples of this methodology is shown in
Figure 3. From the 3D reconstruction, a region of interest is analyzed. In this case, the total area, length and depth of forehead wrinkles and crow’s feet were then assessed. As well as this, skin firmness, moisturizing and TEWL levels were assessed through Cutometer
® MPA 580, Corneometer
® CM 825 and Tewameter
® TM 300, respectively (Bionos Biotech). Anti-irritating activity (i.e., erythema levels) was also quantified after 5 days of treatment (daily) on forearms, using Mexameter
®, after inducing a mild irritation with SLS water solution at 3% (
w/
v). The irritated area was measured 1 h after removal of SLS patch (at time T
1) and was then topically treated with mask PBSK-1 or mask PBSK-2 (twice per day, right or left arm), and measured every 24 h (at times T
2, T
3, and T
4). The mean values obtained by analyzing the 15 volunteers were normalized to their initial basal values (at time T
0).
2.8. Statistical Analysis
For in vitro studies, statistical analyses were carried out by SPSS software (SPSS v.16.0; IBM Corp., Chicago, IL, USA). All data were analyzed using a one-way analysis of variance (ANOVA).
For the in vivo studies, up to five repeated measurements of each parameters were taken at each timepoints (D0, D15, D30). Each technical value at each of the timepoints was normalized to the mean value of the repeated measurements at day 0, being each volunteer its own control. Data were statistically analyzed applying a paired student T-test.
For both studies, probability (p) values < 0.05 were considered as statistically significant differences.
4. Discussion
Cosmetics are very relevant to our society, based on appearance as a means for self-awareness, interaction and communication. Over the past two decades, declining mortality rates have resulted in an increased aged population with a strong desire to maintain a youthful appearance [
28]. This phenomenon has led to a strong demand for anti-aging products able to prevent wrinkles, age-spots, dry skin and uneven skin tone, among other elderly signs of the skin. The cosmetic industry offers a vast armamentarium of skin care products to take care of the skin across ages. Innovative researches are exploring the use of materials of natural origin and bioactive ingredients with demonstrated antiaging efficacy. Interesting sources have been recently obtained by industrial and agri-food waste with high potential to be used for skin care in sanitary, cosmetic and biomedical sectors [
29].
Facial beauty masks represent one of the most largely used cosmetic products. The use of bio-based materials to produce innovative beauty masks aims at combining a green and sustainable approach with skin health and wellbeing, such as protection from oxidative damage and pollution, enable skin microbiota preservation and self-repair capability of the skin [
30]. In this study we developed and fully assessed, both in vitro and in vivo, a novel beauty mask fully from natural-origin materials. The beauty mask was produced via electrospinning using a pullulan/water solution and impregnated with micro-complexes derived from nano-sized chitin, lignin and incorporated a licorice compound, GA, which were investigated in a previous study and are referred as CLA [
18]. Due to the soluble nature of pullulan, this mask, fabricated as a dry nonwoven, turns into a cream-like consistency upon wetting. The final products can be washed out and dissolved in water.
Over the past decade, electrospinning has become a very popular technology to generate bio-based fibrous materials, especially in the fields of biomedicine, pharmaceutics, and cosmetics [
23]. Many natural-occurring materials can be produced to generate fibers via electrospinning [
31,
32]. Electrospun pullulan fibers have been used in combination with other natural-origin biomaterials and showed good capability of supporting and regulating the cell types involved in skin homeostasis [
33]. Under our approach no chemical crosslinking is needed, as the beauty mask will dissolve in water. However, a uniform fiber morphology was required to incorporate CLA. Carriers, such as the pullulan nonwoven, play a critical role in the creation of a cosmetic product by helping to preserve the efficacy, safety and stability of the active ingredients selected. They, in fact, have to load, transport and deliver the actives at the programmed level of the skin, for giving the promised benefits to the customers. The SEM and FTIR-ATR spectroscopy characterization of the beauty masks showed the presence of the impregnated CLA powder in the pullulan electrospun tissue. The peaks attributable to CLA were detected thus highlighting a high CLA content at the mask surface. The load of CLA complexes in the mask was about 12% by weight. This extremely high load was achieved thanks to the exceptionally low density of the electrospun tissue, which is entitled with high porosity [
34]. Having a high loading capacity is fundamental for increasing the beauty mask efficacy. In fact, when the mask is wet, the pullulan tissue is dissolved rapidly thanks to the micrometric dimension of pullulan filaments; hence, the beneficial and abundant CLA complex can be easily and effectively released on the skin. By using HaCaT cells as a model of epidermal response and hMSCs as a model of dermal/hypodermal response, we disclosed the high cytocompatibility of both pullulan and pullulan/CLA beauty masks.
Pullulan beauty mask without CLA was endowed of immunomodulatory properties. Indeed, it was able to upregulate IL-8, IL-6 and TGF-β and to downregulate TNF-α, IL-1α and IL-1β. However, it was unable to induce the production of HBD-2. Differently, after dry impregnation with CLA, the beauty mask was able to upregulate all proinflammatory cytokines, and also have a strong indirect antimicrobial activity by a remarkable upregulation of HBD-2. This is due to CLA, and in particular to the presence of CN-NL complex, acting as an inducer of innate immunity, whereas the effect of GA is more related to inflammation modulation [
18]. Overall, the in vitro tests suggested that the pullulan/CLA beauty mask may act according to the wound healing process, namely, it promotes an initial raise of the pro-inflammatory cytokines able to stimulate skin regeneration and self-defense.
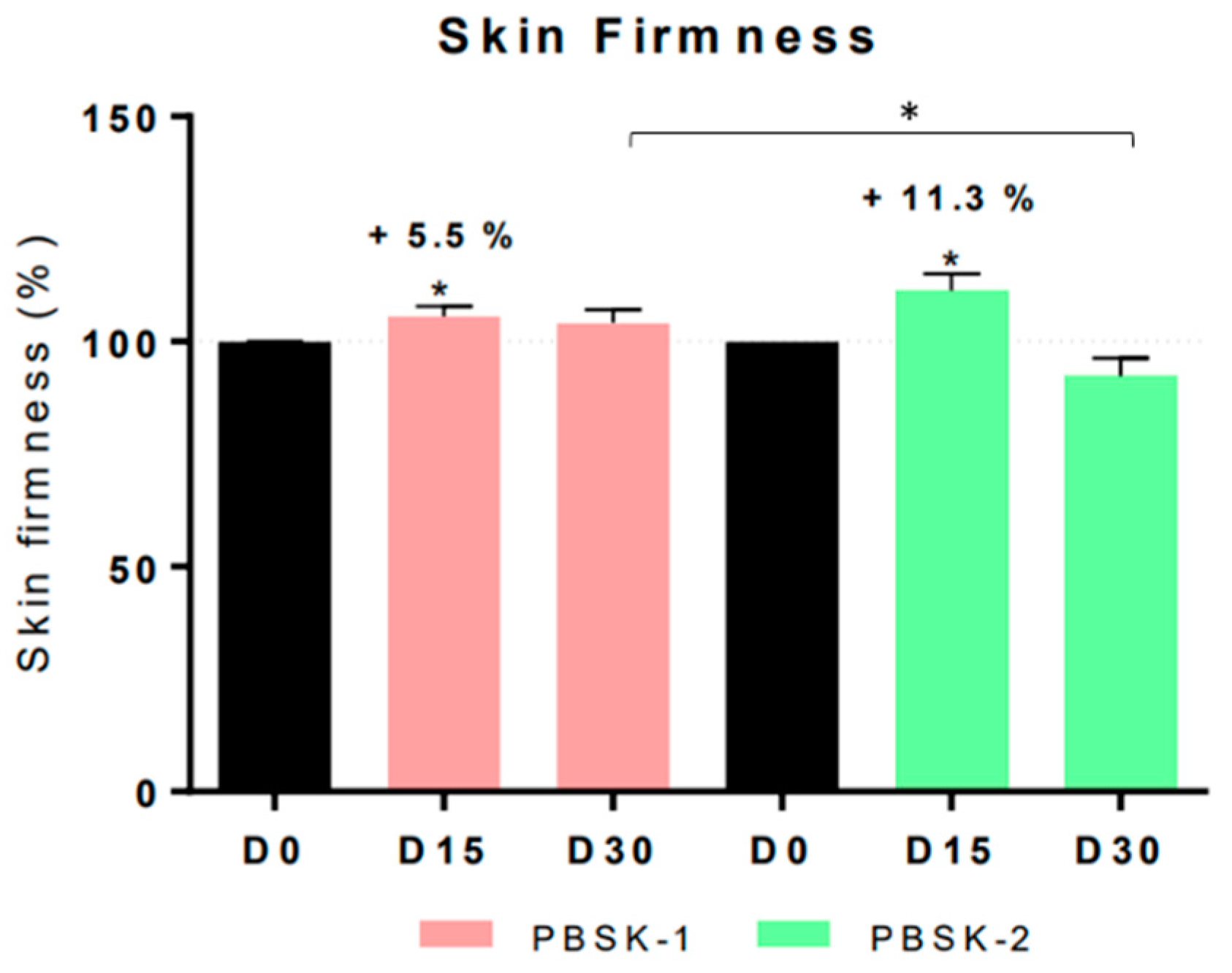
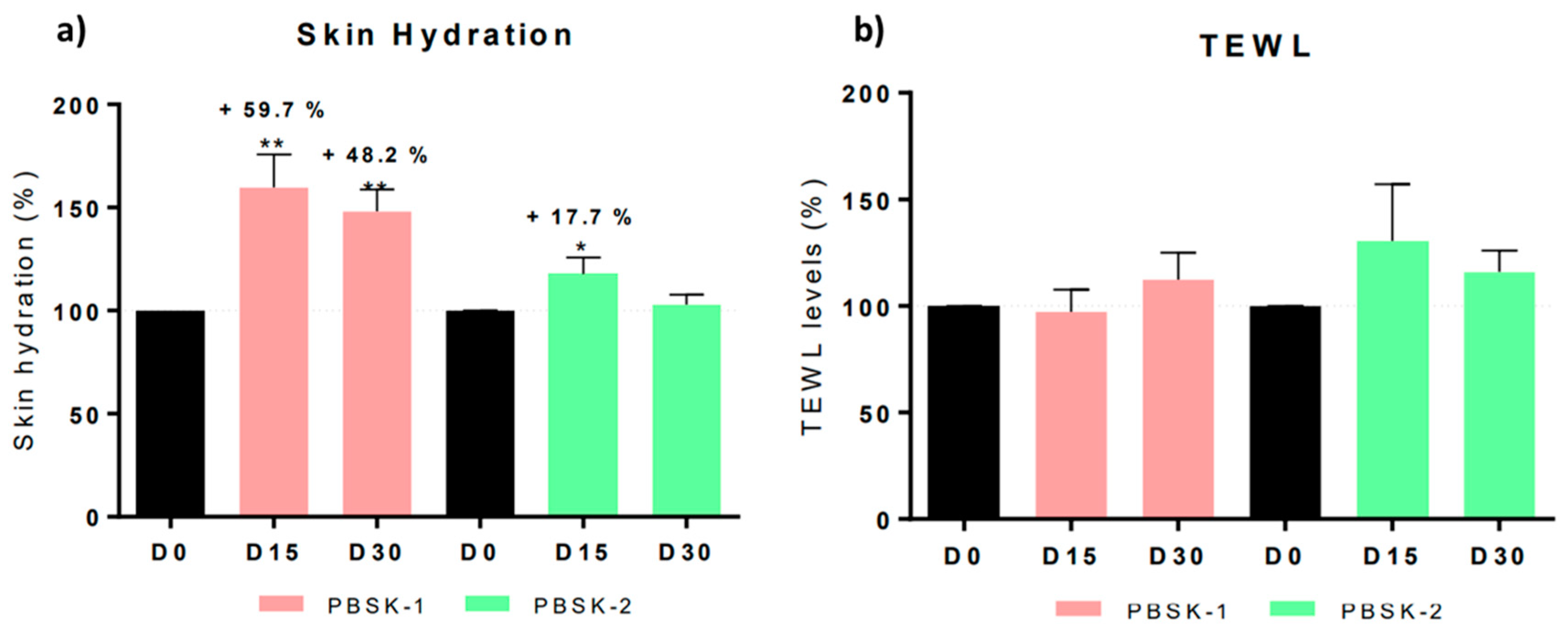
The in vivo effectiveness was tested with the pullulan/CLA (PBSK-1) fibers and pullulan face mask (PBSK-2). Results indicated that treatment with PBSK-1 significantly displayed antiaging effects, after four weeks of topical application in human volunteers, through significant reduction of forehead and crow’s feet wrinkles (area, length, and depth). Moisturizing levels assessed by hydration and TEWL indicated that moisturizing levels significantly increased compared to the placebo (PBSK-2), and TEWL remained similar to the values of the skin previous to the treatment, confirming the high tolerability of these beauty masks. Skin firmness was also improved after two weeks of treatment, for both PBSK-1 and PBSK-2. Concerning the anti-irritation effects, after effectively inducing a mild skin irritation on forearms, no statistically significant differences in erythema levels were yielded upon application of the mask PBSK-1 or the mask PBSK-2, at none of the tested timepoints, thus confirming the safety of the product. Regarding dermatological surveillance, the beauty mask showed good skin compatibility and may claim “Dermatologically tested”, “Clinically Tested” and “Tolerance Tested”. Therefore, the study highlighted that the combination of the hydrophilic nature of pullulan along with the anti-inflammatory and antioxidant properties of the CLA complexes certainly contributed to the improvement in the moisturizing and anti-aging effects, respectively. The pullulan material is a readily water soluble polymer able to solubilize very fast in contact with moisturized skin. This is probably the main factor that contributed to the positive results on moisturizing skin levels. The improvement in wrinkles reduction in such a short timeframe (15–30 days) could be attributed to the efficiently fast release of the CLA from the dissolving mask. The electrospun ultrathin fibers due to their high surface to volume ratio and surface energy stick very efficiently to the inner topology of the skin, delivering the bioactives homogeneously within it, hence presenting an innovative strategy for bioactives delivery in the field of cosmetics.
Bio-based facial masks have been previously studied in an effort to bring to the market innovative bio-friendly solutions. The authors from the study [
35] evaluated the efficacy of various wet bio-cellulose masks in vivo to assess the cosmetic effects of anti-aging, lifting and cell renewal. Various solutions were impregnated into the biocellulose carrier to generate three types of masks: anti-aging, lifting and regenerative mask. In this study, a significant decrease in skin roughness and wrinkle breadth was observed after two months treatment with the anti-aging mask [
35]. Similarly, an improvement in dermal homogeneity and firmness was also observed after two months with the same type of mask. On the other hand, a significant improvement in skin firmness was observed after one month of treatment with the “lifting” masks. Although promising results were obtained, each prepared beauty presented many different active ingredients and they were wet-based masks. In our study, PBSK-1 itself exerted an anti-aging, lifting (firmness) and moisturizing effect obtaining an all-in-one approach beauty mask. Furthermore, PBSK-1 would only be wetted upon application, thus enhancing product stability.
The possible commercialization of such a dry beauty mask is advantageous to avoid the use of high amount of preservatives, generally included in wet mask formulations. Moreover, as the masks are made fully with bio-based and biodegradable components, their final rinsing from face skin do not determine any significant issue in generating solid waste. This beauty mask is thus representing a smart product with both high performance and a strong ecofriendly profile.