Final Publication of the “Regulations on the Supervision and Administration of Cosmetics” and New Prospectives of Cosmetic Science in China
Abstract
:1. Introduction
2. CSAR and Its Regulatory System
3. Definition, Scope and Classification of Cosmetics
3.1. Definition and Scope
3.2. Borderline between Cosmetics and Drugs
3.3. Categorization and Catalog
4. Management of Cosmetic Ingredients
4.1. Ingredient Lists in STSC
4.2. New Cosmetic Ingredients
5. Technical Requirements about Safety and Efficacy
5.1. Safety Evaluation
5.2. Efficacy and Claim Substantiation
6. Technical Evaluation of Cosmetics and New Ingredients
6.1. Introduction of Technical Evaluation Work
6.2. Future of Technical Evaluation
7. New Prospects for Cosmetic Science in China
7.1. Advanced Concepts in CSAR
7.2. Encouragement of Innovation
7.3. Technical Approaches and Scientific Research
7.4. “Regulatory Science” on Cosmetics in China
Author Contributions
Funding
Conflicts of Interest
References
- State Council. Regulations on the Supervision and Administration of Cosmetics. Available online: http://www.gov.cn/zhengce/content/2020-06/29/content_5522593.htm (accessed on 1 December 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Provisions for Cosmetic Registration (Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20200721094201896.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on Three Regulatory Documents Including the Standards of Information File for Cosmetic Product Registration or Notification and Others. Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20201105145412189.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Notes of Guidance for Cosmetic Safety Evaluation (Draft) and the Rules and Catalogue for Categorization of Cosmetics (Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20200729175501268.html (accessed on 29 November 2020).
- China Food and Drug Administration. An Announcement of China Food and Drug Administration on Releasing the Inventory of Existing Cosmetic Ingredients in China (2015 Edition). Available online: https://www.nmpa.gov.cn/hzhp/hzhpggtg/hzhpqtgg/20151223120001361.html (accessed on 29 November 2020).
- China Food and Drug Administration. An Announcement of China Food and Drug Administration on Releasing the Safety and Technical Standards for Cosmetics (2015 Edition). Available online: https://www.nmpa.gov.cn/hzhp/hzhpggtg/hzhpqtgg/20151223120001986.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Provisions for Supervision and Administration of Cosmetic Production and Distribution (Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20200721094301493.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Standards for Management of Cosmetic Production and Quality Control (Draft), the Management Measures for Adverse Reaction Monitoring of Cosmetics (Draft) and the Management Standards for Sampling Inspection of Cosmetics(Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20200928103936177.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Provisions for Supervision and Administration of Toothpaste (Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20201113094757150.html (accessed on 29 November 2020).
- National Medical Products Administration. Notice on Soliciting Public Opinions on the Management Measures for Cosmetic Labeling (Draft). Available online: https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjhzhp/20200921155413149.html (accessed on 29 November 2020).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council, of 30 November 2009, on Cosmetic Products; Official Journal of the European Union, 2009. Available online: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 1 December 2020).
- United States Code-Federal Food, Drug, and Cosmetic Act. Available online: https://uscode.house.gov/browse/prelim@title21&edition=prelim (accessed on 1 December 2020).
- Japan Ministry of Health, Labour and Welfare of Japan. 医薬品、医療機器等の品質、有効性及び安全性の確保等に関する法律, 2014. Available online: https://www.mhlw.go.jp/web/t_doc?dataId=81004000&dataType=0&pageNo=1 (accessed on 2 December 2020).
- Korea Ministry of Food and Drug Safety. Cosmetic Law. Available online: https://www.mfds.go.kr/eng/brd/m_60/view.do?seq=69876 (accessed on 1 December 2020).
- Manual of the Working Group on Cosmetic Products (Sub-group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No 1223/2009. Available online: https://ec.europa.eu/docsroom/documents/29002 (accessed on 1 December 2020).
- Morganti, P.; Paglialunga, S. Eu borderline cosmetic products review of current regulatory status. Clin. Dermatol. 2008, 26, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sonia, S. Overlapping Definitions of Drugs, Topical Medical Devices, Cosmetics. The cosmetic efficacy: Myth or reality? J. Appl. Cosmetol. 2011, 29, 129–133. [Google Scholar]
- Su, Z.; Xing, S.; Wang, G. The Worldwide Regulation of Cosmeceutical and Its Enlightenment to China. Chin. Pharm. Aff. 2019, 33, 1383–1390. [Google Scholar]
- Food and Drug Administration. Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap#Cosmeceutical (accessed on 15 October 2020).
- National Medical Products Administration. Frequently Asked Questions about Cosmetics Supervision and Administration. Available online: https://www.nmpa.gov.cn/xxgk/zhcjd/zhcjdzh/20190110093701592.html (accessed on 28 October 2020).
- Food and Drug Administration. Code of Federal Regulations, Title 21, Part 352: Sunscreen Drug Products for Over-the-counter Human Use. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=58025e96360c4105ae8364a55fedde8f&mc=true&node=pt21.5.352&rgn=div5 (accessed on 3 December 2020).
- Food and Drug Administration. Code of Federal Regulations, Title 21, Part 350: Antiperspirant Drug Products Over-the-counter Human Use. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=58025e96360c4105ae8364a55fedde8f&mc=true&node=pt21.5.350&rgn=div5 (accessed on 3 December 2020).
- Food and Drug Administration. Cosmetic Product Category Codes. Available online: https://www.fda.gov/cosmetics/paper-registration-voluntary-cosmetic-registration-program-vcrp/cosmetic-product-category-codes (accessed on 20 October 2020).
- Cork, M. Cork mjthe importance of skin barrier function. J dermatolog treat 8:S7-s13 (abstr). J. Dermatol. Treat. 2009, 8, S7–S13. [Google Scholar] [CrossRef]
- Draelos, Z. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision (SCCS/1602/18). Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf (accessed on 1 December 2020).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Available online: http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc (accessed on 10 September 2020).
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; Benthem, J.; Zuang, V.; Andersen, K.; Angers-Loustau, A.; Aptula, N.; Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef] [PubMed]
- Cote, I.; Anastas, P.; Birnbaum, L.; Clark, R.; Dix, D.; Edwards, S.; Preuss, P. Advancing the next generation of health risk assessment. Environ. Health Perspect. 2012, 120, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- International Cooperation on Cosmetic Regulation. Integrated Strategies for Safety Assessments of Cosmetic Ingredients—Part I, 2017. Available online: https://iccr-cosmetics.org/files/4715/0824/0761/ICCR-11_JWG_Integrated_Strategies_Integrated_Strategies_for_Safety_Assessments_of_Cosmetic_Ingredients_-_Part_I.pdf (accessed on 28 November 2020).
- International Cooperation on Cosmetic Regulation. Integrated Strategies for Safety Assessments of Cosmetic Ingredients—Part 2, 2018. Available online: https://iccr-cosmetics.org/files/8315/4322/3079/ICCR_Integrated_Strategies_for_Safety_Assessment_of_Cosmetic_Ingredients_Part_2.pdf (accessed on 28 November 2020).
- Luo, F.; Su, Z.; Wu, J.; Zhang, F.; Xing, S.; Wang, G.; Lu, Y. The current status of alternative methods for cosmetics safety assessment in china. ALTEX 2018, 36, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 655/2013 of 10 July 2013 Laying Down Common Criteria for the Justification of Claims Used in Relation to Cosmetic Products; Official Journal of the European Union, 2013. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R0655 (accessed on 1 December 2020).
- Wall, E. A Comprehensive Look at the Fair Packaging and Labeling Act of 1966 and the Fda Regulation of Deceptive Labeling and Packaging Practices: 1906 to Today. 2020. Available online: https://dash.harvard.edu/handle/1/8846774 (accessed on 10 December 2020).
- Weinstein, S.; Drozdenko, R. Objective claims substantiation for cosmetic and pharmaceutical products, based upon measurement of sensory, perceptual, analgesic, pruritic, and irritative effects. Clin. Res. Pract. Drug Regul. Aff. 2008, 4, 143–172. [Google Scholar] [CrossRef]
- Dorato, S. Guidelines for the Cosmetics’ Efficacy Evaluation. The cosmetic efficacy: Myth or reality? J. Appl. Cosmetol. 2011, 29, 119–122. [Google Scholar]
- Task Force Committee for Evaluation of Whitening Function. Guidelines for Evaluation of Quasi-Drug Whitening Products for New Efficacy Claims. J. Jpn. Cosmet. Sci. Soc. 2007, 31, 432–438. [Google Scholar]
- Task Force Committee for Evaluation of Whitening Function. Guidelines for Evaluation of Anti-Wrinkle Products. J. Jpn. Cosmet. Sci. Soc. 2007, 31, 411–431. [Google Scholar]
- Korea Ministry of Food and Drug Safety. Guideline for Efficacy Evaluation of Functional Cosmetics. Available online: http://www.nifds.go.kr/brd/m_15/view.do?seq=8990&srchFr=&srchTo (accessed on 30 November 2020).
- International Organization for Standardization. ISO 24444:2019 Cosmetics—Sun protection test methods—In vivo determination of the sun protection factor (SPF). Available online: https://www.iso.org/standard/72250.html (accessed on 28 November 2020).
- National Institutes for Food and Drug Control. Notice on Soliciting Public Opinions on the Analytical Method for Boric Acid and Borate in Cosmetics and others. Available online: https://www.nifdc.org.cn/nifdc/xxgk/ggtzh/tongzhi/20201112160508189.html (accessed on 2 December 2020).
- Pauwels, M.; Rogiers, V. Database search of safety information on cosmetic ingredients. Regul. Toxicol. Pharmacol. RTP 2008, 49, 208–216. [Google Scholar] [CrossRef] [PubMed]
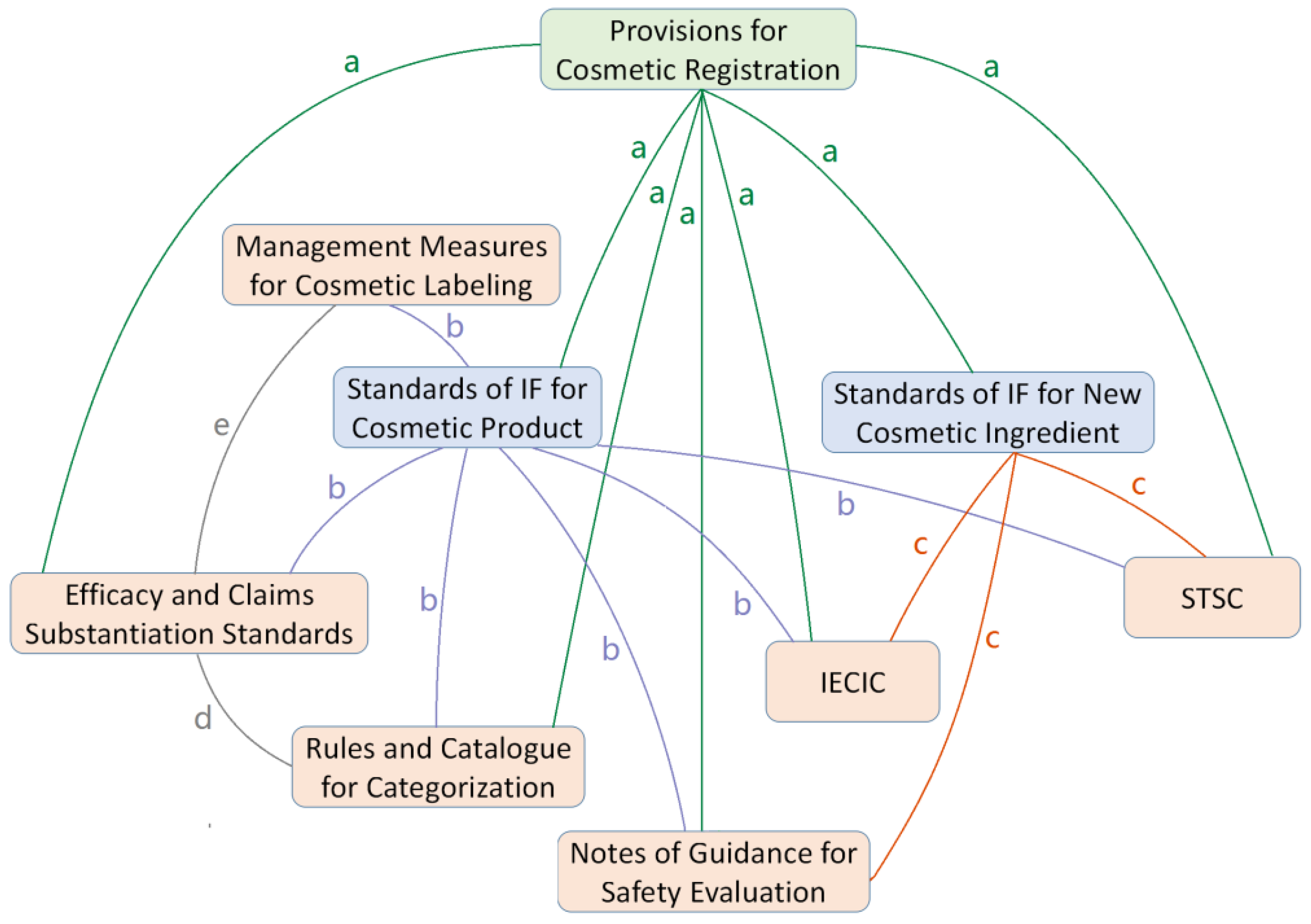
| No. | Legislative Level | Name of the Regulation | Brief Introduction of the Regulation | Current Status |
|---|---|---|---|---|
| About Registration and Notification: | ||||
| 1 | Departmental Regulation | Provisions for Cosmetic Registration | General requirements and procedures about registration and notification of cosmetic products and new ingredients. | Draft published online for public comments on 21 July 2020 [2]. |
| 2 | Normative Document | Standards of Information File for Cosmetic Product Registration or Notification | Detailed requirements about the information file submitted for registration or notification of cosmetic products. | Draft published online for public comments on 4 November 2020 [3]. |
| 3 | Normative Document | Standards of Information File for New Cosmetic Ingredient Registration or Notification | Detailed requirements about the information file submitted for registration or notification of new cosmetic ingredients. | Draft published online for public comments on 4 November 2020 [3]. |
| 4 | Normative Document | Rules and Catalog for Categorization of Cosmetics | Rules and catalog for categorization of cosmetic products, to help give a unique category code for each product. | Draft published online for public comments on 29 July 2020 [4]. |
| 5 | Normative Document | Notes of Guidance for Cosmetic Safety Evaluation | Technical guidelines for the safety evaluation of cosmetic ingredients and cosmetic products. | Draft published online for public comments on 29 July 2020 [4]. |
| 6 | Normative Document | Efficacy and Claims Substantiation Standards for Cosmetics | Technical guidance and basic requirements for the efficacy and claim substantiation of cosmetic products. | Draft published online for public comments on 4 November 2020 [3]. |
| 7 | Normative Document | Guidance for Quantified and Classified Management of General Cosmetics Notification | Rules and guidance for the quantified and classified management of general cosmetics. For example, to grade enterprises according to their historical performance in product notifications. | There is still no public draft, but the mechanism is under experiment. |
| 8 | Normative Document | Inventory of Existing Cosmetic Ingredients in China (IECIC) | An objective collection of ingredients already used in cosmetics in China, but different from a “positive list of cosmetic ingredients”. | Published on 23 December 2015 [5]. |
| 9 | Normative Document | List of Substances Prohibited in Cosmetic Products (collected in the Safety and Technical Standards for Cosmetics, STSC) | STSC is a basic technical regulation for cosmetics covering general safety requirements, list of prohibited substances, list of restricted ingredients, lists of allowed preservatives, UV filters, colorants and hair dyes, and analytical methods and evaluation methods for cosmetics. | Published on 23 December 2015, with several supplementary revisions afterward [6]. |
| About Production and Distribution: | ||||
| 10 | Departmental Regulation | Provisions for Supervision and Administration of Cosmetic Production and Distribution | General requirements and legal responsibilities of the production and distribution activities of cosmetics. | Draft published online for public comments on 21 July 2020 [7]. |
| 11 | Normative Document | Standards for Management of Cosmetic Production and Quality Control | Detailed requirements, standards and key points for the process of cosmetic production and quality control. | Draft published online for public comments on 27 September 2020 [8]. |
| 12 | Normative Document | Management Measures for Adverse Reaction Monitoring of Cosmetics | Basic requirements and responsibilities for adverse reaction monitoring of cosmetics. | Draft published online for public comments on 27 September 2020 [8]. |
| About Toothpaste Management: | ||||
| 13 | Departmental Regulation | Provisions for Supervision and Administration of Toothpaste | General requirements for the supervision and administration of toothpaste. | Draft published online for public comments on 13 November 2020 [9]. |
| 14 | Normative Document | Standards of Information File for Toothpaste Notification | Detailed requirements about the information file submitted for notification of toothpaste. | There is still no public draft by now. According to CSAR, toothpaste shall be regulated in a similar way to general cosmetics. As a result, these regulations (or similar alternatives) can be necessary for toothpaste management. |
| 15 | Normative Document | Catalog of Toothpaste Efficacy | Categorized list of toothpaste efficacy. | |
| 16 | Normative Document | Inventory of Existing Toothpaste Ingredients in China | An objective collection of ingredients already used in toothpaste in China, but different from a “positive list of cosmetic ingredients”. | |
| About Labeling Management: | ||||
| 17 | Normative Document | Management Measures for Cosmetic Labeling | Requirements, principles and key points for the labeling of cosmetic products. | Draft published online for public comments on 21 September 2020 [10]. |
| Country/Region | Definition of Cosmetics |
|---|---|
| China [1] | Daily chemical industry products intended to be applied by spreading, spraying or other similar ways to human body surfaces, such as skin, hair, nails, and lips, for the purpose of cleaning, protecting, beautifying and decorating. |
| EU [11] | Any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odors. |
| US [12] | (1) articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and (2) articles intended for use as a component of any such articles; except that such term shall not include soap. |
| Japan [13] | Applied by spreading, sprinkling or other similar ways for the purpose of cleaning, beautifying, promoting attractiveness, altering appearance, or keeping the health of skin or hair, with a moderate effect on the applied site. Not including the products also having pharmaceutical purpose or quasi-drugs. |
| Korea [14] | Articles applied by spreading, rubbing, sprinkling or other similar ways, with the effect of cleaning or beautifying, in order to promoting attractiveness, altering appearance, and keeping or enhancing the health of skin or hair, with a moderate effect on the human body. Not including pharmaceutical products. |
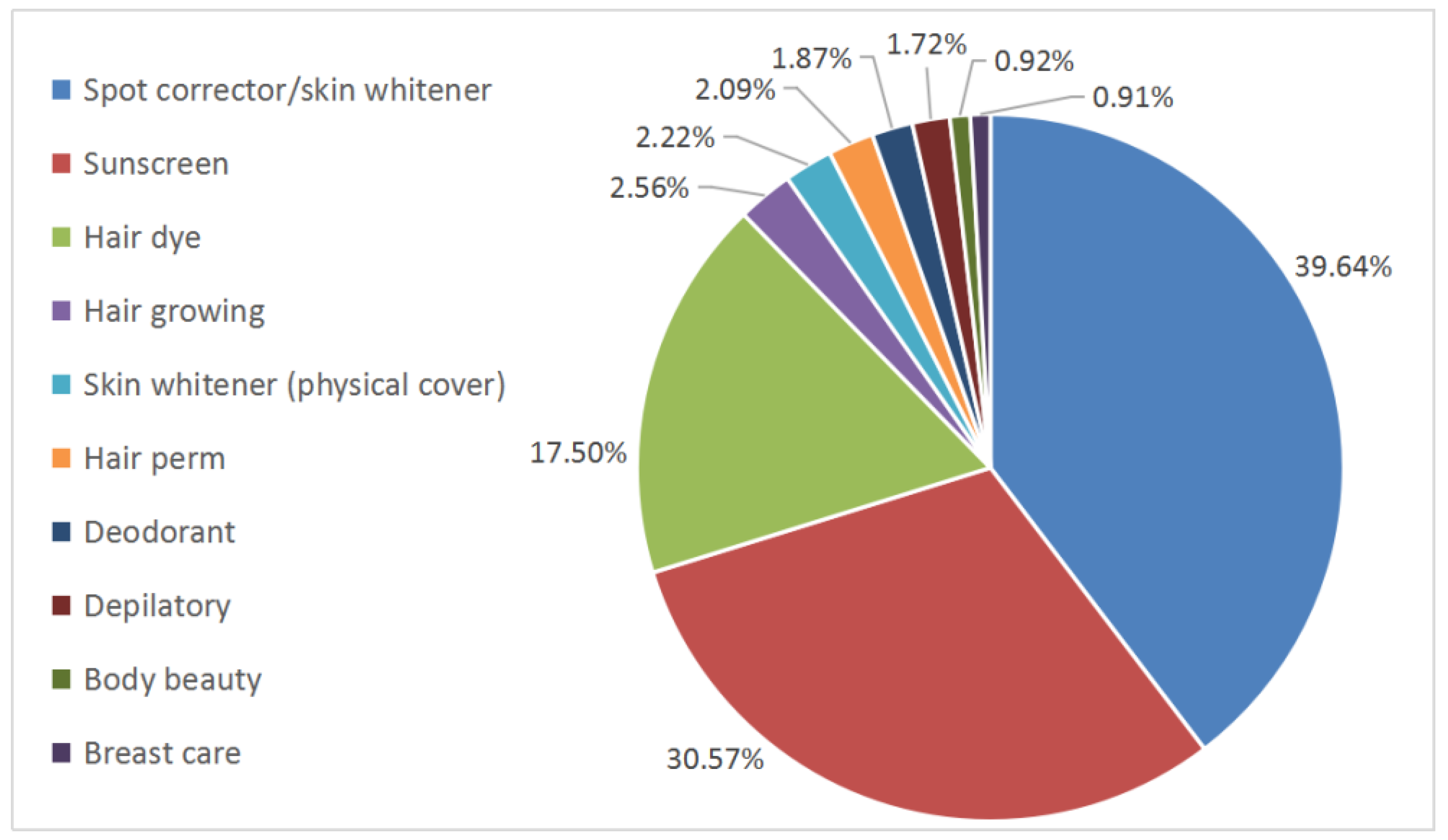
| Function/Claim | 1989 CHSR | 2020 CSAR | EU [11,15] | US [12,15,16] | Japan [13] | Korea [14] |
|---|---|---|---|---|---|---|
| Hair dye | Special cosmetics | Special cosmetics | Cosmetics | Cosmetics | Quasi-drug | General cosmetics (temporary hair dye)/functional cosmetics (permanent hair dye) |
| Hair perm | Special cosmetics | Special cosmetics | Cosmetics | Cosmetics | Quasi-drug | General cosmetics |
| Spot corrector /skin whitener | Special cosmetics | Special cosmetics | Cosmetics/drug | Drug | Quasi-drug | Functional cosmetics |
| Sunscreen | Special cosmetics | Special cosmetics | Cosmetics | Over the counter (OTC) drug | Cosmetics/quasi-drug | Functional cosmetics |
| Hair growth/ Preventing hair loss | Special cosmetics (products for hair growth) | Special cosmetics (products for preventing hair loss) | Cosmetics/drug | Drug | Quasi-drug | Functional cosmetics |
| Depilatory | Special cosmetics | General cosmetics | Cosmetics | Cosmetics | Quasi-drug | Functional cosmetics |
| Breast care | Special cosmetics | No longer cosmetics | Drug | Drug | Depends on the specific case | Drug |
| Body beauty | Special cosmetics | No longer cosmetics | Cosmetics/drug | Drug | Depends on the specific case | Drug |
| Deodorant | Special cosmetics | General cosmetics | Cosmetics | Cosmetics (deodorizer)/OTC drug (sweat inhibitor) | Quasi-drug | Quasi-drug |
| A new special cosmetics: cosmetics with new efficacy claim |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Luo, F.-y.; Pei, X.-r.; Zhang, F.-l.; Xing, S.-x.; Wang, G.-l. Final Publication of the “Regulations on the Supervision and Administration of Cosmetics” and New Prospectives of Cosmetic Science in China. Cosmetics 2020, 7, 98. https://doi.org/10.3390/cosmetics7040098
Su Z, Luo F-y, Pei X-r, Zhang F-l, Xing S-x, Wang G-l. Final Publication of the “Regulations on the Supervision and Administration of Cosmetics” and New Prospectives of Cosmetic Science in China. Cosmetics. 2020; 7(4):98. https://doi.org/10.3390/cosmetics7040098
Chicago/Turabian StyleSu, Zhe, Fei-ya Luo, Xin-rong Pei, Feng-lan Zhang, Shu-xia Xing, and Gang-li Wang. 2020. "Final Publication of the “Regulations on the Supervision and Administration of Cosmetics” and New Prospectives of Cosmetic Science in China" Cosmetics 7, no. 4: 98. https://doi.org/10.3390/cosmetics7040098
APA StyleSu, Z., Luo, F.-y., Pei, X.-r., Zhang, F.-l., Xing, S.-x., & Wang, G.-l. (2020). Final Publication of the “Regulations on the Supervision and Administration of Cosmetics” and New Prospectives of Cosmetic Science in China. Cosmetics, 7(4), 98. https://doi.org/10.3390/cosmetics7040098




