1. Introduction
Essential oils and plant parts containing essential oils have long been valued for their ability to have a positive effect on human health. Essential oils are broadly classified as terpenes and their mono oxygenated analogues are classified as terpenols. These molecules are produced by many plants in nature with two main types of terpenes being found: monoterpenes, molecules with a C10 carbon frame, and sesquiterpenes, with a C15 carbon frame.
Indian sandalwood oil is the essential oil obtained by steam distillation of the aromatic heartwood of
Santalum album [
1], consisting predominately of sesquiterpenes. The oil is renowned for its olfactory characteristics, having a soft, warm, and woody odor. As a result of this odor, the oil has found its way into many applications such as perfumery, attars, and incense [
2].
The major constituents in Indian sandalwood oil are a group of isomers known as santalol, (z-α-santalol, z-β-santalol, trans-α-bergamotol, and epi-β-santalol). These are produced by sesquiterpene synthases acting on farnesene pyrophosphate followed by the subsequent oxidation by P450-dependent monooxygenases. A second group of sesquiterpenols is also produced by the action of monoterpene synthase on farnesene pyrophospahete, (bisabolols, curcemen-12-ols, and lanceol) [
3,
4].
The constituents of the Indian sandalwood oil have been well described and their structures elucidated [
5]. The analysis of constituents can be undertaken by gas chromatography with flame ionization detection (GC FID) as a routine analysis [
3]. It has long been recognized that Indian sandalwood has applications as a cosmetic and medicinal ingredient. In fact, it is one of the oldest recognized cosmetic ingredients, with documented use as far back as 500 BCE where sandalwood was used for cosmetic and health purposes and incorporated into Indian and Chinese traditional medicines [
6]. Cleopatra, whose reputation continues to precede her in modern times, purportedly used sandalwood for its cosmetic benefits. Detailed treatises on cosmetic formulae containing sandalwood were written in the Gupta period of India and the Tang Dynasty of China [
2,
6].
The Ayurvedic system of medicine has listed sandalwood specifically as a substance for the health of skin, highlighting its action beyond the aroma perceived as a perfume. Sandalwood is also used internally as a medicine for blood purifying and energizing in both Traditional Chinese Medicine (TCM) and Ayurveda [
7,
8]. Today, Indian sandalwood is found in the Ayurvedic Pharmacopoeia of India, the China Pharmacopoeia, and it has been recently added to the British Pharmacopoeia.
Prior researchers have also looked into the pharmacological properties of the oil with qualities such as anti-antimicrobial, anti-inflammatory, anti-viral, and anti-proliferation being investigated [
9]. For instance, Mohankumar et al. [
10] reported the anti-oxidative properties of Indian sandalwood oil on human neuroblast cell lines to establish the effect on oxidative neurodegeneration [
10]. Anti-inflammatory properties of Indian sandalwood oil were reported by Sharma et al. [
11] for dermal fibroblast cells when stimulated by bacterial lipopolysaccharides [
11,
12]. It was reported that the therapeutic value of Indian sandalwood oil is related to its high content in natural sesquiterpenes, namely alpha-santalol (50% of the oil composition) and beta-santalol (20% of the oil composition) [
13,
14]. While research into the pharmacological effects of Indian sandalwood has been extensive in recent years, research into the cosmetic attributes of Indian sandalwood has not been as extensive.
The skin being the largest organ and outermost barrier of the body is often directly and frequently exposed to environmental pollutants. This is problematic, as while the primary function of the skin is to protect against environmental stressors, it is also very sensitive toward them. Damaging substances such as pollution and the deleterious synergy of the sun can lead to dermatological conditions such as inflammation, oxidative stress, and poor metabolic activities in the skin [
15]. Recent cosmetic trends have been aimed at retaining skin health and promoting positive aging of skin when exposed to pollution, ultraviolet radiation (UV) and visible light from sun and digital screens [
16]. Advances in the field of dermatology have given rise to multiple studies investigating the cutaneous effects of UV and visible light [
17]. In recent years, a new branch of the literature has grown to demonstrate that besides UV, other stressors such as High-Energy Visible (HEV) light, or blue light in the 400–470 nm region of the visible spectrum, as well atmospheric pollutants, could trigger biological processes at the skin level and lead to premature skin aging [
18]. Blue light is present naturally in sunlight that reaches the earth’s surface and is also emitted through various electronic devices containing light-emitting diodes (LEDs) bulbs [
19]. It has previously been shown that blue light from the sun, together with atmospheric pollutants such as particulate matter, cigarette smoke, and ozone, affect the molecular structure of the skin by inducing significant oxidative stress, inflammation, apoptosis, and collagen degradation, via induction of matrix metalloproteainases such as MMP-1 and DNA damage [
20].
Substances that actively protect or repair the skin from above exposomes are known as adaptogens [
21]. In this study, blue light (412 nm and 450 nm), cigarette smoke, and ozone were selected as environmental stressors in order to quantitatively demonstrate the antioxidant and anti-collagenase capacity of Indian sandalwood oil. The antioxidant capacity was explored by measuring the scavenging activity of Indian sandalwood oil on reactive oxygen species (ROS). Then, the anti-aging effect was investigated by measuring the inhibitory capacity of Indian sandalwood oil on MMP-1. Data obtained would help shed light on the ability of Indian sandalwood oil to be a suitable adaptogen in cosmetic and dermatological care by exerting a protective effect on cells in vitro and on the skin ex vivo.
2. Materials and Methods
2.1. Test and Control Substances
Indian sandalwood oil was provided by Quintis Sandalwood (Perth, WA, Australia). This oil was steam distilled from the aromatic heartwood of the tree
Santalum album, which was grown in plantations located in Kununurra, Western Australia. The oil was tested and confirmed to comply with ISO 3518 [
22]. Constituent analysis by GC FID using the method detailed in ISO 3518 can be seen in
Table 1. Quercetin (Sigma-Aldrich, St. Louis, MO, USA) and alpha-tocopherol (Vitamin E) (Sigma-Aldrich, St. Louis, MO, USA) were used as positive controls.
2.2. Cells and Reagents
The human immortalized keratinocyte cell line, HaCaT (ATCC®, Manassas, VA, USA) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Scientific, Waltham, MA, USA) and supplemented with 100 µg/mL streptomycin, 100 U/mL penicillin (Pen-Strep), 2 mm L-glutamine, 10% heat-inactivated FBS, and 0.25% sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA). The cell line was maintained at 37 °C with 5% CO2. Cells were serially passaged at 70–80% confluence. When performing experiments, HaCaT cells were grown near 100% confluency, and all treatments were performed in the same medium.
2.3. Human Skin Explants Preparation
Human skin explants were obtained, with research consent, from residual skin following mammoplasty and trimmed to remove any subcutaneous fat. Eight-millimeter skin biopsies were taken from skin composed of dermis and epidermis using a sterile dermal biopsy punch (Kai Medical, Dallas, TX, USA) and rapidly placed into a 24-well plate. Then, the skin was maintained under air–liquid interface culture conditions in skin culture medium, Gibco™ DMEM, without glutamine or phenol red (Thermo Fisher, Waltham, MA, USA). The skin was maintained at 37 °C in a 5% CO2 atmosphere for sufficient adaptation time.
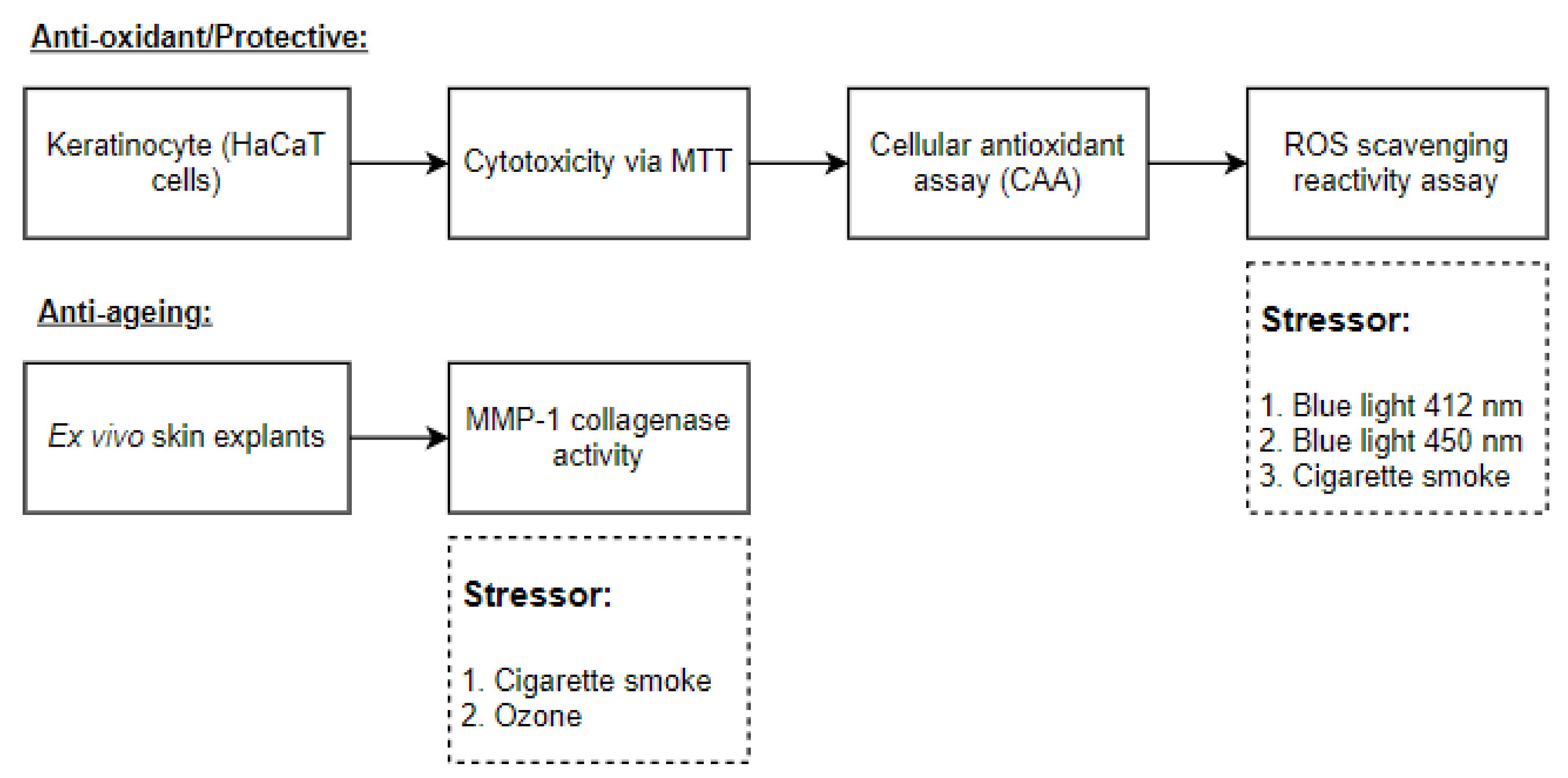
2.4. Experimental Design
The experimental design is shown in
Figure 1.
2.5. Environmental Stressors (Exposure to Blue Light (412/450 nm) and Cigarette Smoke)
2.5.1. Blue Light Source
Each blue light lamp (412 nm and 450 nm) consisted of 10 identical LEDs (Honglitronic, Guangzhou, China) emitting continuous visible radiation embedded in a reflector, which was covered by a transparent glass window. A single peak with a maximum wavelength of either 412 nm or 450 nm could be observed for the lamps. The aperture on the light source was 4.5 cm × 4.5 cm. The array at the surface of exposure was approximately 10 cm × 10 cm, at an approximate distance of 5 cm from the light source. A thermopile detector (Gentec EO USA Inc., Lake Oswego, OR, USA) was used to measure the precise intensity of the light source in Watt/cm2 at the level of the investigational site. The time of exposure was adjusted to ensure that 1 J/cm2 of blue light was delivered to the investigational site.
2.5.2. Cigarette Smoke
A transparent exposure chamber designed to accommodate the well plates and a cigarette connected to an air pump were used (Tarsons, Kolkata, India). During 30 min exposure, three cigarettes were used. The cigarette was connected to a pump that mimics the aspiration of the smoker, and the smoke released in the chamber corresponds to exhaled smoke. One cigarette was lit at the start of the exposure and then every 10 min for a total of three cigarettes per exposure of 30 min.
2.6. Ozone Exposure
Ozone was produced using an ozone generator. The skin explants were exposed to 1.6 parts per million (ppm) for a total exposure time of 30 min.
2.7. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Assay for Cell Viability and Proliferation
In vitro cell viability evaluation on the HaCaT cell line was performed whereby 1 × 104 cells in complete medium were added to each well of flat-bottom 96-well plates and incubated at 37 °C in a humidified 5% CO2 for 16 h of incubation. The cells were treated with different concentrations of Indian sandalwood oil in ethanol for 24 h or 48 h at 37 °C. The supernatants were removed, and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) solution (1 mg/mL in DMEM) was added to each well. Cells were incubated for two hours at 37 °C. After incubation, the MTT solution was removed, and isopropanol was added to dissolve the formazan crystals. Then, the absorbance was measured at 570 nm using a Synergy HTX multimode microplate reader (BioTek, Winooski, VT, USA).
2.8. Cellular Antioxidant Assay
First, 1 × 105 cells in complete medium were added to each well of a 24-well plate and incubated at 37 °C in a humidified 5% CO2 for 16 h of incubation. Based on the data obtained from the cytotoxicity and solubility tests, the optimal concentration of Indian sandalwood oil was determined by testing the Indian sandalwood oil at eight different concentrations: namely, 0.2%, 0.1%, 0.07%, 0.05%, 0.025%, 0.01%, 0.005%, and 0.001%. As a positive control, quercetin (Sigma-Aldrich, St. Louis, MO, USA) was also tested in this assay at six different concentrations of 0.0075%, 0.00375%, 0.001875%, 0.0009375%, 0.00047%, and 0.00023%. Then, the HaCaT cells were treated with 1 mm of the non-fluorescent probe, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Sigma, St. Louis, MO, USA) for three hours at 37 °C and 5% CO2. The oxidation reaction of DCFH was started by the addition of 600 µM AAPH (2,2’-azobis(2-amidinopropane) dihydrochloride) (Sigma, St. Louis, MO, USA). Lysis buffer (2% Triton X-100 in PBS) was added to the wells. Then, fluorescence was measured. The wavelength of excitation of 485/88 nm and emission 528/30 nm was measured using the Synergy HTX multimode microplate reader (BioTek, Winooski, VT, USA).
2.9. Intracellular Reactive Oxygen Species (ROS) Scavenging Activity Assay
Approximately 1 × 105 HaCaT cells were seeded per well in different 24-well plates. After the adaptation incubation period of 16 h at 37 °C, the Indian sandalwood oil was added to the cells at three different concentrations (0.2%, 0.1%, and 0.05%) for 24 h at 37 °C and supplemented with 5% CO2. The positive control alpha-tocopherol (vitamin E) (Sigma-Aldrich, St. Louis, MO, USA) was also tested at three different concentrations: namely, 15 units (corresponding to 2%), 7.5 units (corresponding to 1%), and 3.75 units (corresponding to 0.5%). Then, the cells were treated with DCFH-DA (Sigma, St. Louis, MI, USA) for three hours at 37 °C and 5% CO2. After the DCFH-DA treatment, the cells were washed three times with 1X PBS. Then, the 96-well plates were exposed to 412 nm and 450 nm blue light at 1J/cm2 or cigarette smoke as an environmental stressor and left unexposed in a dark environment as a control. Following exposure, lysis solution (with 2% Triton X-100) are added to all 96 wells. Then, the well plates were shaken, and the fluorescence (wavelength of excitation of 485/88 nm and emission 528/30 nm) was measured using the Synergy HTX multimode microplate reader (BioTek, Winooski, VT, USA).
2.10. Collagenase Inhibition Assay via Matrix Metalloproteinase-1 (MMP-1)
Following skin biopsy and adaptation time, the test item was applied and incubated for 24 h at 37 °C and 5% CO2. Then, the 24-well plates were exposed to cigarette smoke or ozone (1.6 ppm) for 30 min. The remaining plates were left unexposed in a dark environment as a control. Then, the supernatant was transferred to an antibody-coated well plate to assess the level of matrix metalloproteinase-1 (MMP-1) following the manufacturer’s instruction of the human MMP-1 ELISA Kit (Sigma, St. Louis, MI, USA). An increase in the color intensity was monitored by the spectrometry at 450 nm (BioTek, Winooski, VT, USA).
2.11. Statistical Analysis
Experiments were independently repeated in biological triplicate. Error bars in the graphical data represent standard estimation of the mean (SEM). A one-way ANOVA was used for the statistical analysis using the software GraphPad Prism Version 7 (GraphPad Software Inc., San Diego, CA, USA), and a statistical significance was claimed when the p-value was lower than 0.01 (p < 0.01).
3. Results
3.1. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Assay for Cell Viability and Proliferation
Prior to the determination of the efficacy of Indian sandalwood oil to protect against the oxidative stress induced in HaCaT cells, an MTT assay was performed to establish the optimum concentration of sandalwood oil that would not induce any defect in cell cycle or cell toxicity. Thus, HaCaT cells were treated with different concentrations of Indian sandalwood oil for either 24 h or 48 h (
Figure 2A,B).
At 24 h post treatment, the highest concentrations of Indian sandalwood oil tested (10%, 2%, 0.6%, 0.5%, 0.4%, and 0.3%) revealed a cell viability count lower than 70% compared to the cells that were only treated with cell culture media. At concentrations of ≤0.2% of Indian sandalwood oil, a cell viability higher than 70% could be observed. In parallel, the cell count of HaCaT cells treated for 48 h with Indian sandalwood oil was also evaluated (
Figure 2B). At concentrations of 10%, 2%, 0.6%, 0.5%, 0.4%, and 0.3%, a decrease lower than 70% of the cell count could be observed. However, the cells treated with 0.2% of Indian sandalwood oil or less showed cell count results showing more than 70% cell viability, which was reminiscent to what was previously observed for a treatment time of 24 h. Based on these results, all subsequent efficacy experiments performed in HaCaT cells and necessitating 24 h or 48 h treatment with Indian sandalwood oil were performed at 0.2% as the highest concentration.
3.2. Cellular Antioxidant Assay
Then, the potential of Indian sandalwood oil to protect against the oxidative stress induced by the peroxyl initiator AAPH was monitored in HaCaT cells. As determined in the MTT assay, the highest concentration of Indian sandalwood oil used was 0.2%, and the lowest concentration used was 0.001%. Quercetin, a known antioxidant, was used as a positive control. The highest concentration used was 0.0075%, while the lowest concentration used was 0.00023% (
Figure 3A,B).
Indian sandalwood oil showed antioxidant potential at the five highest concentrations tested (0.2%, 0.1%, 0.07%, 0.05%, and 0.025%). The antioxidant potency of Indian sandalwood oil was equivalent to what was observed with the three highest concentrations of the positive control quercetin. These results suggested that at a concentration of 0.2%, Indian sandalwood oil has an antioxidant activity that was as potent as the antioxidant activity of the positive control quercetin at 0.0075%. IC
50 determination was found to be 0.03% for Indian sandalwood oil and 0.002% for quercetin (
Figure 3B).
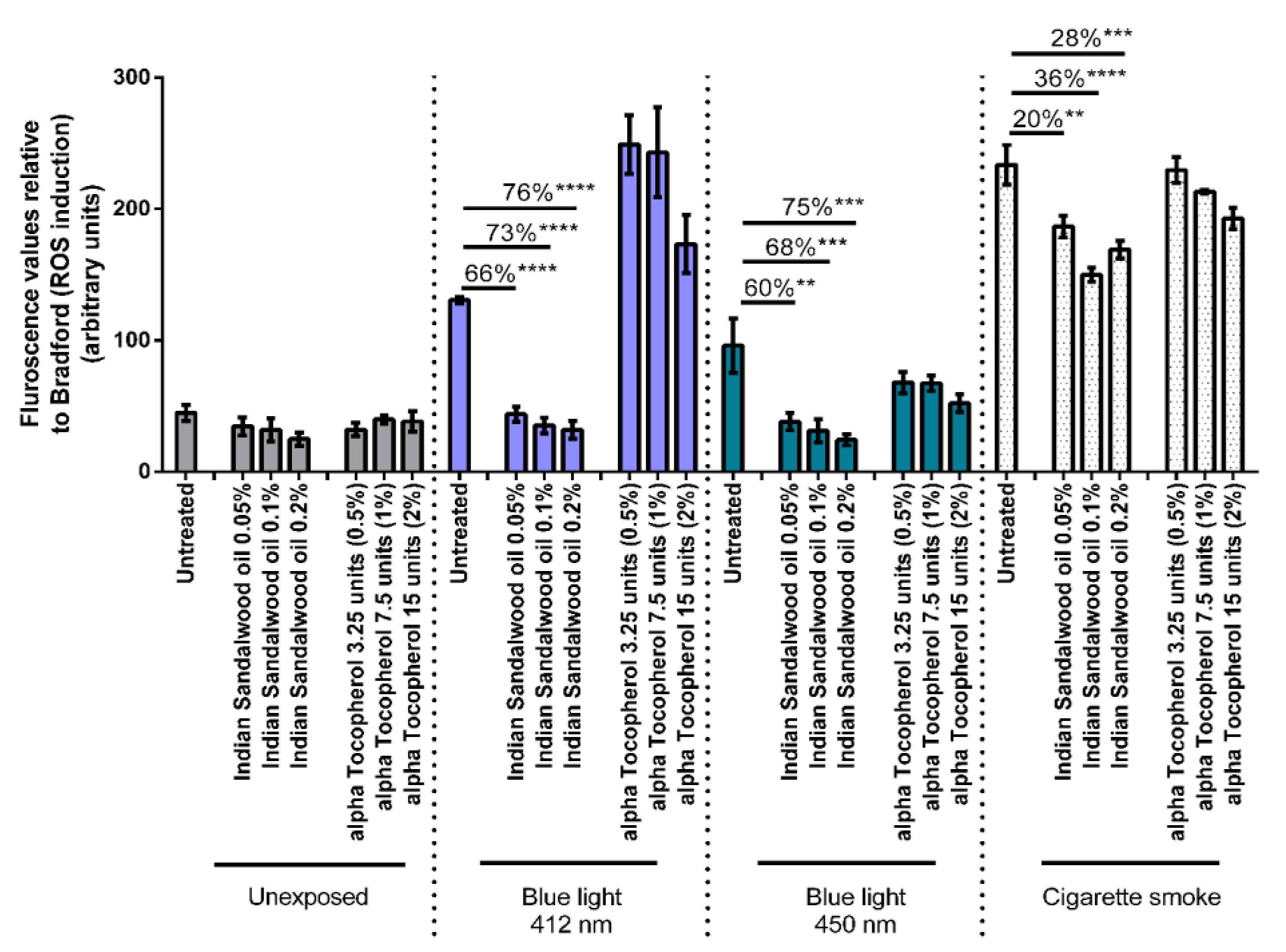
3.3. Intracellular Reactive Oxygen Species (ROS) Scavenging Activity Assay
The antioxidant potential of Indian sandalwood oil to protect against oxidative stress induced by environmental stressors was subsequently monitored. Three different environmental stressors were used in this study namely blue light at 412 nm, blue light at 450 nm, and cigarette smoke. Both wavelengths of blue light were tested at a dose of 1 J/cm
2. Three concentrations of Indian sandalwood oil showing good cellular antioxidant efficacy from the MTT assay were used for this assay: namely, 0.05%, 0.1%, and 0.2%. Alpha-tocopherol, a lipophilic antioxidant, was used as the positive control at concentrations of 0.5%, 1%, and 2% (
Figure 4).
A sharp induction in the levels or ROS can be observed basally when the untreated HaCaT cells were exposed to the stressors: blue light at 412 nm, blue light at 450 nm, and cigarette smoke. When the HaCaT cells were treated with the three concentrations of Indian sandalwood oil (0.05%, 0.1% and 0.2%), a significant reduction in the oxidative stress induced by either blue light (412 nm or 450 nm) or cigarette smoke could be observed. Indeed, 66% (p < 0.0001), 73% (p < 0.0001), and 76% (p < 0.0001) decreases in the level of ROS could be observed in cells treated with 0.05%, 0.1%, and 0.2% of Indian sandalwood oil, respectively prior to exposure to blue light at 412 nm. In cells exposed to blue light at 450 nm, a similar trend could be observed where 60% (p = 0.0012), 68% (p = 0.0005), and 75% (p = 0.0002) decreases could be observed for similar concentrations of Indian sandalwood oil, respectively. Moreover, in HaCaT cells that were exposed to cigarette smoke, Indian sandalwood oil was observed to significantly protect against the ROS induced, although the decrease was more modest in comparison to blue light at 412 nm and 450 nm. Thus, at the highest concentration (0.2%) of Indian sandalwood oil, only a 28% (p = 0.001) decrease in the level of ROS was detected.
While all three concentrations (0.5%, 1%, and 2%) of the positive control, alpha-tocopherol, could significantly reduce the ROS induced by blue light 450 nm, only the two highest concentrations of alpha-tocopherol (1% and 2%) could induce a protective effect against the levels of ROS induced by cigarette smoke when compared to the untreated samples. However, none of the three concentrations tested for alpha-tocopherol could significantly decrease the ROS induced by blue light 412 nm. Interestingly, in HaCaT cells treated with the highest concentration of alpha-tocopherol prior to blue light 412 nm exposure, an increase in the levels of ROS can be observed compared to untreated but exposed cells. This suggests that an interference might be occurring between the DCFH-DA assay, alpha-tocopherol, and blue light at 412 nm.
3.4. Collagenase Inhibition Assay (MMP-1)
Since the protective effect of Indian sandalwood oil against the oxidative stress induced by environmental stressors was demonstrated, the evaluation of whether Indian sandalwood oil could protect against the detrimental effect of pollution by monitoring a more downstream effector, MMP-1, was also investigated (
Figure 5).
There was a drastic increase in the levels of MMP-1 in the untreated samples exposed to the environmental stressors when compared to the untreated and unexposed samples. When exposed to ozone, Indian sandalwood oil inhibited the expression of MMP-1 by 88% (p < 0.0001). Indian sandalwood oil also displayed an inhibitory effect on the levels of MMP-1 when exposed to cigarette smoke. For this exposure, the difference between the untreated and treated samples was 70% (p = 0.0003), which was statistically significant.
4. Discussion
Indian sandalwood has been in use for over 2500 years, and its commercial usage dates back to 300 BCE [
2,
6]. It has been previously reported for its antioxidant, anti-tyrosinase, anti-inflammatory, anti-proliferative, and anti-microbial properties [
10,
11,
12,
23,
24]. Despite reports of the pharmacological activity of sandalwood, only a few studies have evaluated its benefits as a cosmetic ingredient. In particular, the potential benefits of Indian sandalwood oil in protecting the skin against the detrimental effect of environmental stressors has never been reported. In this study, we demonstrated that Indian sandalwood oil showed a significant protective effect against pollutants such as cigarette smoke and ozone in skin explants. Indian sandalwood oil was also shown to protect HaCaT cells against the oxidative stress induced by environmental provocation by both blue light and cigarette smoke. We further demonstrated that Indian sandalwood oil decreases the collagenase MMP-1 levels in skin explants.
Cellular antioxidant assays were performed revealing the inhibitory effect of Indian sandalwood oil. In this study, 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) was utilized as a free radical generating compound that could mimic oxidative stress in the HaCaT cells. This was used to induce a constant rate of radical generation, allowing sufficient time to assess the free-radical-scavenging activity [
25]. Quercetin was chosen as the positive control. Our study showed that Indian sandalwood oil has equal antioxidant capacity to the established antioxidant quercetin, where a 95–85% decrease in the level of ROS could be observed at the highest concentration used for both test products.
Then, HaCaT cells were subjected to environmental oxidative stressors, utilizing cigarette smoke and subsequently two distinct wavelengths of blue light. Cigarette smoke was shown to impart a far greater stress on HaCaT cells compared to the two wavelengths of blue light used. Indeed, cigarette smoke constituted a ubiquitous environmental hazard as a source of human exposure to chemically active pollutants. Ozone was used similarly in the ex vivo experiment. Both stressors generated high levels of ROS, including hydroxyl radicals and hydrogen peroxide, which can be linked to damaging pathological processes in the skin [
26,
27]. The protective effect of Indian sandalwood oil was more modest in cells exposed to cigarette smoke compared to blue light. This could be explained by the fact that in the different experiments performed in this study, cigarette smoke induced approximately twice more ROS than blue light. Thus, even at the highest concentration tested (0.2%) of Indian sandalwood oil, as well as the control of alpha-tocopherol at 2% (15 units), they were likely overwhelmed by the elevated amount of ROS induced.
Exposure to UV, in conjunction with the natural functional changes due to aging, causes dermal fibroblasts to increase the production of MMP-1 [
28,
29]. Matrix metalloproteinase-1 (MMP-1) is a zinc and calcium-dependent endopeptidase that is synthesized and released from both dermal fibroblast and keratinocytes. It works by breaking down collagen found in the extracellular matrix (ECM). Then, MMP-1 is released as an inactive proenzyme, which is later activated via proteolytic cleavage, leading to the release of its active form. An elevated expression of its activity caused by an upregulation has been implicated with a degradation of the extracellular matrix and leads to premature photoaging of the human skin as well as skin cancer [
30].
In skin tissue or cultured fibroblasts, both the active and inactivated form of MMP-1 is released into the culture media [
28]. However, it should be noted that the high-throughput ELISA assay used only quantified the pro-domain and the active form of the MMP-1 but did not quantify the inactivated form of MMP-1 [
28]. Notably, Indian sandalwood oil was able to decrease the level of matrix metalloproteinase-1 (MMP-1) in the ex vivo skin by a significant amount. As such, we can posit that by showing a significant drop in MMP-1, Indian sandalwood oil likely acted on the activated form of the MMP-1 enzyme. Thus, it can be concluded that Indian sandalwood oil effectively prevented an increase in the activity of at least the activated form of MMP-1. In the future, more studies could be performed to further explore the pathways by which the activity of MMP-1 was disrupted by Indian sandalwood oil.
It is well documented those environmental factors contribute to vulnerability in the skin, and this in turn leads to premature skin aging [
16]. The factors responsible for accelerated skin aging are contributed largely by the over-expression of ROS and the upregulation of MMPs [
16]. Indeed, as the skin ages, and due to the imbalance in the expression of free radicals, the turnover number of keratinocytes in the epidermis dropped. This led to the subsequent decrease in collagen found in the skin. These changes were reported to loop back and contribute to an increased production in free radicals [
31]. In addition, another factor of premature skin aging was the increased expression of MMPs and a decrease in the level of its inhibitors (TIMP). These were found to be related to the upregulation of ROS [
32]. Therefore, the level of ROS induced in the skin played a central role in the responses toward premature skin aging.
Here, we demonstrated that Indian sandalwood oil protected against the oxidation caused by blue light at 412 nm and 450 nm and the effect of cigarette smoke on keratinocytes with an approximate decrease of 75% in ROS activity observed with blue light and an approximate decrease of around 30% in ROS activity observed for cigarette smoke. This decrease in ROS and MMP-1 suggested that Indian sandalwood oil likely possessed skin anti-aging properties. These skin elements that were affected by the pollutants could be targeted by a cosmetic ingredient such as Indian sandalwood oil in the prevention of early skin aging. Ultimately, skin aging caused by environmental stress is a crucial element to consider in maintaining good skin health.
As reported by Velasco et al. [
15], cosmetic ingredients may work by either preventing contact between the skin and pollutants or by triggering biochemical processes that hinder the oxidative primary product. To achieve an ideal cosmetic formulation, both characteristics are heavily sought after. These would give rise to cosmetic products that can lower short-term damages such as inflammation and upregulate the signaling pathway to increase metabolic activity and cell differentiation [
15]. In this study, we reported promising preliminary results attesting to the protective and anti-aging effect of Indian sandalwood oil. Thus, in order to be a successful candidate as a cosmetic ingredient, further efficacy tests should be carried in order to further assess the functional characteristic of Indian sandalwood oil. Furthermore, in vivo assessment of the dermatological activity and usage levels of the oil remains to be carried out to examine the long-term and short-term effects of exposure to the environmental exposomes.
5. Conclusions
We described in this study a novel property of Indian sandalwood oil as a protective active ingredient against the detrimental effect of environmental stressors in vitro and on human skin ex vivo.
The antioxidant efficacy of Indian sandalwood oil using a cellular antioxidant assay was explored whereby the test substance significantly reduced the level of ROS induced by the peroxyl initiator AAPH. Reminiscent to the latter results, Indian sandalwood oil was also shown to subsequently protect HaCaT cells against the oxidative stress induced by environmental stressors such as blue light at 412 nm and 450 nm and cigarette smoke.
The protective effect of Indian sandalwood oil against the detrimental effect of pollution (cigarette smoke and ozone) was also monitored using a more complex model, human skin explants. Our results have shown that Indian sandalwood oil was capable of significantly decreasing the level of MMP-1 induced by either cigarette smoke or ozone.
The results presented in this study suggested that Indian sandalwood oil has the potential to serve as an active ingredient in dermatology for protection against environmental stressors in addition to its already existing fragrance and aromatherapy applications. Following more in-depth studies, Indian sandalwood oil may also serve as a promising candidate in its use as a multipurpose ingredient in cosmetics care.