The Potential of 2-aza-8-Oxohypoxanthine as a Cosmetic Ingredient
Abstract
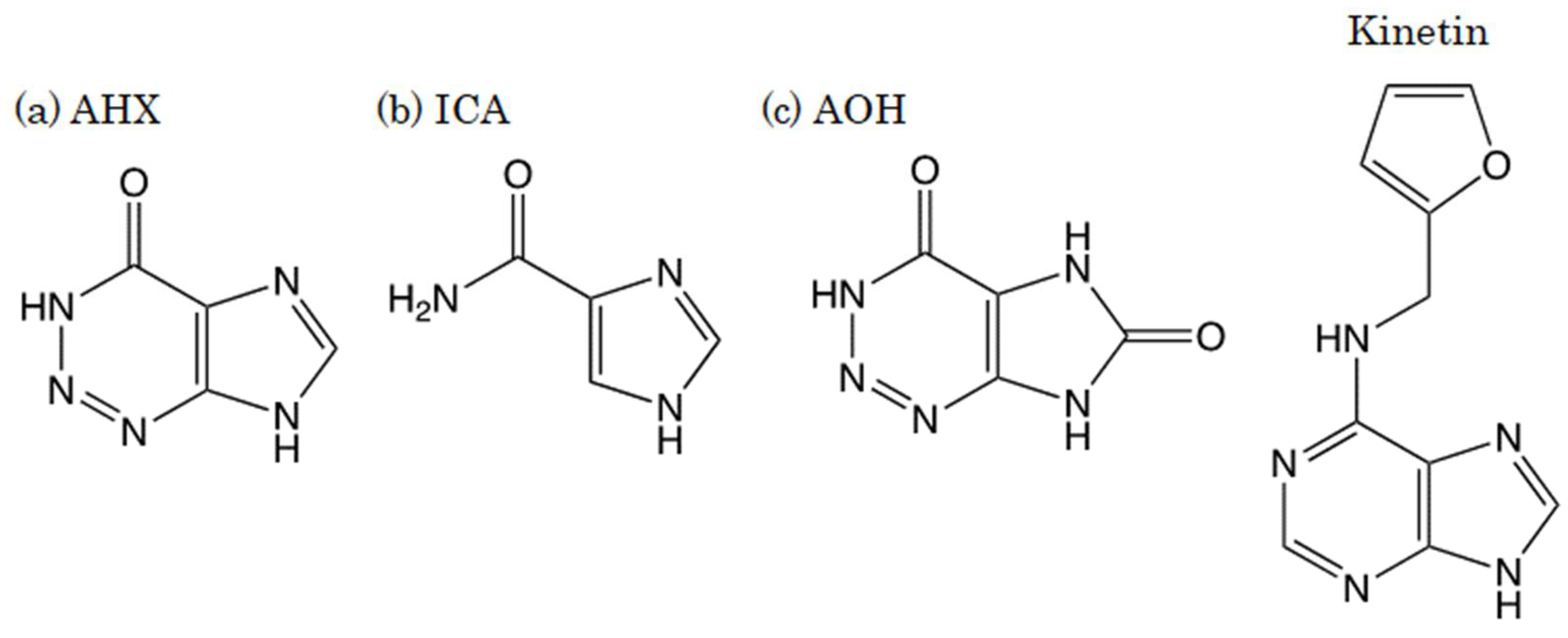
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Viability Assay
2.3. DNA Microarray Analysis
2.3.1. Cell Culture and Treatment with AOX
2.3.2. RNA Isolation
2.3.3. Microarray Analysis
2.4. Statistical Analysis
3. Results
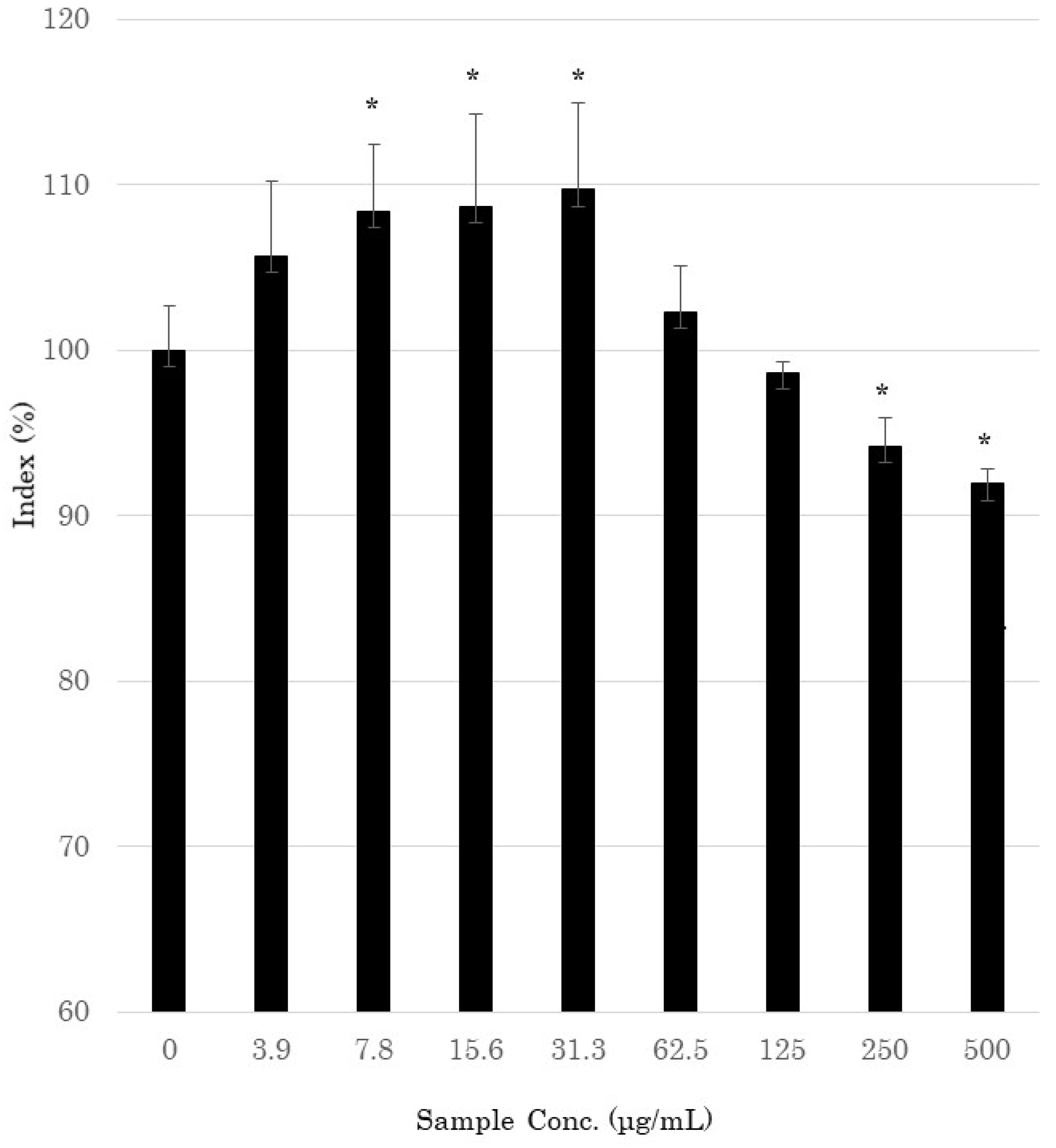
3.1. Cell Viability Assay
3.2. DNA Microarray Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsbottom, J. Rate of growth of fungus rings. Nature 1926, 117, 158–159. [Google Scholar] [CrossRef]
- Shantz, H.L.; Piemeisel, R.L. Fungus fairy rings in Eastern Colorado and their effect on vegetation. J. Agric. Res. 1917, 11, 191–245. [Google Scholar]
- Smith, J.D. Fungi and turf diseases. J. Sports Turf. Res. Inst. 1957, 10, 324–352. [Google Scholar]
- Evershed, H. Fairy rings. Nature 1884, 29, 384–385. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Fushimi, K.; Abe, N.; Tanaka, H.; Maeda, S.; Morita, A.; Hara, M.; Motohashi, R.; Matsunaga, J.; Eguchi, Y.; et al. Disclosure of the “fairy” of fairy-ring-forming fungus Lepista sordida. Chembiochem 2010, 11, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-growth regulator, imidazole-4-carboxamide, produced by the fairy ring forming fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ohnishi, T.; Yamakawa, Y.; Takeda, S.; Sekiguchi, S.; Maruyama, W.; Yamashita, K.; Suzuki, T.; Morita, A.; Ikka, T.; et al. The source of “fairy rings”: 2-azahypoxanthine and its metabolite found in a novel purine metabolic pathway in plants. Angew. Chem. Int. Ed. Engl. 2014, 53, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Mitchinson, A. Fairy chemicals. Nature 2014, 505, 298. [Google Scholar] [CrossRef]
- Asai, T.; Choi, J.H.; Ikka, T.; Fushimi, K.; Abe, N.; Tanaka, H.; Yamakawa, Y.; Kobori, H.; Kiriiwa, Y.; Motohashi, R.; et al. Effect of 2-azahypoxanthine (AHX) produced by the fairy-ring-forming fungus on the growth and the grain yield of rice. JARQ 2015, 49, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Tobina, H.; Choi, J.H.; Asai, T.; Kiriiwa, Y.; Asakawa, T.; Kan, T.; Morita, A.; Kawagishi, H. 2-Azahypoxanthine and imidazole-4-carboxamide produced by the fairy-ring-forming fungus increase yields of wheat. Field Crop. Res. 2014, 162, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Kawagishi, H. Fairy chemicals—A candidate for a new family of plant hormones and possibility of practical use in agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 752–758. [Google Scholar] [CrossRef]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baran, R.; Maibach, H.I. Textbook of Cosmetic Dermatology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 113–116. [Google Scholar]
- Berge, U.; Kristensen, P.; Rattan, S.I. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann. N. Y. Acad. Sci. 2006, 1067, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Berge, U.; Kristensen, P.; Rattan, S.I. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp. Gerontol. 2008, 43, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Chan, C.C.; Lin, H.M.; Chiu, H.C. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: A randomized, double-blind, placebo-controlled, split-face comparative trial. J. Cosmet. Dermatol. 2007, 6, 243–249. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.L.; Weinstein, G.D. Clinical study of safety and efficacy of using topical kinetin 0.1% (Kinerase) to treat photodamaged skin. Cosmet. Dermatol. 2002, 15, 29–32. [Google Scholar]
- Wu, J.J.; Weinstein, G.D.; Kricorian, G.J.; Kormeili, T.; McCullough, J.L. Topical kinetin 0.1% lotion for improving the signs and symptoms of rosacea. Clin. Exp. Dermatol. 2007, 32, 693–695. [Google Scholar] [CrossRef]
- Aoshima, H.; Hyodo, S.; Ibuki, R.; Wu, J.; Choi, J.H.; Kawagishi, H. Safety evaluation of 2-aza-8-oxohypoxanthine based on in vitro and human patch tests. Fundam. Toxicol. Sci. 2020, 7, 207–214. [Google Scholar] [CrossRef]
- Choi, J.H.; Kikuchi, A.; Pumkaeo, P.; Hirai, H.; Tokuyama, S.; Kawagishi, H. Bioconversion of AHX to AOH by resting cells of Burkholderia contaminans CH-1. Biosci. Biotechnol. Biochem. 2016, 80, 2045–2050. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, K.; Fujii, R.; Sugiyama, S.; Asakawa, T.; Inai, M.; Hamashima, Y.; Choi, J.H.; Suzuki, T.; Kawagishi, H.; Kan, T. Practical synthesis of natural plant-growth regulator 2-azahypoxanthine, its derivatives, and biotin-labeled probes. Org. Biomol. Chem. 2014, 12, 3813–3815. [Google Scholar] [CrossRef] [Green Version]
- Motojima, H.; O Villareal, M.; Han, J.; Isoda, H. Microarray analysis of immediate-type allergy in KU812 cells in response to fulvic acid. Cytotechnology 2011, 63, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isozaki, K.; Imamura, M.; Fukushima, K.; Tanaami, T.; Kawamur, S.; Negishi, H.; Otsuki, S.; Tomosada, N. Micro-measurement and manipulation technologies. Yokogawa Tech. Rep. Engl. Ed. 2006, 41, 7–18. [Google Scholar]
- Roth, W.; Kumar, V.; Beer, H.D.; Richter, M.; Wohlenberg, C.; Reuter, U.; Thiering, S.; Staratschek-Jox, A.; Hofmann, A.; Kreusch, F.; et al. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J. Cell Sci. 2012, 125, 5269–5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Cha, H.J.; Ko, J.M.; Han, H.; Kim, S.Y.; Kim, K.S.; Lee, S.J.; An, I.S.; Kim, S.; Youn, H.J.; et al. Kinetin Improves Barrier Function of the Skin by Modulating Keratinocyte Differentiation Markers. Ann. Dermatol. 2017, 29, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzeciak, M.; Olszewska, B.; Sakowicz-Burkiewicz, M.; Sokołowska-Wojdyło, M.; Jankau, J.; Nowicki, R.J.; Pawełczyk, T. Expression Profiles of Genes Encoding Cornified Envelope Proteins in Atopic Dermatitis and Cutaneous T-Cell Lymphomas. Nutrients 2020, 12, 862. [Google Scholar] [CrossRef] [Green Version]

| Function | Effect | Gene Symbol | Gene Name (Homo sapiens) | Cont. | AOH (30 μg/mL) | AOH (100 μg/mL) | AOH (300 μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | p | Mean ± S.D. | p | Mean ± S.D. | p | Mean ± S.D. | p | ||||
| Intercellular adhesion | Formation of tight junctions | CLDN1 | Claudin-1 | 1.00 ± 0.12 | 1.000 | 1.19 ± 0.08 | 0.090 | 1.49 ± 0.07 | 0.006 | 1.43 ± 0.18 | 0.031 |
| DSC1 | Desmocollin-1 | 1.00 ± 0.06 | 1.000 | 1.26 ± 0.23 | 0.170 | 1.28 ± 0.01 | 0.012 | 1.59 ± 0.09 | 0.001 | ||
| DSG1 | Desmocollin-1 | 1.00 ± 0.02 | 1.000 | 1.09 ± 0.14 | 0.363 | 1.13 ± 0.04 | 0.020 | 1.15 ± 0.01 | 0.000 | ||
| Cell adhesion factor (E-cadherin) | CDH1 | Desmoglein-1 | 1.00 ± 0.07 | 1.000 | 1.11 ± 0.08 | 0.152 | 1.09 ± 0.04 | 0.130 | 1.16 ± 0.03 | 0.038 | |
| Stratum corneum differentiation | Stratum corneum protease | KLK5 | Homo sapiens Kallikrein-5 | 1.00 ± 0.02 | 1.000 | 1.11 ± 0.07 | 0.087 | 1.16 ± 0.02 | 0.001 | 1.24 ± 0.06 | 0.013 |
| KLK7 | Homo sapiens Kallikrein-7 | 1.00 ± 0.08 | 1.000 | 1.11 ± 0.21 | 0.430 | 1.24 ± 0.05 | 0.016 | 1.17 ± 0.07 | 0.045 | ||
| Inhibitor of stratum corneum protease | SPINK5 (LEKTI1) | Serine peptidase inhibitor Kazal type 5 | 1.00 ± 0.04 | 1.000 | 1.15 ± 0.14 | 0.197 | 1.28 ± 0.03 | 0.002 | 1.38 ± 0.07 | 0.030 | |
| SPINK9 (LEKTI2) | Serine peptidase inhibitor Kazal type 9 | 1.00 ± 0.03 | 1.000 | 1.02 ± 0.16 | 0.859 | 1.13 ± 0.02 | 0.004 | 1.09 ± 0.08 | 0.176 | ||
| Epidermal differentiation | Filaggrin biosynthesis | BLMH | Bleomycin hydrolase | 1.00 ± 0.02 | 1.000 | 0.98 ± 0.06 | 0.648 | 1.11 ± 0.03 | 0.009 | 1.10 ± 0.05 | 0.068 |
| Induction of stratum corneum differentiation | KRT1 | Keratin 1 | 1.00 ± 0.04 | 1.000 | 1.468 ± 0.16 | 0.033 | 1.60 ± 0.11 | 0.006 | 2.07 ± 0.25 | 0.016 | |
| Induction of stratum corneum differentiation | KRT10 | Keratin 10 | 1.00 ± 0.03 | 1.000 | 1.19 ± 0.07 | 0.034 | 1.33 ± 0.08 | 0.010 | 1.44 ± 0.14 | 0.029 | |
| Formation of CE | TGM1 | Transglutaminase 1 | 1.00 ± 0.10 | 1.000 | 1.16 ± 0.14 | 0.173 | 1.29 ± 0.05 | 0.019 | 1.57 ± 0.08 | 0.002 | |
| IVL | Involucrin | 1.00 ± 0.04 | 1.000 | 1.35 ± 0.16 | 0.055 | 1.49 ± 0.03 | 0.000 | 1.75 ± 0.11 | 0.004 | ||
| SPRR1B | Small proline-rich protein 1 B | 1.00 ± 0.04 | 1.000 | 1.27 ± 0.05 | 0.003 | 1.42 ± 0.05 | 0.000 | 1.60 ± 0.14 | 0.012 | ||
| Biosynthesis of hyaluronic acid | Hyaluronic acid synthase (epidermis) | HAS3 | Hyaluronan synthase 3 | 1.00 ± 0.11 | 1.000 | 1.09 ± 0.19 | 0.510 | 1.27 ± 0.08 | 0.030 | 1.60 ± 0.13 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoshima, H.; Ito, M.; Ibuki, R.; Kawagishi, H. The Potential of 2-aza-8-Oxohypoxanthine as a Cosmetic Ingredient. Cosmetics 2021, 8, 60. https://doi.org/10.3390/cosmetics8030060
Aoshima H, Ito M, Ibuki R, Kawagishi H. The Potential of 2-aza-8-Oxohypoxanthine as a Cosmetic Ingredient. Cosmetics. 2021; 8(3):60. https://doi.org/10.3390/cosmetics8030060
Chicago/Turabian StyleAoshima, Hisae, Masayuki Ito, Rinta Ibuki, and Hirokazu Kawagishi. 2021. "The Potential of 2-aza-8-Oxohypoxanthine as a Cosmetic Ingredient" Cosmetics 8, no. 3: 60. https://doi.org/10.3390/cosmetics8030060
APA StyleAoshima, H., Ito, M., Ibuki, R., & Kawagishi, H. (2021). The Potential of 2-aza-8-Oxohypoxanthine as a Cosmetic Ingredient. Cosmetics, 8(3), 60. https://doi.org/10.3390/cosmetics8030060






