The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Bioactive Compound Extraction
2.4. Total Phenolic Content (TPC) Analysis
2.5. Cell Culture
2.6. Determination of Cell Cytotoxicity
2.7. Wound Healing Assay
2.8. Antioxidant Assays
2.8.1. DPPH Radical Scavenging Activity of the S. macrophylla Extract
2.8.2. ABTS Radical Scavenging Assay
2.8.3. Ferrous Ion Chelating Assay
2.9. UVA and UVB Absorption Spectrum of Extract and Its Fractions
2.10. Statistical Analysis
3. Results
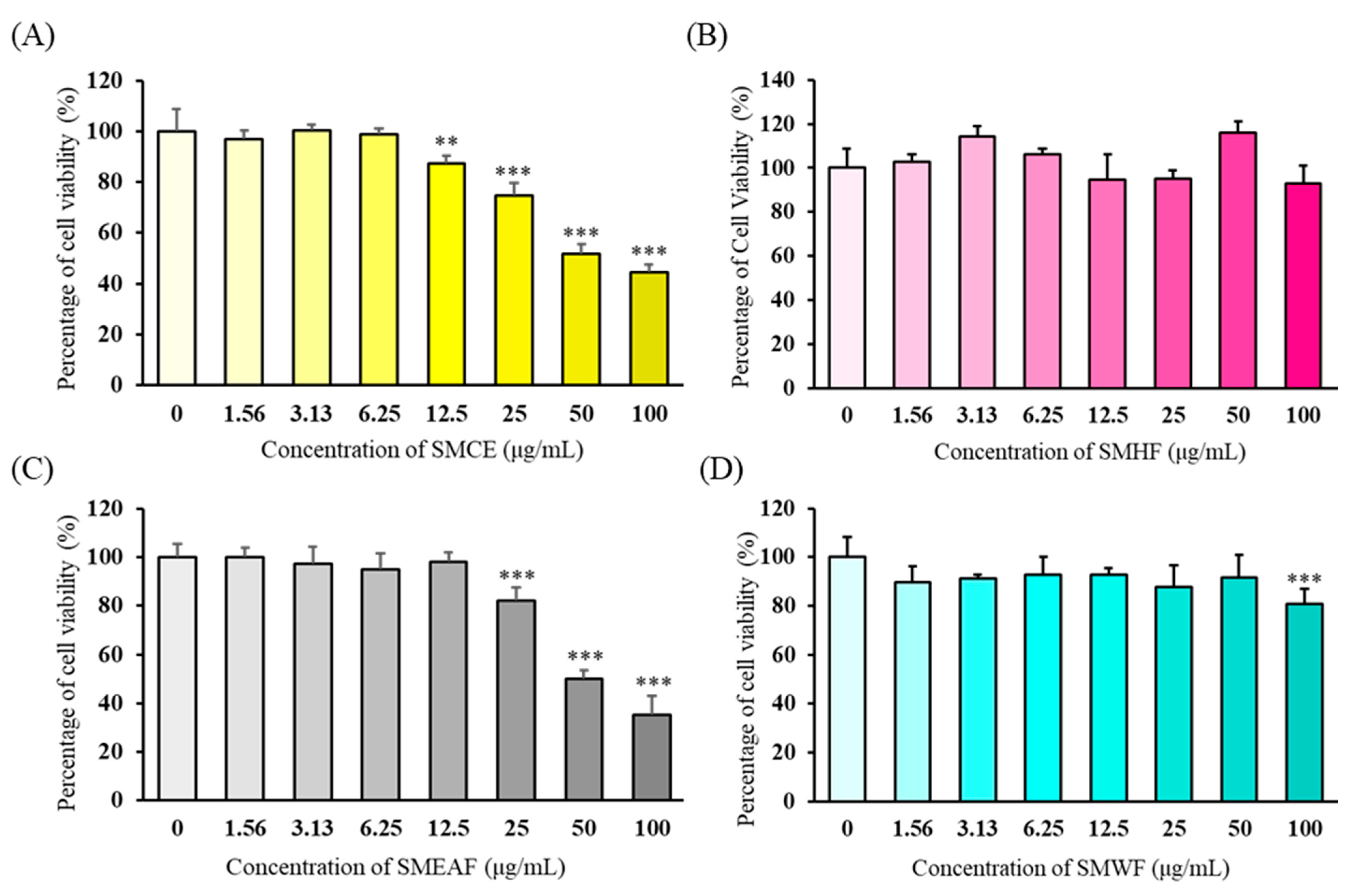
3.1. Assessment of Cytotoxicity of the Extract and Its Fraction on HaCaT Cells
3.2. Wound Healing Properties of S. macrophylla Seeds
3.3. Analysis of Antioxidant Properties of S. macrophylla Seed Extract
3.4. Potential Photoprotective Properties of S. macrophylla Extract and Its Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.-C.; Chiu, C.L.; Mansumitrchai, S.; Quarles, B.J. Hedonic and utilitarian value as a mediator of men’s intention to purchase cosmetics. J. Glob. Fash. Mark. 2020, 11, 71–89. [Google Scholar] [CrossRef]
- Scharffetter–Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Draelos, Z.D. The latest cosmeceutical approaches for anti-aging. J. Cosmet. Dermatol. 2007, 6, 2–6. [Google Scholar] [CrossRef]
- Guinot, C.; Malvy, D.J.M.; Ambroisine, L.; Latreille, J.; Mauger, E.; Tenenhaus, M.; Morizot, F.; Lopez, S.; Le Fur, I.; Tschachler, E. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch. Dermatol. 2002, 138, 1454–1460. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. MO Med. 2011, 108, 60–63. [Google Scholar] [PubMed]
- Smirnova, M.H. A will to youth: The woman’s anti-aging elixir. Soc. Sci. Med. 2012, 75, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, E.; Soon, P.C.; Mutum, D.S.; Nguyen, B. Health and cosmetics: Investigating consumers’ values for buying organic personal care products. J. Retial. Consum. Serv. 2017, 39, 154–163. [Google Scholar] [CrossRef]
- Dorni, A.I.C.; Augustine, A.; Sreeraj, G.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Gangemi, E.N.; Gregori, D.; Berchialla, P.; Zingarelli, E.; Cairo, M.; Bollero, D.; Ganem, J.; Capocelli, R.; Cuccuru, F.; Cassano, P.; et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch. Facial Plast. Surg. 2008, 10, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Rivera, A.E. Acne scarring: A review and current treatment modalities. J. Am. Acad. Dermatol. 2008, 59, 659–676. [Google Scholar] [CrossRef]
- Yu, P.-X.; Diao, W.-Q.; Qi, Z.-L.; Cai, J.-L. Effect of dermabrasion and ReCell® on large superficial facial scars caused by burn, trauma and acnes. Chin. Med. Sci. J. 2016, 31, 173–179. [Google Scholar] [CrossRef]
- Garg, V.K.; Sinha, S.; Sarkar, R. Glycolic acid peels versus salicylic–mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: A comparative study. Dermatol. Surg. 2009, 35, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Z.; Ding, J.-P.; Yang, M.-Y.; Chen, D.-W.; Chen, B. Treatment of facial post-burn hyperpigmentation using micro-plasma radiofrequency technology. Lasers Med. Sci. 2015, 30, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Akino, K.; Imaizumi, T.; Hirano, A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008, 16, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Lin, C.K.; Chuang, Y.S.; Chang, F.R.; Tseng, C.K.; Wu, Y.C.; Lee, J.C. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 2012, 19, 364–370. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Chien, Y.-T.; Lee, J.-C.; Tseng, C.-K.; Wang, H.-C.; Lo, I.-W.; Wu, Y.-H.; Wang, S.-Y.; Wu, Y.-C.; Chang, F.-R. Limonoids from the seeds of Swietenia macrophylla with inhibitory activity against dengue virus 2. J. Nat. Prod. 2014, 77, 2367–2374. [Google Scholar] [CrossRef]
- Goh, B.H.; Abdul Kadir, H. In vitro cytotoxic potential of Swietenia macrophylla King seeds against human carcinoma cell lines. J. Med. Plant Res. 2011, 5, 1395–1404. [Google Scholar] [CrossRef]
- Low, D.Y.S.; Mahendra, C.K.; Supramaniam, J.; Tan, L.T.H.; Lee, L.H.; Manickam, S.; Goh, B.H.; Tan, K.W.; Tang, S.Y. Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity. Nanotechnol. Rev. 2021, 10, 572–585. [Google Scholar] [CrossRef]
- Sayyad, M.; Tiang, N.; Kumari, Y.; Goh, B.H.; Jaiswal, Y.; Rosli, R.; Williams, L.; Shaikh, M.F. Acute toxicity profiling of the ethyl acetate fraction of Swietenia macrophylla seeds and in-vitro neuroprotection studies. Saudi Pharm. J. 2017, 25, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, A.; Yam, M.F.; Hor, S.Y.; Lim, C.P.; Asmawi, M.Z.; Sadikun, A. Anti-hyperglycaemic activity of Swietenia macrophylla King (Meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin. Med. 2013, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Goh, B.H.; Chan, C.K.; Kamarudin, M.N.A.; Abdul Kadir, H. Swietenia macrophylla King induces mitochondrial-mediated apoptosis through p53 upregulation in HCT116 colorectal carcinoma cells. J. Ethnopharmacol. 2014, 153, 375–385. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.-I.; Jang, J.-H. Sargassum fulvellum protects HaCaT cells and BALB/c mice from UVB-induced proinflammatory responses. Evid. Based Complementary Altern. Med. 2013, 2013, 747846. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Zainal, N.; Palanisamy, U.D.; Goh, B.-H.; Yin, W.-F.; Chan, K.-G.; Lee, L.-H. Streptomyces gilvigriseus sp. nov., a novel actinobacterium isolated from mangrove forest soil. Antonie van Leeuwenhoek 2015, 107, 1369–1378. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Mahendra, C.K.; Mahendra, C.K.; Pusparajah, P.; Htar, T.-T.; Lay-Hong, C.; Duangjai, A.; Khan, T.M.; Yoon Yen, Y.; Kumari, Y.; Bey Hing, G. Simplified, cost effective, and accurate calculation of critical wavelength via the MATLAB software. Prog. Drug Discov. Biomed. Sci. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Diffey, B.L. A method for broad spectrum classification of sunscreens. Int. J. Cosmet. Sci. 1994, 16, 47–52. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 1–34. [Google Scholar]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cellular Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, S.; Udupa, S.; Udupa, L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evid. Based Complementary Altern. Med. 2008, 5, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.-G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef] [Green Version]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Reddy, B.S.; Reddy, R.K.K.; Naidu, V.G.M.; Madhusudhana, K.; Agwane, S.B.; Ramakrishna, S.; Diwan, P.V. Evaluation of antimicrobial, antioxidant and wound-healing potentials of Holoptelea integrifolia. J. Ethnopharmacol. 2008, 115, 249–256. [Google Scholar] [CrossRef]
- Nilugal, K.C.; Fattepur, S.; Asmani, M.F.; Abdullah, I.; Vijendren, S.; Ugandar, R.E. Evaluation of wound healing acitivity of Swietenia macrophylla (Meliaceae) seed extract in albino rats. J. Pharm. Tech. Res. 2017, 7, 113–124. [Google Scholar]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef] [PubMed]
- Musalmah, M.; Fairuz, A.H.; Gapor, M.T.; Wan Ngah, W.Z. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac. J. Clin. Nutr. 2002, 11, S448–S451. [Google Scholar] [CrossRef] [PubMed]
- Fowles, R.G.; Mootoo, B.S.; Ramsewak, R.S.; Reynolds, W.F.; Nair, M.G. Limonoids from Meliaceae with lipid peroxidation inhibitory activity. Nat. Prod. Commun. 2011, 6, 785–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiming, Z.; Mak, K.-K.; Balijepalli, M.K.; Chakravarthi, S.; Pichika, M.R. Swietenine potentiates the antihyperglycemic and antioxidant activity of Metformin in Streptozotocin induced diabetic rats. Biomed. Pharmacother. 2021, 139, 111576. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salucci, S.; Burattini, S.; Buontempo, F.; Martelli, A.M.; Falcieri, E.; Battistelli, M. Protective effect of different antioxidant agents in UVB-irradiated keratinocytes. Eur. J. Histochem. 2017, 61, 2784. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-H.; Lee, D.-H.; Yoon, J.-H.; Nkajima, S.; Yasui, A.; Pfeifer, G.P. Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J. Biol. Chem. 2001, 276, 44688–44694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, D.; Raffa, S.; Flori, E.; Aspite, N.; Briganti, S.; Cardinali, G.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J. Dermatol. Sci. 2009, 54, 106–113. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Senthil Kumar, K.J.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Filip, G.A.; Postescu, I.D.; Bolfa, P.; Catoi, C.; Muresan, A.; Clichici, S. Inhibition of UVB-induced skin phototoxicity by a grape seed extract as modulator of nitrosative stress, ERK/NF-kB signaling pathway and apoptosis, in SKH-1 mice. Food Chem. Toxicol. 2013, 57, 296–306. [Google Scholar] [CrossRef]
- Nylander, K.; Bourdon, J.-C.; Bray, S.E.; Gibbs, N.K.; Kay, R.; Hart, I.; Hall, P.A. Transcriptional activation of tyrosinase and TRP-1 by p53 links UV irradiation to the protective tanning response. J. Pathol. 2000, 190, 39–46. [Google Scholar] [CrossRef]
- Imokawa, G. Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: A pivotal role of fibrobrast-derived elastase. Arch. Dermatol. Res. 2008, 300, 7–20. [Google Scholar] [CrossRef]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. UVB and pro-inflammatory cytokines synergistically activate TNF-α production in keratinocytes through enhanced gene transcription. J. Investig. Dermatol. 2009, 129, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in uv-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. Engl. 2017, 56, 9812–9815. [Google Scholar] [CrossRef]
- Serpone, N.; Salinaro, A.; Emeline, A.V.; Horikoshi, S.; Hidaka, H.; Zhao, J. An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents. Photochem. Photobiol. Sci. 2002, 1, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Djalil, A.D.; Ambarwati, T.; Genatrika, E. Characterization of sunscreen cream containing benzophenone-3,3′,4,4′-tetracarboxylate dianhydride. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012090. [Google Scholar] [CrossRef]
- ISO. ISO 24444:2010 Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). Available online: https://www.iso.org/obp/ui/#iso:std:iso:24444:ed-1:v1:en (accessed on 10 August 2018).
- FDA. Guidance for Industry Labelling and Effectiveness Testing: Sunscreen Drug Products for Over-the Counter Human Use—Small Entity Compliance Guide. Available online: https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm (accessed on 10 August 2018).
- Pelizzo, M.; Zattra, E.; Nicolosi, P.; Peserico, A.; Garoli, D.; Alaibac, M. In Vitro evaluation of sunscreens: An update for the clinicians. ISRN Dermatol. 2012, 2012, 352135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- Mahendra, C.K.; Abidin, S.A.Z.; Htar, T.T.; Chuah, L.H.; Khan, S.U.; Ming, L.C.; Tang, S.Y.; Pusparajah, P.; Goh, B.H. Counteracting the ramifications of UVB irradiation and photoaging with Swietenia macrophylla King seed. Molecules 2021, 26, 2000. [Google Scholar] [CrossRef] [PubMed]



| Sample | Concentration (μg/mL) | DPPH Radical Scavenging Activity (%) | ABTS Radical Scavenging Activity (%) | Iron Chelating Activity (%) |
|---|---|---|---|---|
| SMCE | 125 | ND | 3.878 ± 1.26 | 1.738 ± 0.55 |
| 250 | ND | ND | ND | |
| 500 | ND | ND | ND | |
| 1000 | 3.581 ± 3.45 | ND | ND | |
| 2000 | 4.523 ± 1.64 | ND | ND | |
| SMHF | 125 | ND | ND | ND |
| 250 | ND | ND | ND | |
| 500 | ND | 0.662 ± 2.00 | 3.830 ±0.27 | |
| 1000 | ND | 2.523 ± 1.55 | 4.638 ± 1.56 | |
| 2000 | ND | 6.082 ± 0.54 | 14.073 ± 0.18 * | |
| SMEAF | 125 | ND | 3.130 ±1.14 | 0.959 ± 2.38 |
| 250 | ND | ND | ND | |
| 500 | ND | ND | ND | |
| 1000 | ND | ND | ND | |
| 2000 | 0.377 ± 1.15 | ND | ND | |
| SMWF | 125 | ND | 12.796 ± 2.01 * | 8.014 ± 2.51 * |
| 250 | ND | 14.252 ± 3.37 * | 10.366 ± 0.38 * | |
| 500 | 0.452 ± 1.82 | 18.297 ± 3.01 * | 12.01 ± 1.95 * | |
| 1000 | 2.978 ± 0.90 | 20.077 ± 0.93 * | 15.531± 2.19 * | |
| 2000 | 6.332 ± 0.80 * | 29.946 ± 0.47 * | 23.523 ± 1.94 * | |
| Gallic acid 1 | 10 | 42.158 ± 2.03 * | 44.578 ± 0.58 * | N/A |
| EDTA 1 | 50 | N/A | N/A | 24.821 ± 1.14 * |
| Antioxidant Activities | Total Phenolic Content |
|---|---|
| DPPH radical scavenging activity | r = 0.935 * |
| ABTS radical scavenging activity | r = 0.903 * |
| Iron chelating activity | r = 0.915 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahendra, C.K.; Tan, L.T.H.; Mahendra, C.K.; Ser, H.-L.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Yap, W.H.; Tang, S.Y.; Ming, L.C.; et al. The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent. Cosmetics 2021, 8, 79. https://doi.org/10.3390/cosmetics8030079
Mahendra CK, Tan LTH, Mahendra CK, Ser H-L, Pusparajah P, Htar TT, Chuah L-H, Yap WH, Tang SY, Ming LC, et al. The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent. Cosmetics. 2021; 8(3):79. https://doi.org/10.3390/cosmetics8030079
Chicago/Turabian StyleMahendra, Camille Keisha, Loh Teng Hern Tan, Cayvern Kishen Mahendra, Hooi-Leng Ser, Priyia Pusparajah, Thet Thet Htar, Lay-Hong Chuah, Wei Hsum Yap, Siah Ying Tang, Long Chiau Ming, and et al. 2021. "The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent" Cosmetics 8, no. 3: 79. https://doi.org/10.3390/cosmetics8030079
APA StyleMahendra, C. K., Tan, L. T. H., Mahendra, C. K., Ser, H.-L., Pusparajah, P., Htar, T. T., Chuah, L.-H., Yap, W. H., Tang, S. Y., Ming, L. C., Yow, Y.-Y., & Goh, B. H. (2021). The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent. Cosmetics, 8(3), 79. https://doi.org/10.3390/cosmetics8030079