Topical Use of Cannabis sativa L. Biochemicals
Abstract
:1. Introduction
2. Cannabis sativa L. Botany
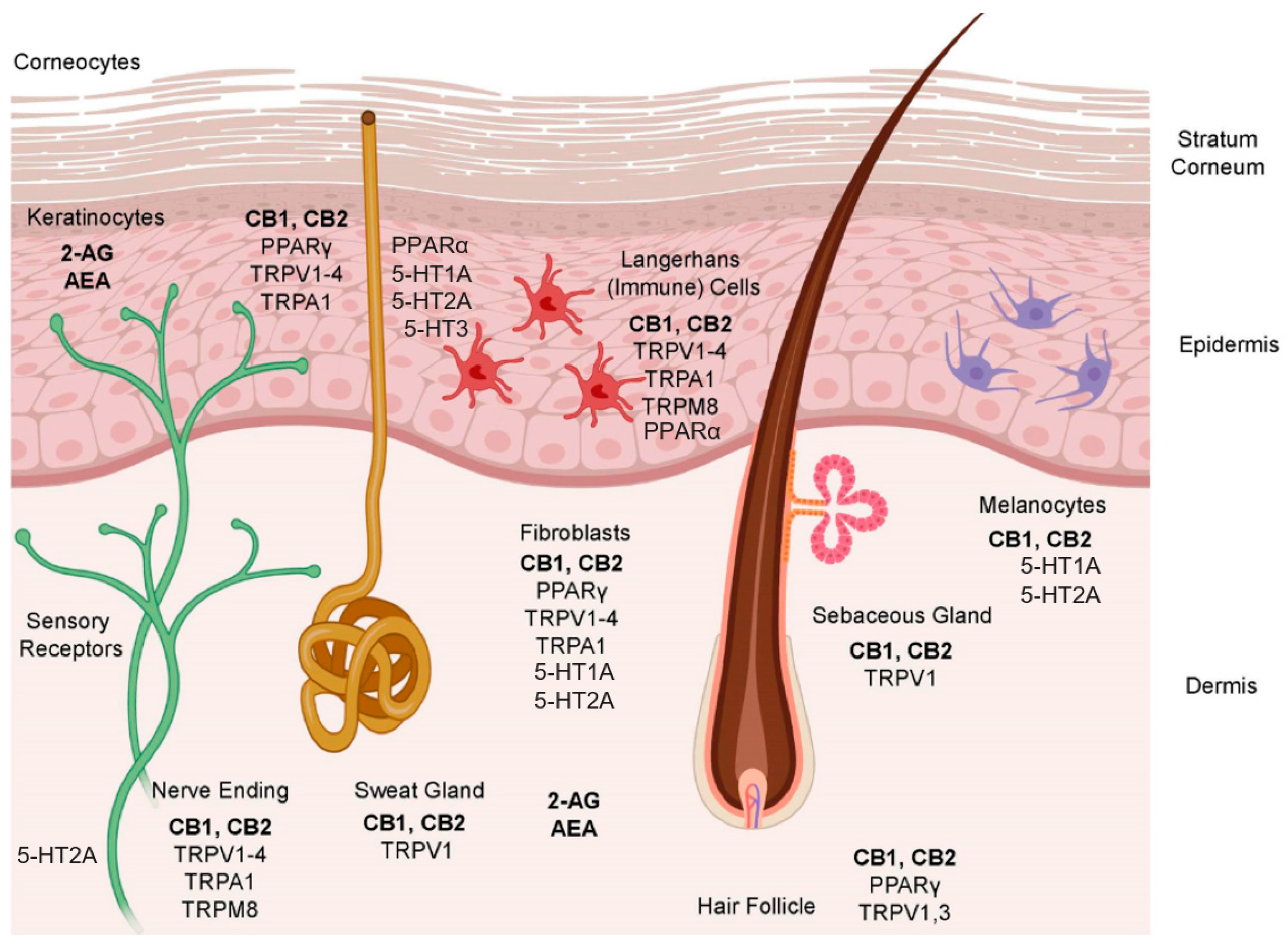
3. The Endocannabinoid System
4. Secondary Cannabinoid Target
5. Cannabinoids
6. Hemp Seed Oil
6.1. Generalities
6.2. Biological Value of the Minor Compounds of Hemp Seed Oil
6.2.1. Essential Fatty Acids
6.2.2. Carotenoids
6.2.3. Tocopherols
6.2.4. Phytosterols
6.2.5. Chlorophyll
6.2.6. Flavonoids
6.2.7. Terpenes
6.3. The Entourage Effect
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Grof, C.P.L. Cannabis, from plant to pill. Br. J. Clin. Pharmacol. 2018, 84, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2019, 31, 839–845. [Google Scholar] [CrossRef]
- Vogl, C.R.; Mölleken, H.; Lissek-Wolf, G.; Surböck, A.; Kobert, J. Hemp (Cannabis sativa L.) as a resource for green cosmetics: Yield of seed and fatty acid compositions of 20 varieties under the growing conditions of organic farming in Austria. J. Ind. Hemp 2004, 9, 51–68. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, L.; Gu, X.; Wang, J. Study on the Oxidation Products of Hemp Seed Oil and its Application in Cosmetics. Tenside Surfactants Deterg. 2020, 57, 230–236. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Kiralan, M.; Gül, V.; Kara, S.M. Fatty acid composition of hempseed oils from different locations in Turkey. Span. J. Agric. Res. 2010, 8, 385. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Nikolaou, P.; Mitakou, S.; Skaltsounis, A. Exploitation of Vitis vinifera, Foeniculum vulgare, Cannabis sativa and Punica granatum By-Product Seeds as Dermo-Cosmetic Agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef]
- Bakro, F.; Jedryczka, M.; Wielgusz, K.; Sgorbini, B.; Inchingolo, R.; Cardenia, V. Simultaneous determination of terpenes and cannabidiol in hemp (Cannabis sativa L.) by fast gas chromatography with flame ionization detection. J. Sep. Sci. 2020, 43, 2817–2826. [Google Scholar] [CrossRef]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Klumpers, L.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Cannabis sativa L.: Botany and Horticulture. In Cannabis sativa L.—Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 79–100. [Google Scholar] [CrossRef]
- Addo, P.W.; Brousseau, V.D.; Morello, V.; MacPherson, S.; Paris, M.; Lefsrud, M. Cannabis chemistry, post-harvest processing methods and secondary metabolite profiling: A review. Ind. Crop. Prod. 2021, 170, 113743. [Google Scholar] [CrossRef]
- EB, R. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef] [Green Version]
- del Río, C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef]
- Caterina, M.J. TRP Channel Cannabinoid Receptors in Skin Sensation, Homeostasis, and Inflammation. ACS Chem. Neurosci. 2014, 5, 1107–1116. [Google Scholar] [CrossRef]
- Tüting, T.; Gaffal, E. Regulatory Role of Cannabinoids for Skin Barrier Functions and Cutaneous. Inflammation 2017, 543–549. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Jorgačević, B.; Vučević, D.; Samardžić, J.; MladenoviĆ, D.; Vesković, M.; Vukićević, D.; Ješić, R.; Radosavljević, T. The Effect of CB1 Antagonism on Hepatic Oxidative/Nitrosative Stress and Inflammation in Nonalcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 28, 169–180. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25, 417. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.-K.; Zhang, M.; Li, J.-Y.; Wang, L.-L.; Guan, D.-W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef] [Green Version]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Alba, R.; Barragan-Iglesias, P.; González-Hernández, A.; Valdez-Moráles, E.E.; Granados-Soto, V.; Condés-Lara, M.; Rodríguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2019, 9, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E. Jagerovic NTherapeutic Exploitation of GPR18: Beyond the Cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55-A putative “type 3” cannabinoid receptor in inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Perez-Gomez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sanchez, C. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2012, 32, 2534–2542. [Google Scholar] [CrossRef] [Green Version]
- Fabisiak, A.; Fabisiak, N.; Mokrowiecka, A.; Malecka-Panas, E.; Jacenik, D.; Kordek, R.; Zielińska, M.; Kieć-Kononowicz, K.; Fichna, J. Novel selective agonist of GPR18, PSB-KK-1415 exerts potent anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain. Neurogastroenterol. Motil. 2020, 33, e14003. [Google Scholar] [CrossRef]
- Qin, Y.; Verdegaal, E.M.E.; Siderius, M.; Bebelman, J.P.; Smit, M.J.; Leurs, R.; Willemze, R.; Tensen, C.P.; Osanto, S. Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: The constitutively active orphan GPCR GPR18 as novel drug target. Pigment. Cell Melanoma Res. 2011, 24, 207–218. [Google Scholar] [CrossRef]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 173–192. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2006, 160, 1–24. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2016, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, O.; Cayirli, M.; Sakin, Y.S.; Seyrek, M.; Akar, A.; Dogrul, A. Systemic and spinal administration of FAAH, MAGL inhibitors and dual FAAH/MAGL inhibitors produce antipruritic effect in mice. Arch. Dermatol. Res. 2016, 308, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. Endocannabinoid Modulation of Scratching Response in an Acute Allergenic Model: A New Prospective Neural Therapeutic Target for Pruritus. J. Pharmacol. Exp. Ther. 2009, 329, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Sasso, O.; Summa, M.; Armirotti, A.; Pontis, S.; De Mei, C.; Piomelli, D. The N-Acylethanolamine Acid Amidase Inhibitor ARN077 Suppresses Inflammation and Pruritus in a Mouse Model of Allergic Dermatitis. J. Investig. Dermatol. 2018, 138, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Nordlind, K.; Azmitia, E.; Slominski, A. The skin as a mirror of the soul: Exploring the possible roles of serotonin. Exp. Dermatol. 2008, 17, 301–311. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.; Strübing, C. The trp ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Rigamonti, E.; Chinetti, G.; Staels, B. Regulation of Macrophage Functions by PPAR-α, PPAR-γ, and LXRs in Mice and Men. Arter. Thromb. Vasc. Biol. 2008, 28, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Bagood, M.; Isseroff, R. TRPV1: Role in Skin and Skin Diseases and Potential Target for Improving Wound Healing. Int. J. Mol. Sci. 2021, 22, 6135. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Bonchak, J.G.; Swerlick, R.A. Emerging therapies for atopic dermatitis: TRPV1 antagonists. J. Am. Acad. Dermatol. 2018, 78, S63–S66. [Google Scholar] [CrossRef]
- Ishii, T.; Uchida, K.; Hata, S.; Hatta, M.; Kita, T.; Miyake, Y.; Okamura, K.; Tamaoki, S.; Ishikawa, H.; Yamazaki, J. TRPV2 channel inhibitors attenuate fibroblast differentiation and contraction mediated by keratinocyte-derived TGF-β1 in an in vitro wound healing model of rats. J. Dermatol. Sci. 2018, 90, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid signaling in the skin: Therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019, 24, 918. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Yang, F.; Zheng, J.; Wang, K. Intracellular Proton-mediated Activation of TRPV3 Channels Accounts for the Exfoliation Effect of α-Hydroxyl Acids on Keratinocytes. J. Biol. Chem. 2012, 287, 25905–25916. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the Warm Temperature-Activated Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel Attenuates Atopic Dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef]
- Sokabe, T.; Tominaga, M. The TRPV4 cation channel. Commun. Integr. Biol. 2010, 3, 619–621. [Google Scholar] [CrossRef]
- Goswami, R.; Cohen, J.; Sharma, S.; Zhang, D.X.; Lafyatis, R.; Bhawan, J.; Rahaman, S.O. TRPV4 ION Channel Is Associated with Scleroderma. J. Investig. Dermatol. 2017, 137, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Maglie, R.; de Araujo, D.S.M.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [Green Version]
- Dubrac, S.; Schmuth, M. PPAR-alpha in cutaneous inflammation. Dermato-Endocrinology 2011, 3, 23–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrofrancesco, A.; Kovacs, D.; Sarra, M.; Bastonini, E.; Cardinali, G.; Aspite, N.; Camera, E.; Chavatte, P.; Desreumaux, P.; Monteleone, G.; et al. Preclinical Studies of a Specific PPARγ Modulator in the Control of Skin Inflammation. J. Investig. Dermatol. 2014, 134, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K. Pharmacological activation of PPAR-γ: A potential therapy for skin fibrosis. Int. J. Dermatol. 2020, 60, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.G.; Selvi, E.; Balistreri, E.; Akhmetshina, A.; Palumbo, K.; Lorenzini, S.; Lazzerini, P.E.; Montilli, C.; Capecchi, P.L.; Lucattelli, M.; et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Correia-Sá, I.; Paiva, A.; Carvalho, C.M.; Vieira-Coelho, M.A. Cutaneous endocannabinoid system: Does it have a role on skin wound healing bearing fibrosis? Pharmacol. Res. 2020, 159, 104862. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. Current and Future Therapies for Psoriasis with a Focus on Serotonergic Drugs. Mol. Neurobiol. 2020, 57, 2391–2419. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. The Brain–Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System. Cells 2020, 9, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawana, S.; Kato, Y.; Omi, T. Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin. Exp. Dermatol. 2010, 35, 835–840. [Google Scholar] [CrossRef]
- Sadiq, A.; Menchetti, I.; Shah, A.; Jeschke, M.G.; Belo, C.; Carlos-Alcalde, W.; Hayat, M.Q.; Amini-Nik, S. 5-HT1A Receptor Function Makes Wound Healing a Happier Process. Front. Pharmacol. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Wetterberg, J.; Taher, C.; Azmitia, E.; Elnour, H. Time-dependent modulation of serotonin and its receptors 1A and 2A expression in allergic contact dermatitis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1200–1205. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.V.; Christensen, A.; Morgan, D.; Basso, K.B. Gas chromatography/electron ionization mass spectrometry (GC/EI-MS) for the characterization of phytocannabinoids in Cannabis sativa. Compr. Anal. Chemistry 2020, 90, 235–274. [Google Scholar] [CrossRef]
- Site How Biosynthesis of Cannabinoids Could Impact the Cannabis Industry–New Cannabis Ventures. Available online: https://www.newcannabisventures.com/how-biosynthesis-of-cannabinoids-could-impact-the-cannabis-industry/ (accessed on 12 July 2021).
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and Hemp. In Nutraceuticals: Efficacy, Safety and Toxicity; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 735–754. ISBN 9780128021477. [Google Scholar]
- Brousseau, V.D.; Wu, B.-S.; MacPherson, S.; Morello, V.; Lefsrud, M. Cannabinoids and Terpenes: How Production of Photo-Protectants Can Be Manipulated to Enhance Cannabis sativa L. Phytochemistry. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Phytocannabinoids 2017, 103, 61–101. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Palomares, B.; Garrido-Rodriguez, M.; Gonzalo-Consuegra, C.; Gómez-Cañas, M.; Saen-Oon, S.; Soliva, R.; Collado, J.A.; Fernández-Ruiz, J.; Morello, G.; Calzado, M.A.; et al. Δ 9 -Tetrahydrocannabinolic acid alleviates collagen-induced arthritis: Role of PPARγ and CB 1 receptors. Br. J. Pharmacol. 2020, 177, 4034–4054. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Invest. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [Green Version]
- Steenson, S.; Chambers, L. Cannabidiol: A budding industry! Nutr. Bull. 2019, 44, 228–240. [Google Scholar] [CrossRef]
- Rosenthaler, S.; Pöhn, B.; Kolmanz, C.; Nguyen Huu, C.; Krewenka, C.; Huber, A.; Kranner, B.; Rausch, W.D.; Moldzio, R. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 2014, 46, 49–56. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2020, 376, 204–212. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; de Medina, V.S.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB 2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Hallak, J.E.C.; Crippa, J.A.S. Neuropharmacological Effects of the Main Phytocannabinoids: A Narrative Review. Cannabinoids Neuropsychiatr. Disord. 2020, 1264, 29–45. [Google Scholar] [CrossRef]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ 9-tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N. Cannabinol Inhibits Proliferation and Induces Cell Cycle Arrest and Apoptosis in Glioblastoma, Hepatocellular Carcinoma and Breast Cancer Cells. Master’s Thesis, University of Lethbridge, Lethbridge, Alta, 2020. [Google Scholar]
- Anavi-Goffer, S.; Baillie, G.; Irving, A.J.; Gertsch, J.; Greig, I.R.; Pertwee, R.G.; Ross, R.A. Modulation of l-α-Lysophosphatidylinositol/GPR55 Mitogen-activated Protein Kinase (MAPK) Signaling by Cannabinoids. J. Biol. Chem. 2012, 287, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enrichedCannabisextracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2011, 204, 255–266. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Prados, M.E.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Fellous, T.; Iannotti, F.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.G.; Carrillo-Salinas, F.J.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.M.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (‒)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Limonta, V.; Malabarba, L.; Zani, A.; Sala, M. 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats. Eur. J. Pharmacol. 2007, 555, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.G.; Zamberletti, E.; Marini, P.; Parolaro, D.; Pertwee, R.G. The phytocannabinoid, Δ9-tetrahydrocannabivarin, can act through 5-HT1Areceptors to produce antipsychotic effects. Br. J. Pharmacol. 2015, 172, 1305–1318. [Google Scholar] [CrossRef] [Green Version]
- Rock, E.M.; Sullivan, M.T.; Collins, S.A.; Goodman, H.; Limebeer, C.L.; Mechoulam, R.; Parker, L.A. Evaluation of repeated or acute treatment with cannabidiol (CBD), cannabidiolic acid (CBDA) or CBDA methyl ester (HU-580) on nausea and/or vomiting in rats and shrews. Psychopharmacology 2020, 237, 2621–2631. [Google Scholar] [CrossRef]
- Bolognini, D.; Rock, E.M.; Cluny, N.L.; Cascio, M.G.; Limebeer, C.L.; Duncan, M.; Stott, C.G.; Javid, F.; Parker, L.A.; Pertwee, R.G. Cannabidiolic acid prevents vomiting inSuncus murinusand nausea-induced behaviour in rats by enhancing 5-HT1Areceptor activation. Br. J. Pharmacol. 2013, 168, 1456–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, P.J. The Chemistry of Cannabis and Cannabinoids. Aust. J. Chem. 2021, 74, 369. [Google Scholar] [CrossRef]
- Russo, E.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Xiong, W.; Koo, B.-N.; Morton, R.; Zhang, L. Psychotropic and nonpsychotropic cannabis derivatives inhibit human 5-HT3A receptors through a receptor desensitization-dependent mechanism. Neuroscience 2011, 184, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PPucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Pollastro, F.; Caprioglio, D.; Prete, D.D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1189–1194. [Google Scholar] [CrossRef] [Green Version]
- Zamberletti, E.; Rubino, T.; Parolaro, D. Therapeutic potential of cannabidivarin for epilepsy and autism spectrum disorder. Pharmacol. Ther. 2021, 226, 107878. [Google Scholar] [CrossRef]
- Fuziwara, S.; Suzuki, A.; Inoue, K.; Denda, M. Dopamine D2-Like Receptor Agonists Accelerate Barrier Repair and Inhibit the Epidermal Hyperplasia Induced by Barrier Disruption. J. Investig. Dermatol. 2005, 125, 783–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughn, A.R.; Davis, M.J.; Sivamani, R.K.; Isseroff, R.R. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules 2017, 23, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrado, A.C.; Salaverry, L.S.; Mangone, F.M.; Apicella, C.E.; Gentile, T.; Canellada, A.; Rey-Roldán, E.B. Differential Response of Dopamine Mediated by β-Adrenergic Receptors in Human Keratinocytes and Macrophages: Potential Implication in Wound Healing. Neuroimmunomodulation 2017, 24, 282–291. [Google Scholar] [CrossRef]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. Analgesic and antiinflammatory activity of constituents ofCannabis sativa L. Inflammation 1988, 12, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.P.D.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASX/Media Release BTX 1503 acne Phase 2 study results presentation; Philadelphia PA and Syndey Australia. 2019. Available online: https://www.asx.com.au/asxpdf/20191023/pdf/449s36d2bz6vfr.pdf (accessed on 11 August 2021).
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Ben-Nun, A.; Coppola, G.; Vogel, Z. Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J. Neuroinflammation 2015, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffal, E.; Cron, M.; Glodde, N.; Tüting, T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef]
- Maor, Y.; Yu, J.; Kuzontkoski, P.M.; Dezube, B.J.; Zhang, X.; Groopman, J.E. Cannabidiol Inhibits Growth and Induces Programmed Cell Death in Kaposi Sarcoma-Associated Herpesvirus-Infected Endothelium. Genes Cancer 2012, 3, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Glodde, N.; Jakobs, M.; Bald, T.; Tüting, T.; Gaffal, E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015, 138, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Changoer, L.; Anastassov, G. METHOD TO TREAT PSORIASIS 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US238100786&docAn=16106420 (accessed on 11 August 2021).
- Muscarà, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; La Camera, E.; Occhiuto, C.; Grassi, G.; Circosta, C. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytotherapy Res. 2020, 35, 1099–1112. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodriguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Wardle, J. Linking Chemical Phenotypes of Different Varieties of Cannabis Sativa to Their Antimicrobial Activities against a Range of Microorganisms. Master’s Thesis, Manchester Metropolitan University, Manchester, UK, September 2019. [Google Scholar]
- Turner, C.E.; ElSohly, M.A. Biological Activity of Cannabichromene, its Homologs and Isomers. J. Clin. Pharmacol. 1981, 21, 283S–291S. [Google Scholar] [CrossRef] [PubMed]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant function of phytocannabinoids: Molecular basis of their stability and cytoprotective properties under UV-irradiation. Free. Radic. Biol. Med. 2021, 164, 258–270. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, A.; Musazzi, U.M.; Centin, G.; Franzè, S.; Minghetti, P. Topical Administration of Cannabidiol: Influence of Vehicle-Related Aspects on Skin Permeation Process. Pharmaceuticals 2020, 13, 337. [Google Scholar] [CrossRef]
- Ali, A.; Akhtar, N. The safety and efficacy of 3% Cannabis seeds extract cream for reduction of human cheek skin sebum and erythema content. Pak. J. Pharm. Sci. 2015, 28, 1389–1395. [Google Scholar]
- Adusumilli, N.C.; Hazuka, E.L.; Friedman, A.J. Nanotechnology to deliver cannabinoids in dermatology. Precis. Nanomed. 2021, 4, 787–794. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crop. Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Uluata, S.; Özdemir, N. Antioxidant Activities and Oxidative Stabilities of Some Unconventional Oilseeds. J. Am. Oil Chem. Soc. 2012, 89, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, G.; Skrajda, M. Frakcja Lipidowa I Białkowa Nasion Konopi Siewnych (C. sativa L.) Oraz Jej Korzystny Wpływ Na Zdrowie Człowieka = Lipid and Protein Fraction of Hemp Seed (C. sativa L.) And Its Beneficial Influence On Human Health. J. Educ. Health Sport. 2016, 6, 357–366. [Google Scholar] [CrossRef]
- Kazlauskienė, D.; Kasparavičienė, G.; Nenortienė, P.; Marksa, M.; Jukilaitytė, J.; Velžienė, S.; Ževžikovas, A. Determination of fatty acid composition and antioxidant activity in vegetable oils. Chemija 2021, 32. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Wydro, D.M. Omega-3 and 6 Acids in Medicine and Cosmetology. The Effect of Oral Supplementation with Omega-3 and 6 Acids on Skin Condition–Pilot Study. Available online: https://www.researchgate.net/publication/270960751_Kwasy_omega_3_i_6_w_medycynie_i_kosmetologii_Wplyw_doustnej_suplementacji_kwasami_omega-3_i_6_na_kondycje_skory_-_badanie_pilotazowe_Omega-3_and_6_acids_in_medicine_and_cosmetology_The_effect_of_oral_ (accessed on 15 June 2021).
- Pei, L.; Luo, Y.; Gu, X.; Wang, J. Formation, Stability and Properties of Hemp Seed Oil Emulsions for Application in the Cosmetics Industry. Tenside Surfactants Deterg. 2020, 57, 451–459. [Google Scholar] [CrossRef]
- Kurek-górecka, A.; Balwierz, R.; Mizera, P.; Nowak, M.; Żurawska-płaksej, E. L’importance thérapeutique et cosmétique de l ’ huile de chanvre. BioResources 2018, 16, 704–708. [Google Scholar]
- Metwally, S.; Ura, D.P.; Krysiak, Z.J.; Kaniuk, Ł.; Szewczyk, P.K.; Stachewicz, U. Electrospun PCL Patches with Controlled Fiber Morphology and Mechanical Performance for Skin Moisturization via Long-Term Release of Hemp Oil for Atopic Dermatitis. Membranes 2021, 11, 26. [Google Scholar] [CrossRef]
- Nowak, I.; Zielińska, A.K. tłuszczowe w olejach roślinnych i ich znaczenie w kosmetyce. Chemik 2014, 68, 103–110. [Google Scholar]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Cunliffe, W.J.; Holland, D.; Jeremy, A. Comedone formation: Etiology, clinical presentation, and treatment. Clin. Dermatol. 2004, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Aachary, A.A.; Thiyam-Holländer, U. Hemp Seed Oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Wisniewska, A.; Widomska, J.; Subczynski, W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006, 53, 475–484. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leveret, J.; Krutmann, J. Role of Ingestible Carotenoids in Skin Protection: A Review of Clinical Evidence. Photodermatol. Photoimmunol. Photomed. 2021. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Quirin, K. Phytosterol-Rich Soy Germ and Guggul Extracts Provide Anti-Ageing Benefits. Cosmet. Sci. Technol. 2011, 8, 18–27. [Google Scholar]
- Smith, L.W. The Present Status of topical Chlorophyll Therapy. NY State J. Med. 1995, 14, 2041–2050. [Google Scholar]
- Stephens, T.J.; McCook, J.P.; Herndon, J.H. Pilot Study of Topical Copper Chlorophyllin Complex in Subjects With Facial Acne and Large Pores. J. Drugs Dermatol. 2015, 14, 589–592. [Google Scholar] [PubMed]
- McCook, J.P.; Stephens, T.J.; Jiang, L.I.; Law, R.M.; Gotz, V. Ability of sodium copper chlorophyllin complex to repair photoaged skin by stimulation of biomarkers in human extracellular matrix. Clin. Cosmet. Investig. Dermatol. 2016, 9, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.D.S.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, S.E. Marijuana and the Cannabinoids, 1st ed.; ElSohly, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2007; ISBN 978-1-58829-456-2. [Google Scholar]
- McPartland, J.M.; Russo, E.B. Cannabis and Cannabis Extracts: Greater than The Sum of Their Parts. In Cannabis Therapeutics in HIV/AIDS; Taylor and Francis Inc.: Abingdon, UK, 2012; pp. 103–132. ISBN 9780203049105. [Google Scholar]
- Barrett, M.; Gordon, D.; Evans, F. Isolation from Cannabis sativa L. of cannflavin—A novel inhibitor of prostaglandin production. Biochem. Pharmacol. 1985, 34, 2019–2024. [Google Scholar] [CrossRef]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins–From plant to patient: A scoping review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakshlag, J.J.; Cital, S.; Eaton, S.J.; Prussin, R.; Hudalla, C. Cannabinoid, Terpene, and Heavy Metal Analysis of 29 Over-the-Counter Commercial Veterinary Hemp Supplements. Veter-Med. Res. Rep. 2020, 11, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef]
- Anand, U.; Pacchetti, B.; Anand, P.; Sodergren, M.H. Cannabis-based medicines and pain: A review of potential synergistic and entourage effects. Pain Manag. 2021, 11, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Cogan, P.S. The ‘entourage effect’ or ‘hodge-podge hashish’: The questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Rev. Clin. Pharmacol. 2020, 13, 835–845. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]


| Type of Target | Name of Target | Cell | Biodynamic Effect upon Activation |
|---|---|---|---|
| Transient potential ion channel | TRPV1 [18,40,41,42] | Sweat glands, Sebaceous glands, Hair follicle, Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Pro-inflammatory activity in cases of psoriasis, atopic dermatitis [42], fungal infection, burn wound, and UVB wound Anti-inflammatory activity in case of allergic contact dermatitis, barrier tissue infection, and corneal wound |
| TRPV2 [18,41,43] | Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Anti-inflammatory activity Reduces chronic pain Slows wound healing [43] | |
| TRPV3 [18,41,44,45,46] | Hair follicle, Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Promotes epidermal differentiation and hair growth [18]. Pro-inflammatory activity in sebaceous glands and epidermal keratinocytes [44]. Pro-inflammatory activity in case of atopic dermatitis [46]. | |
| TRPV4 [18,41,47,48] | Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Repairs damaged skin Downregulates lipid synthesis Involved in the pathogenesis of scleroderma [48] | |
| TRPA1 [18,41,49] | Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Contributes to the pathogenesis of atopic dermatitis and allergic contact dermatitis itch Protective role against psoriasis | |
| TRPM8 [18,41,50] | Keratinocytes, Nerve ending, Fibroblasts, Langerhans Cells | Anti-inflammatory activity | |
| Peroxisome proliferator-activated receptor transcription faction | PPARα [51] | Keratinocytes, Langerhans Cells | Anti-inflammatory activity via NFκ-B 1 pathway |
| PPARγ [52,53,54,55] | Hair follicle, Keratinocytes, Fibroblasts, Melanocytes | Anti-inflammatory activity via NFκ-B 1 pathway [52] Modulates potential of fibrotic skin disease [53,54,55] | |
| Serotonin receptor | 5-HT1A [56,57,58,59,60] | Keratinocytes, Fibroblasts, Melanocytes | Promotes wound healing [59] Anti-inflammatory activity in psoriatic skin [56,57,58,60] |
| 5-HT2A [56,57,60] | Keratinocytes, Fibroblasts, Nerve ending, Melanocytes | Pro-inflammatory activity in psoriatic skin | |
| 5-HT3 [57] | Basal keratinocytes | Mediates the pruritus reaction |
| Cannabinoid | Δ9-THC | Δ9-THCV | Δ9-THCA | Δ9-THCVA | CBD | CBDA | CBDV | CBG | CBGA | CBGV | CBC | CBN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CB1 | PAG 1 [67] | AN 3 [68] | AG 2 [69] | NCAG 4 [70,71] | wAG 2,6 [72] | / 9 [72] | wAG 2,6 [73] | / 9 [74] | IAG 5 [74] | / 9 [75] | PAG 1 [76] | |
| CB2 | PAG 1 [67] | PAG 1 [68] | IAG 5 [69] | IAG 5 [70,71] | wAG 2,6 [68] | AG 2 [68,74] | wPAG 1,6 [72,73] | / 9 [74] | IAG 5 [74] | AG 2 [75] | PAG 1 [76] | |
| GPR18 | AG 2 [77] | AN 3 [77] | AG 2 [78] | |||||||||
| GPR55 [79,80] | AN 3 [28] | wAG 2,6 [79] | wAG 2,6 [79] | wAN 3,6 [79,80] | wAN 3,6 [79] | PoAN 3,7 [79] | AN 3 [79] | PoAN 3,7 [79] | PoAN 3,7 [79] | AN 3 [78] | ||
| TRPV1 [81] | wAG 2,6 | PoAG 2,7 | wAG 2,6 | wAG 2,6 | PoAG 2,7 | wAG 2,6 | wAG 2,6 | AG 2 | PoAG2,7 | PoAG 2,7 | wAG 2,6 | wAG 2,6 |
| TRPV2 [81] | PoAG 2,7 | PoAG 2,7 | PoAG 2,7 | wAG 2,6 | AG 2 | wAG 2,6 | AG 2 | PoAG 2,7 | wAG 2,6 | PoAG 2,7 | wAG 2,6 | AG 2 |
| TRPV3 [82] | wAG 2,6 | PoAG 2,7 | wAG 2,6 | wAG 2,6 | AG 2 | wAG 2,6 | wAG 2,6 | wAG 2,6 | wAG 2,6 | wAG 2,6 | wAG 2,6 | wAG 2,6 |
| TRPV4 [82] | wAG 2,6 | PoAG 2,7 | wAG 2,6 | wAG 2,6 | wAG 2,6 | wAG 2,6 | AG 2 | wAG 2,6 | AG 2 | wAG 2,6 | wAG 2,6 | wAG 2,6 |
| TRPA1 [81] | AG 2 | wAG 2,6 | wAG 2,6 | wAG 2,6 | PoAG 2,7 | AG 2 | AG 2 | AG 2 | wAG 2,6 | AG 2 | PoAG 2,7 | PoAG 2,7 |
| TRPM8 [81] | PoAN 3,7 | AN 3 | PoAN 3,7 | AN 3 | PoAN 3,7 | AN 3 | AN 3 | AN 3 | AN 3 | AN 3 | AG 2 | PoAN 3,7 |
| PPARα | AG 2 [83] | / 9 [84] | / 9 [85] | PAG 1 [85] | PAG 1 [85] | AG 2 [85] | ||||||
| PPARγ [83,85] | AG 2 [83,86] | / 9 [83] | PAG 1 [69,83] | AG 2 [86,87] | PAG 1 [85,88] | PAG 1 [73,85] | PAG 1 [85] | wAG 2,6 [86] | ||||
| 5-HT1A | / 9 [89] | AG 2 [90] | AG 2 [76] | AG 2 [91,92,93] | PoAN 3,7 [73] | |||||||
| 5-HT2A | / 9 [94] | PAG 1 [76] | ||||||||||
| 5HT3 | AN 3 [76,95] | AN 3 [76] | ||||||||||
| MAGL [81] | IN 8 | PoIN 7,8 | IN 8 | / 9 | IN 8 | / 9 | PoIN 7,8 | IN 8 | wIN 6,8 | PoIN 8 | PoIN 8 | |
| NAAA [81] | wIN 6,8 | IN 8 | wIN 6,8 | IN 8 | PoIN 7,8 | IN 8 | wIN 6,8 | PoIN 7,8 | PoIN 7,8 | IN 8 | IN 8 | |
| FAAH [81] | wIN 6,8 | PoIN 7,8 | wIN 6,8 | PoIN 7,8 | PoIN 7,8 | IN 8 | IN 8 | PoIN 7,8 | PoIN 7,8 | IN 8 | IN 8 |
| Disease | Phytocannabinoid | Model | Outcome | Reference |
|---|---|---|---|---|
| Acne | THC and CBD | In vivo (mouse) | Inhibition of cyclooxygenase and lipoxygenase | [104] |
| CBD | In vitro (human sebocytes) | Inhibition of pro-inflammatory p65 NF-kB 1 pathway | [70] | |
| THCV CBC, CBDV CBG, CBGV | In vitro (human sebocytes) | THCV suppresses sebocytes proliferation, arachidonic acid-induced seborrhoea lipogenesis, and has a strong anti-inflammatory action. CBC, CBDV suppresses arachidonic acid-induced seborrhoea lipogenesis. CBG and CBGV increase the sebaceous lipid production (pro-lipogenic and pro-acne effect). | [105] | |
| BTX 1503 (CBD) | Phase 2 successfully ended (5 dose groups, 368 subjects) | 40% reduction of lesions after 12 weeks | [106] | |
| Allergic Contact Dermatitis | CBD | In vitro (HaCaT cells) | Suppresses inflammatory reaction by the inhibition of MCP2 2, IL-6 3, IL-8 3, and TNF-α 4 | [107] |
| CBD | In vitro (Splenocytes) | Suppresses inflammatory reaction by the inhibition of IL-6 3, IL-8 3, IL-17 3, TNF-α 4, and IFN-γ 5. Inhibition of T-cells and B-cells mediated response | [108] | |
| THC | In vivo (CBR-deficient mouse) | Suppresses inflammatory reaction by the inhibition of IFN-γ 5, MCP1 2, MCP2 2, and IP-10 6. | [109] | |
| Kaposi Sarcoma | CBD | In vitro (infected endothelial cells) | Induction of apoptosis by the reduction of GRO-α 7, the inhibition of vGPCR 8, and by the reduction of VEGF-C 9 and VEGFR-3 10 | [110] |
| Melanoma | THC THC and CBD (Sativex) | In vitro (melanoma cells) and in vivo (mouse) | Reduction in tumor size with THC+CBD more effective than THC alone | [111] |
| THC | In vivo (mouse) | Inhibition of melanoma growth | [112] | |
| CBD | In vivo (mouse) | Reduction in tumor size | [113] | |
| Psoriasis | THC and CBD | In vitro (human-skin keratinocytes) | Inhibition of keratinocytes proliferation | [114] |
| CBD and CBG | Human trial (2 subjects) | 16–33% reduction of lesions after 6 weeks | [115] |
| Cannabinoid | Δ9-THC | Δ9-THCV | Δ9-THCA | Δ9-THCVA | CBD | CBDA | CBDV | CBG | CBGA | CBGV | CBC | CBN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antioxidant activity | + [62] | + [116] | + [62] | + [116] | + [62,117] | + [62,118] | + [116] | + [62] | + [62] | + [62] | ||
| Antibacterial gram-positive activity | + [61,119,120] | + [116,120] | + [121] | + [116] | + [61,119,120] | + [121] | + [116,120] | + [61,119,120] | + [122] | + [120] | + [119,120,121] | + [61,119] |
| Photoprotectant activity | − [123] | + (UVA) [123] | + (UVA) [123] | + (UVB) [123] | + (UVB) [123] |
| Terpene | Biodynamic Effect |
|---|---|
| β-caryophyllene | Anti-viral, antioxidant, anti-inflammatory, anticancerogenic, analgesic, anxiolytic, antibacterial, antifungal, antiproliferation, antidepressant, neuroprotective and gastroprotective [161] |
| β-myrcene | Antioxidant, anti-inflammatory, anticancerogenic, analgesic, sedative, muscle relaxant and antipsychotic [161] |
| α-pinene | Anti-inflammatory, antibacterial, bronchodilator, antiseptic and gastroprotective [161] |
| β-pinene | Antiseptic [161] |
| α-humulene | Anti-inflammatory, anticancerogenic, antifungal and analgesic [161] |
| limonene | Antibacterial, antifungal, antimicrobial, antiproliferative, anxiolytic, antidepressant, antispasmodic and gastroprotective [161] |
| linalool | Antineoplastic, antiepileptic, anticonvulsant, sedative, anxiolytic, antipsychotic, analgesic, antidepressant and anesthetic [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnekin, L.; Ripoll, L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics 2021, 8, 85. https://doi.org/10.3390/cosmetics8030085
Mnekin L, Ripoll L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics. 2021; 8(3):85. https://doi.org/10.3390/cosmetics8030085
Chicago/Turabian StyleMnekin, Léonid, and Lionel Ripoll. 2021. "Topical Use of Cannabis sativa L. Biochemicals" Cosmetics 8, no. 3: 85. https://doi.org/10.3390/cosmetics8030085
APA StyleMnekin, L., & Ripoll, L. (2021). Topical Use of Cannabis sativa L. Biochemicals. Cosmetics, 8(3), 85. https://doi.org/10.3390/cosmetics8030085





