Detection of Raspberry Ketone after Percutaneous Absorption of Rhododendrol-Containing Cosmetics and Its Mechanism of Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Test Solution
2.3. Measurement of Skin Permeability after Repeated Application of Solutions Containing RD
2.4. Measurements of the Skin Permeation Rate with Different Cosmetic Formulations
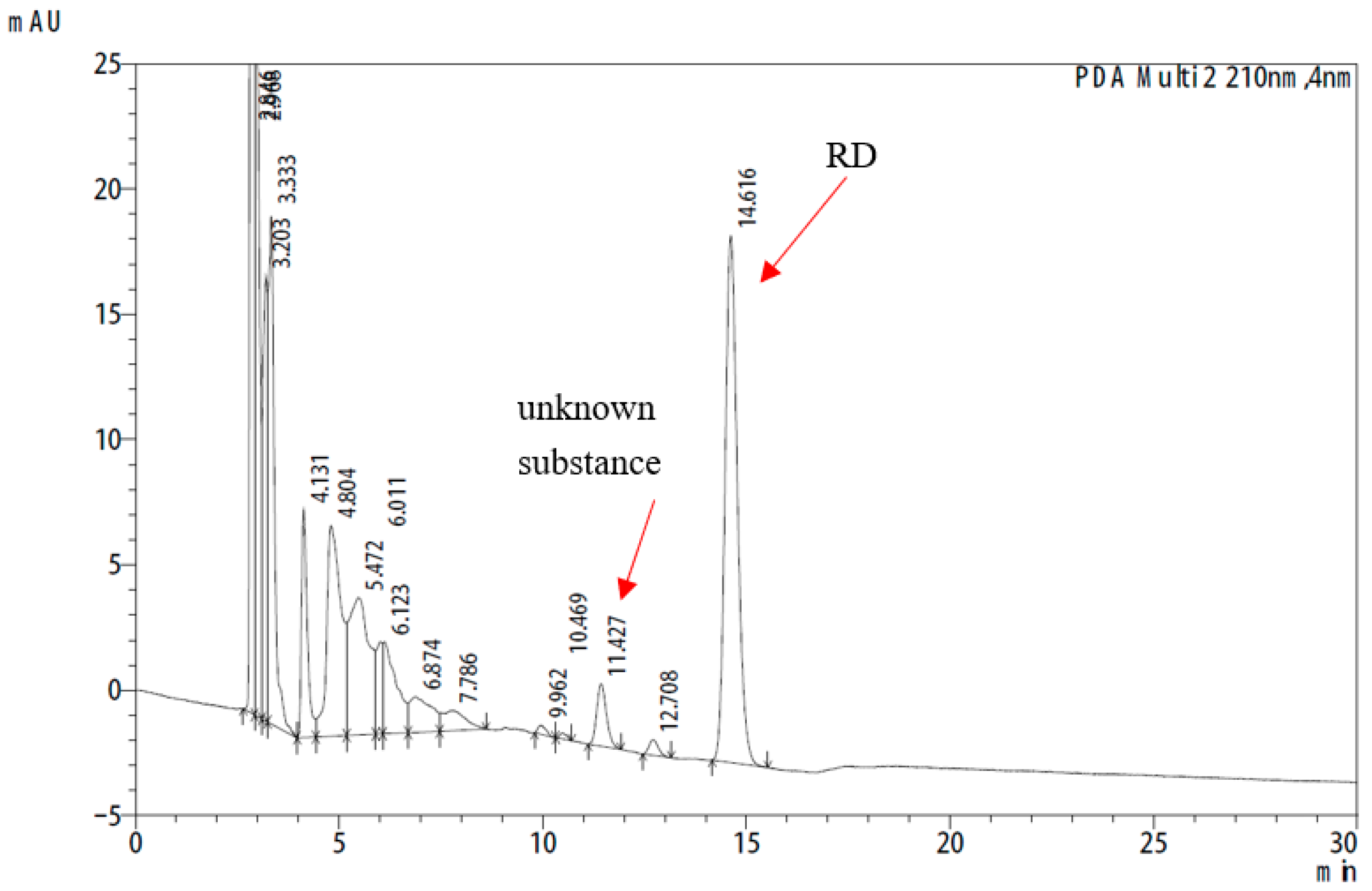
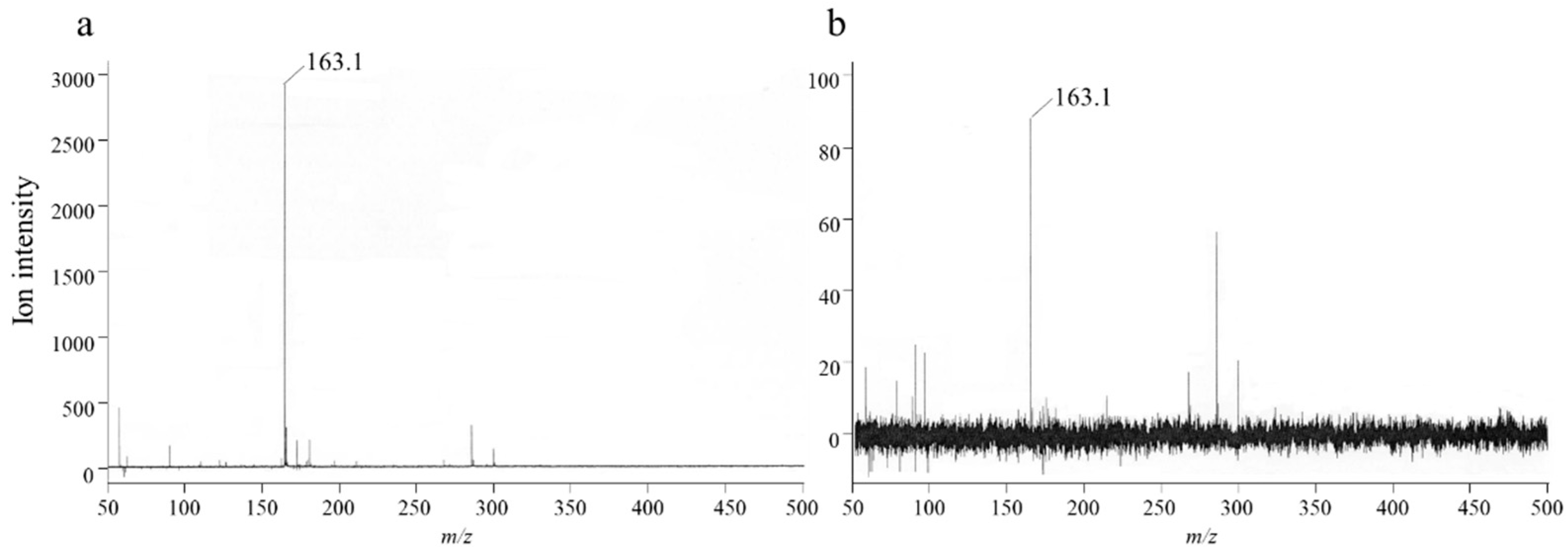
2.5. Analysis of the Unknown Substance
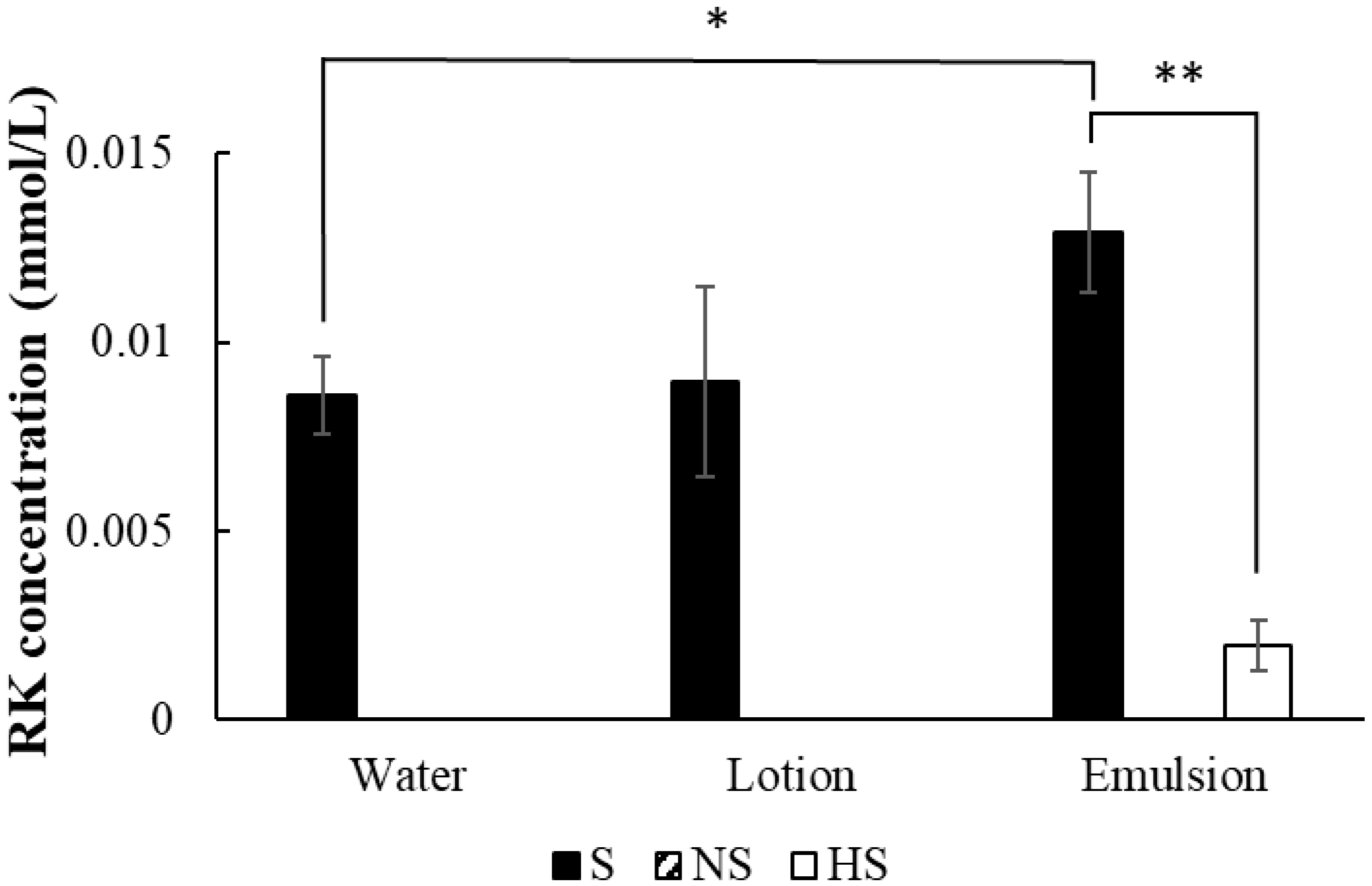
2.6. RK Generation by the Skin with Different Treatments
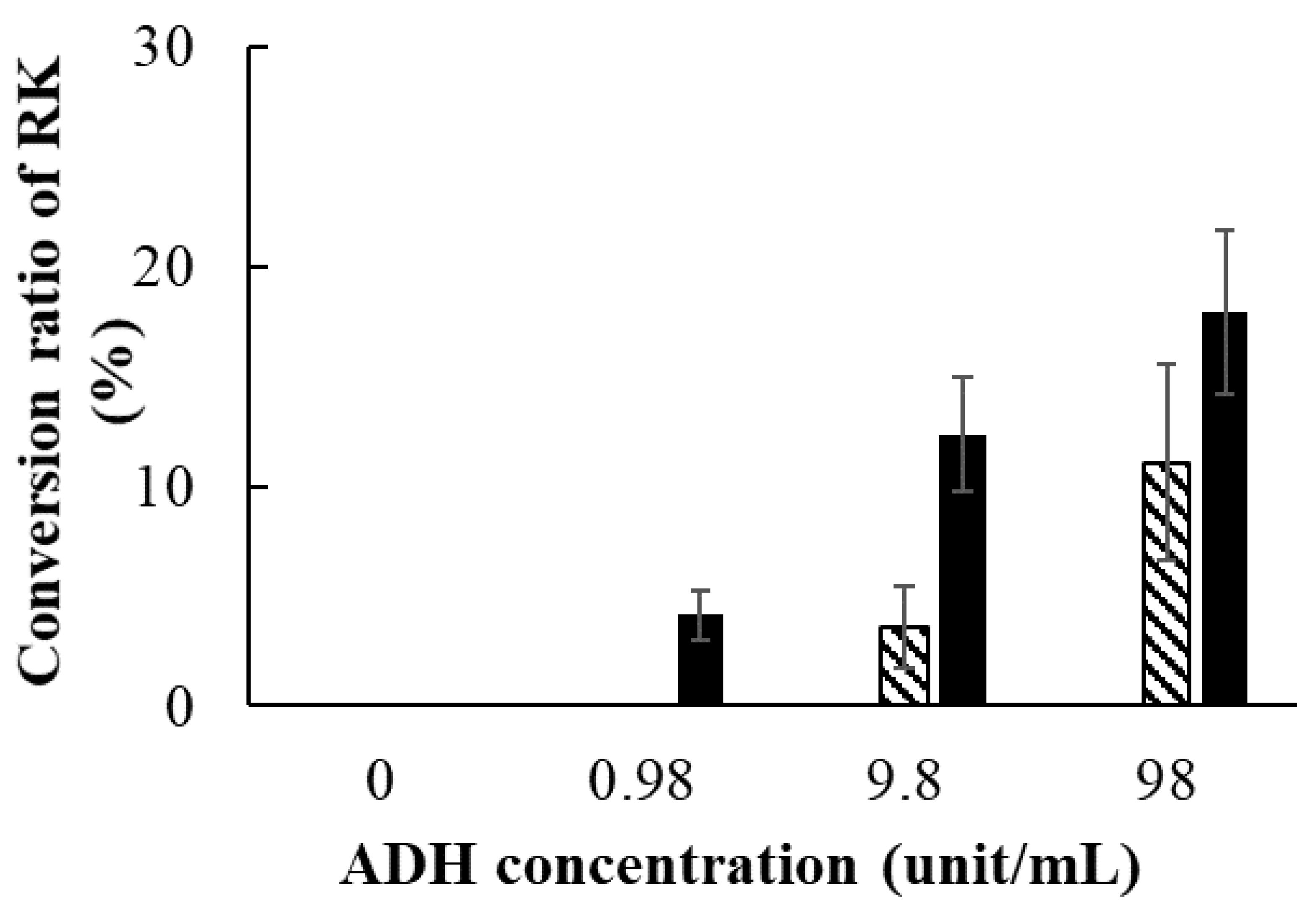
2.7. Conversion of RD to RK by ADH
2.8. Statistical Analysis
3. Results
3.1. Skin Permeability of RD-Containing Solutions after Repeated Application
3.2. Skin Permeation Rates of Different Cosmetic Formulations
3.3. Analysis of the Unknown Substance
3.4. RK Generation by the Skin with Different Treatments
3.5. Conversion of RD to RK by ADH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, K. Advances in development of skin whitening agents. Fragr. J. 2008, 36, 65–67. (In Japanese) [Google Scholar]
- Homepage of Kanebo Cosmetics, Inc. The Number of Checks of the White Spots’ Condition, and Recovery, a Reconciliation Situation/the Number of Object Recall. Available online: http://www.kanebo-cosmetics.jp/information/correspondence/data_2016.html (accessed on 25 August 2021).
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cells: Production of RD-pheomelanin and covalent binding with thiol proteins. Pigment Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Okura, M.; Wakamatsu, K.; Yamashita, T. The potent pro-oxidant activity of rhododendrol–eumelanin induces cysteine depletion in B16 melanoma cells. Pigment Cell Melanoma Res. 2017, 30, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokura, Y.; Fujiyama, T.; Ikeya, S.; Tatsuno, K.; Aoshima, M.; Kasuya, A.; Ito, T. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 146–149. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef]
- Gu, L.; Zeng, H.; Takahashi, T.; Maeda, K. In vitro methods for predicting chemical leukoderma caused by quasi-drug cosmetics. Cosmetics 2017, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Nagano, M.; Futatsuka, M. Occupational leukoderma in workers engaged in 4-(p-hydroxyphenyl)-2-butanone manufacturing. J. Occup. Health 1998, 40, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Nagano, M.; Tsukamoto, K.; Futatsuka, M. In vitro studies on the depigmenting activity of 4-(p-hydroxyphenyl)-2-butanone. J. Occup. Health 1998, 40, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Okmura, Y.; Shirai, T. Vitiliginous lesions occurring among workers in a phenol derivative factory. Jpn. J. Dermatol. 1962, 7, 617–619. [Google Scholar]
- James, O.; Mayes, R.W.; Stevenson, C.J. Occupational vitiligo induced by p-tert-butylphenol, a systemic disease? Lancet 1977, 2, 1217–1219. [Google Scholar] [CrossRef]
- Ebner, H.; Helletzgruber, M.; Höfer, R.; Kolbe, H.; Weissel, M.; Winker, N. Vitiligo from p-tert. butylphenol; A contribution to the problem of the internal manifestations of this occupational disease. Dermatosen Beruf Umw. 1979, 27, 99–104. [Google Scholar]
- Budde, J.; Stary, A. Skin and systemic disease caused by occupational contact with p-tert-butylphenol. Case reports. Dermatosen Beruf Umw. 1988, 36, 17–19. [Google Scholar]
- Rodermund, O.E.; Jörgens, H.; Müller, R.; Marsteller, H.J. Systemic changes in occupational vitiligo. Hautarzt 1975, 26, 312–316. [Google Scholar] [PubMed]
- Ikeda, M.; Ohtsuji, H.; Miyahara, S. Two cases of leucoderma, presumably due to nonyl or octylphenol in synthetic detergents. Ind. Health 1970, 8, 192–196. [Google Scholar] [CrossRef]
- Russell, O.; Richard, H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar]
- Hisatomi, A.; Kimura, M.; Maeda, M.; Matsumoto, M.; Ohara, K.; Noguchi, H. Toxicity of polyoxyethylene hydrogenated castor oil 60 (HCO-60) in experimental animals. J. Toxicol. Sci. 1993, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.S.; Jamil, K.; Madhusudhan, P.; Anjani, G.; Das, B. Antibacterial activity of isolates from piper longum and Taxus baccata. Pharm. Biol. 2001, 39, 236–238. [Google Scholar] [CrossRef]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef]
- Parmar, V.S.; Vardhan, A.; Taneja, P.; Sinha, R.; Patnaik, G.K.; Tripathi, S.C.; Boll, P.M.; Larsen, S. Absolute configuration of epi-rhododendrin and (–)-rhododendrol [=(–)-betuligenol] and X-ray crystal and molecular structure of rhododendrin [= betuloside], a hepatoprotective constituent of Taxus baccata. J. Chem. Soc. Perkin Trans. 1991, 1, 2687–2690. [Google Scholar] [CrossRef]
- Becker, A.; Böttcher, D.; Katzer, W.; Siems, K.; Müller-Kuhrt, L.; Bornscheuer, U.T. An ADH toolbox for raspberry ketone production from natural resources via a biocatalytic cascade. Appl. Microbiol. Biotechnol. 2021, 105, 4189–4197. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Maibach, H.I. Skin metabolism: Relevance of skin enzymes for rational drug design. Skin Pharmacol. Physiol. 2019, 32, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Fronza, G.; Fuganti, C.; Pedrocchi-Fantoni, G.; Serra, S.; Zucchi, G.; Fauhl, C.; Guillou, C.; Reniero, F. Stable isotope characterization of raspberry ketone extracted from Taxus baccata and obtained by oxidation of the accompanying alcohol (Betuligenol). J. Agric. Food Chem. 1999, 47, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Davies, N.G.; Hoog, J.O.; Hotchkiss, S.A.M.; Pease, C.K.S. Species variations in cutaneous alcohol dehydrogenases and aldehyde dehydrogenases may impact on toxicological assessments of alcohols and aldehydes. Toxicology 2003, 184, 97–112. [Google Scholar] [CrossRef]
- Takemoto, M.; Achiwa, K. Deracemization of the racemic 4-pyridyl-1-ethanol by Catharanthus roseus cell cultures. Phytochemistry 1998, 49, 1627–1629. [Google Scholar] [CrossRef]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Köhler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers. Arzneimittelforschung 1979, 29, 1640–1642. [Google Scholar] [PubMed]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the thalidomide chirality in biological processes by the self-disproportionation of enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Jacques, V.; Czarnik, A.W.; Judge, T.M.; Van der Ploeg, L.H.; DeWitt, S.H. Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl. Acad. Sci. USA 2015, 112, E1471–E1479. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, D.G.; Vangiessen, G.J.; Reischer, R.J.; Wechter, W.J. Isomeric inversion of ibuprofen (R)-enantiomer in humans. J. Pharm. Sci. 1976, 65, 269–273. [Google Scholar] [CrossRef]
- Ikuta, H.; Kawase, A.; Iwaki, M. Stereoselective pharmacokinetics and chiral inversion of ibuprofen in adjuvant-induced arthritic rats. Drug Metab. Dispos. 2017, 45, 316–324. [Google Scholar] [CrossRef]
- Sabitha, G.; Thirupathaiah, B.; Yadav, J.S. Asymmetric synthesis of (R)-(-)-rhododendrol, the aglycone of the hepatoprotective agent rhododendron. Synth. Commun. 2007, 37, 1683–1688. [Google Scholar] [CrossRef]
- Yuasa, Y.; Shibuya, S.; Yuasa, Y. Resolution of racemic rhododendrol by lipase-catalyzed enantioselective acetylation. Synth. Commun. 2003, 33, 1469–1475. [Google Scholar] [CrossRef]
- Karume, I.; Takahashi, M.; Hamdan, S.M.; Musa, M.M. Deracemization of secondary alcohols by using a single alcohol dehydrogenase. ChemCatChem 2016, 8, 1459–1463. [Google Scholar] [CrossRef]



| Water | Lotion | Emulsion | |
|---|---|---|---|
| RD | 0.2 | 0.2 | 0.2 |
| Glycerin | - | 0.4 | 0.4 |
| 1,3-butanediol | - | 0.6 | 0.6 |
| PEG-60 hydrogenated castor oil | - | 0.02 | 0.02 |
| Phenoxyethanol | - | 0.035 | 0.035 |
| Squalene | - | - | 1 |
| Sodium acrylate–sodium acryloyldimethyl taurate copolymer/isohexadecane/polysorbate 80 | - | - | 0.25 |
| Water | 9.8 | 8.745 | 7.495 |
| RD Recovery Ratio in Receptor Chamber (%) | RD Recovery Ratio in Mice Skin (%) | Total RD Recovery Ratio (%) | |
|---|---|---|---|
| Water | 106.6 ± 6.3 | 0.480 ± 0.065 | 107.1 ± 6.4 |
| Lotion | 100.0 ± 3.2 | 0.331 ± 0.210 | 100.3 ± 3.4 |
| Emulsion | 87.0 ± 9.1 | 0.408 ± 0.104 | 87.4 ± 9.2 |
| Time (h) | Cumulative Skin Permeability (μg/cm2) | Permeation Rate (μg/cm2/h) | Apparent Permeability Coefficient # | |
|---|---|---|---|---|
| Water | 2 | 40.52 | 20.26 | 0.1013 |
| 4 | 65.03 | 12.26 | 0.0613 | |
| 8 | 97.56 | 8.13 | 0.0406 | |
| 12 | 127.65 | 7.52 | 0.0376 | |
| 24 | 213.16 | 7.13 | 0.0356 | |
| Lotion | 2 | 28.80 | 14.40 | 0.0720 |
| 4 | 47.10 | 9.15 | 0.0457 | |
| 8 | 86.31 | 9.80 | 0.0490 | |
| 12 | 121.67 | 8.84 | 0.0442 | |
| 24 | 200.06 | 6.53 | 0.0327 | |
| Emulsion | 2 | 24.36 | 12.18 | 0.0609 |
| 4 | 35.94 | 5.79 | 0.0289 | |
| 8 | 59.32 | 5.84 | 0.0292 | |
| 12 | 89.55 | 7.56 | 0.0378 | |
| 24 | 173.91 | 7.03 | 0.0351 |
| Comparison between Formulations | p Value |
|---|---|
| Water vs. Lotion | 0.3534 |
| Water vs. Emulsion | 0.0032 |
| Lotion vs. Emulsion | 0.0877 |
| Time after Application | Comparison between Formulations | p Value |
|---|---|---|
| 2 h | Water vs. Lotion | 0.0239 |
| Water vs. Emulsion | 0.0016 | |
| Lotion vs. Emulsion | 0.5471 | |
| 4 h | Water vs. Lotion | 0.3133 |
| Water vs. Emulsion | 0.0118 | |
| Lotion vs. Emulsion | 0.2614 | |
| 8 h | Water vs. Lotion | 0.7070 |
| Water vs. Emulsion | 0.5284 | |
| Lotion vs. Emulsion | 0.1600 | |
| 12 h | Water vs. Lotion | 0.8068 |
| Water vs. Emulsion | 0.9999 | |
| Lotion vs. Emulsion | 0.8158 | |
| 24 h | Water vs. Lotion | 0.9569 |
| Water vs. Emulsion | 0.9988 | |
| Lotion vs. Emulsion | 0.9696 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Fujisawa, A.; Maeda, K. Detection of Raspberry Ketone after Percutaneous Absorption of Rhododendrol-Containing Cosmetics and Its Mechanism of Formation. Cosmetics 2021, 8, 97. https://doi.org/10.3390/cosmetics8040097
Gu L, Fujisawa A, Maeda K. Detection of Raspberry Ketone after Percutaneous Absorption of Rhododendrol-Containing Cosmetics and Its Mechanism of Formation. Cosmetics. 2021; 8(4):97. https://doi.org/10.3390/cosmetics8040097
Chicago/Turabian StyleGu, Lihao, Akio Fujisawa, and Kazuhisa Maeda. 2021. "Detection of Raspberry Ketone after Percutaneous Absorption of Rhododendrol-Containing Cosmetics and Its Mechanism of Formation" Cosmetics 8, no. 4: 97. https://doi.org/10.3390/cosmetics8040097
APA StyleGu, L., Fujisawa, A., & Maeda, K. (2021). Detection of Raspberry Ketone after Percutaneous Absorption of Rhododendrol-Containing Cosmetics and Its Mechanism of Formation. Cosmetics, 8(4), 97. https://doi.org/10.3390/cosmetics8040097







