Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. AGSE and E-AGSE Production
2.3. PP2A Demethylation Assay
2.4. Cell Culture and Viability
2.5. UVB-Induced Inflammation
2.6. Tissue Histology
2.7. Aging Marker Expression
2.8. Dermal–Epidermal Junction (DEJ) Marker Expression
2.9. Melanin Assay
2.10. Tyrosinase Assay
2.11. Clinical Study
2.12. Statistical Anlysis
3. Results
3.1. Chemical Encapsulation of AGSE does Not Alter PP2A Activity
3.2. E-AGSE Upregulates the Expression of Key Anti-Aging Extracellular Matrix Markers
3.3. E-AGSE Upregulates Dermal–Epidermal Junction (DEJ) Marker Expression
3.4. E-AGSE Protects against UVB-induced DNA Damage and Promotes Epidermal Integrity
3.5. E-AGSE Demonstrates Skin-Brightening Properties
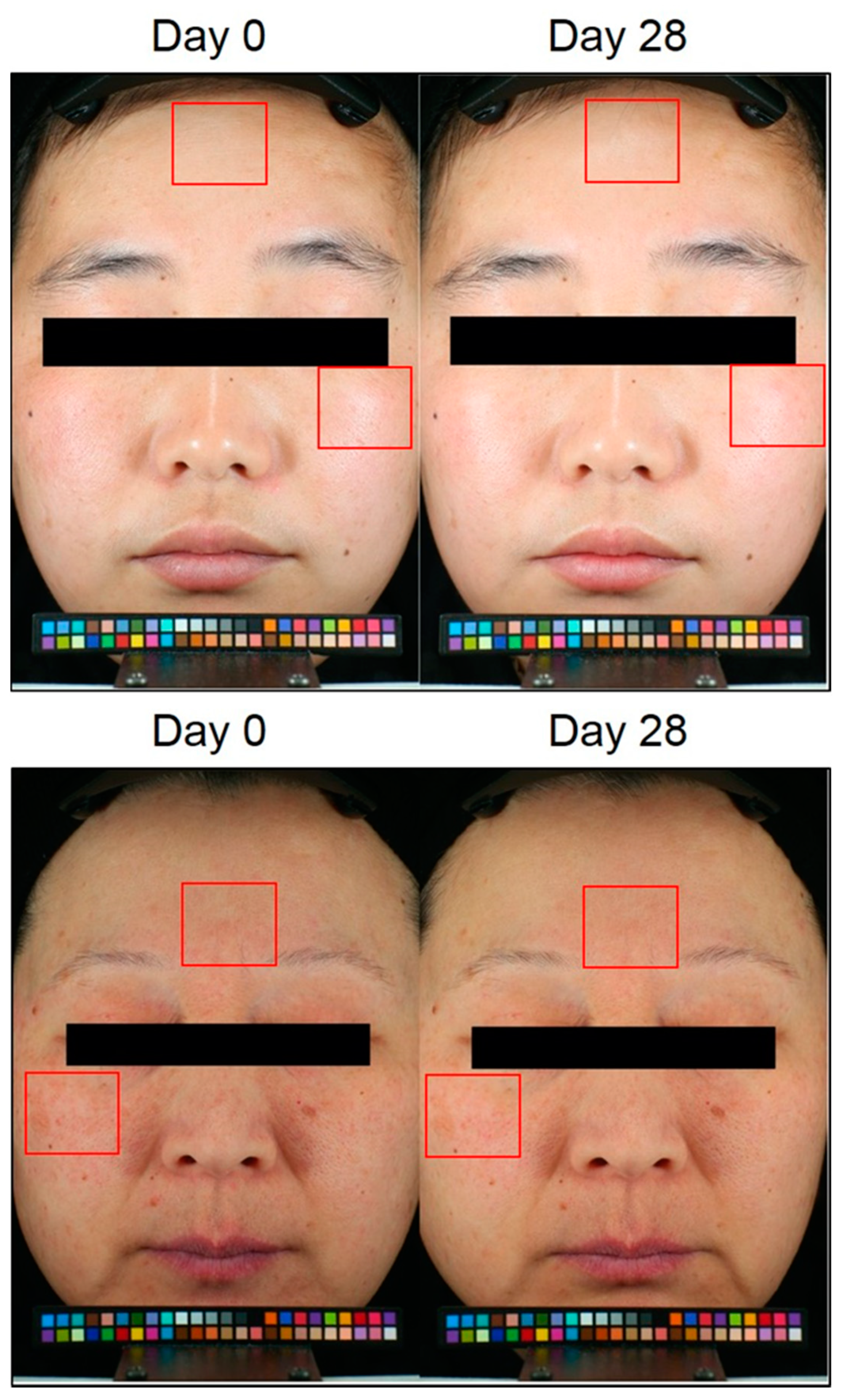
3.6. E-AGSE Improves Skin Aging, Brightening, and Hydration in Human Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The Regulation of Skin Pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, E.M.; Lee, E.K.; Song, C.H.; Song, J.; Chung, H.Y.; Chae, C.H.; Jung, K.J. Ferulate, an Active Component of Wheat Germ, Ameliorates Oxidative Stress-Induced PTK/PTP Imbalance and PP2A Inactivation. Toxicol. Res. 2018, 34, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Mhamdi, A.; Trotta, A.; Kangasjarvi, S.; Noctor, G. The protein phosphatase subunit PP2A-B’gamma is required to suppress day length-dependent pathogenesis responses triggered by intracellular oxidative stress. New Phytol. 2014, 202, 145–160. [Google Scholar] [CrossRef]
- Elgenaidi, I.S.; Spiers, J.P. Regulation of the phosphoprotein phosphatase 2A system and its modulation during oxidative stress: A potential therapeutic target? Pharmacol. Ther. 2019, 198, 68–89. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.F.; Welti, J.C.; Sully, K.; Byrne, C. Akt-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development. Development 2009, 136, 3423–3431. [Google Scholar] [CrossRef] [Green Version]
- Kam, E.; Nirunsuksiri, W.; Hager, B.; Fleckman, P.; Dale, B.A. Protein phosphatase activity in human keratinocytes cultured from normal epidermis and epidermis from patients with harlequin ichthyosis. Br. J. Dermatol. 1997, 137, 874–882. [Google Scholar] [CrossRef]
- Dobrowsky, R.T.; Kamibayashi, C.; Mumby, M.C.; Hannun, Y.A. Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 1993, 268, 15523–15530. [Google Scholar] [CrossRef]
- Voronkov, M.; Braithwaite, S.P.; Stock, J.B. Phosphoprotein phosphatase 2A: A novel druggable target for Alzheimer’s disease. Future Med. Chem. 2011, 3, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Okadaic acid suppresses melanogenesis via proteasomal degradation of tyrosinase. Biol. Pharm. Bull. 2013, 36, 1503–1508. [Google Scholar] [CrossRef] [Green Version]
- Weseler, A.R.; Bast, A. Masquelier’s grape seed extract: From basic flavonoid research to a well-characterized food supplement with health benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Cronin, H.; Draelos, Z.D. Original Contribution: Top 10 botanical ingredients in 2010 anti-aging creams. J. Cosmet. Dermatol. 2010, 9, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.L.; Fernández, J.R.; Webb, C.; Rouzard, K.; Healy, J.; Tamura, M.; Stock, J.B.; Stock, M.; Pérez, E. AGSE: A Novel Grape Seed Extract Enriched for PP2A Activating Flavonoids That Combats Oxidative Stress and Promotes Skin Health. Mol. 2021, 26, 6351. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.R.; Huber, K.L.; Webb, C.; Rouzard, K.; Tamura, M.; Wang, Y.; Liao, Z.; Sun, P.; Nie, J.; Zhang, Z.; et al. TIRACLE™ and ACTIVITIS™: A novel anti-aging blend. J. Investig. Dermatol. 2019, 139, PB17. [Google Scholar] [CrossRef] [Green Version]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef]
- Schlupp, P.; Blaschke, T.; Kramer, K.; Höltje, H.-D.; Mehnert, W.; Schäfer-Korting, M. Drug Release and Skin Penetration from Solid Lipid Nanoparticles and a Base Cream: A Systematic Approach from a Comparison of Three Glucocorticoids. Ski. Pharmacol. Physiol. 2011, 24, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramovits, W.; Granowski, P.; Arrazola, P. Applications of nanomedicine in dermatology: Use of nanoparticles in various therapies and imaging. J. Cosmet. Dermatol. 2010, 9, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, 12–16. [Google Scholar]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, F.; Palacios, S.; Alemañ, N.; Guerrero, F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215. [Google Scholar] [CrossRef]
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef]
- Gallo, R. Proteoglycans and glycosaminoglycans of skin. Dermatol. Gen. Med. 1999, 1, 283–288. [Google Scholar]
- Pummi, K.; Malminen, M.; Aho, H.; Karvonen, S.-L.; Peltonen, J.; Peltonen, S. Epidermal Tight Junctions: ZO-1 and Occludin are Expressed in Mature, Developing, and Affected Skin and In Vitro Differentiating Keratinocytes. J. Investig. Dermatol. 2001, 117, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Pineau, N.; Bernerd, F.; Cavezza, A.; Dalko-Csiba, M.; Breton, L. A new C-xylopyranoside derivative induces skin expression of glycosaminoglycans and heparan sulphate proteoglycans. Eur. J. Dermatol. 2008, 18, 36–40. [Google Scholar]
- Pageon, H.; Azouaoui, A.; Zucchi, H.; Rocois, S.; Tran, C.; Asselineaau, D. Potentially beneficial effects of rhamnose on skin ageing: An in vitro and in vivo study. Int. J. Cosmet. Sci. 2019, 41, 213–220. [Google Scholar] [CrossRef]
- Wegrowski, Y.; Maquart, F.; Borel, J. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex Glycyl-L-histidyl-L-lysine-Cu2+. Life Sci. 1992, 51, 1049–1056. [Google Scholar] [CrossRef]
- Strickland, I.; Rhodes, L.E.; Flanagan, B.F.; Friedmann, P.S. TNF-α and IL-8 Are Upregulated in the Epidermis of Normal Human Skin after UVB Exposure: Correlation with Neutrophil Accumulation and E-Selectin Expression. J. Investig. Dermatol. 1997, 108, 763–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mildner, M.; Jin, J.; Eckhart, L.; Kezic, S.; Gruber, F.; Barresi, C.; Stremnitzer, C.; Buchberger, M.; Mlitz, V.; Ballaun, C.; et al. Knockdown of Filaggrin Impairs Diffusion Barrier Function and Increases UV Sensitivity in a Human Skin Model. J. Investig. Dermatol. 2010, 130, 2286–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.H.; Neudecker, B.A.; DiNardo, J.C.; Lewis, J.A.; Maibach, H.I. Idebenone: A new antioxidant—Part, I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J. Cosmet. Dermatol. 2005, 4, 10–17. [Google Scholar] [CrossRef]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing Muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2014, 37, 253–258. [Google Scholar] [CrossRef]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of Tyrosinase as the Determinant of Pigmentation in Cultured Human Melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Foroozan, M.; Houshmand, G.; Moosavi, Z.B.; Bahadoram, M.; Maram, N.S. The Topical Effect of Grape Seed Extract 2% Cream on Surgery Wound Healing. Glob. J. Health Sci. 2014, 7, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Giacomelli, L.; Togni, S.; Meneghin, M.; Eggenhooffner, R.; Maramaldi, G. In vivo validation of the multicomponent powder (Vitachelox(R)) against the deposition of polluting ions. Clin. Cosmet. Investig. Dermatol. 2018, 11, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S.; Saraf, S. Assessment of viscoelasticity and hydration effect of herbal moisturizers using bioengineering techniques. Pharmacogn. Mag. 2010, 6, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: Results from a vehicle controlled, double-blind, randomized study. Photochem. Photobiol. 2015, 91, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Barisic, S.; Strozyk, E.; Peters, N.; Walczak, H.; Kulms, D. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: Its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell. Death Differ. 2008, 15, 1681–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
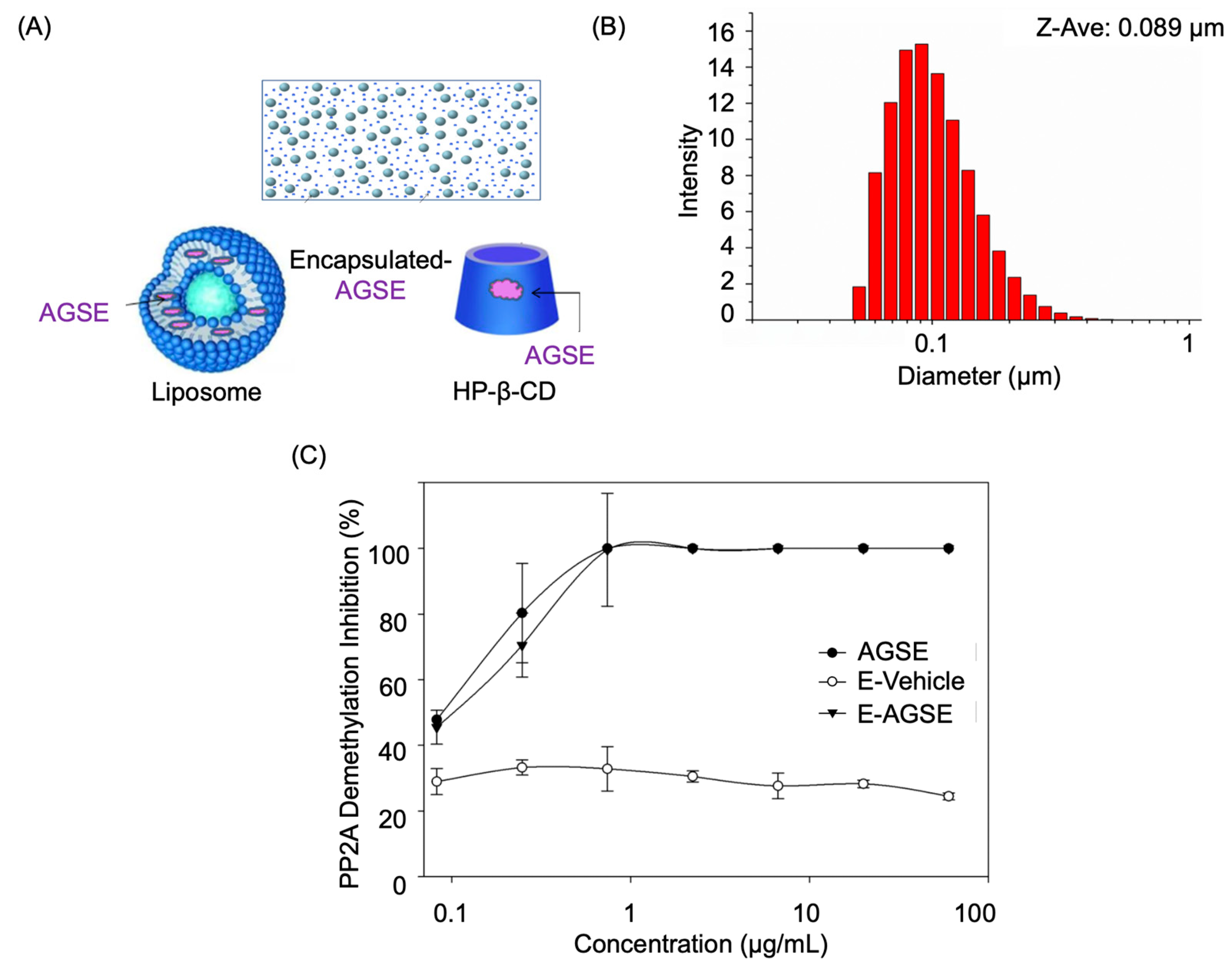
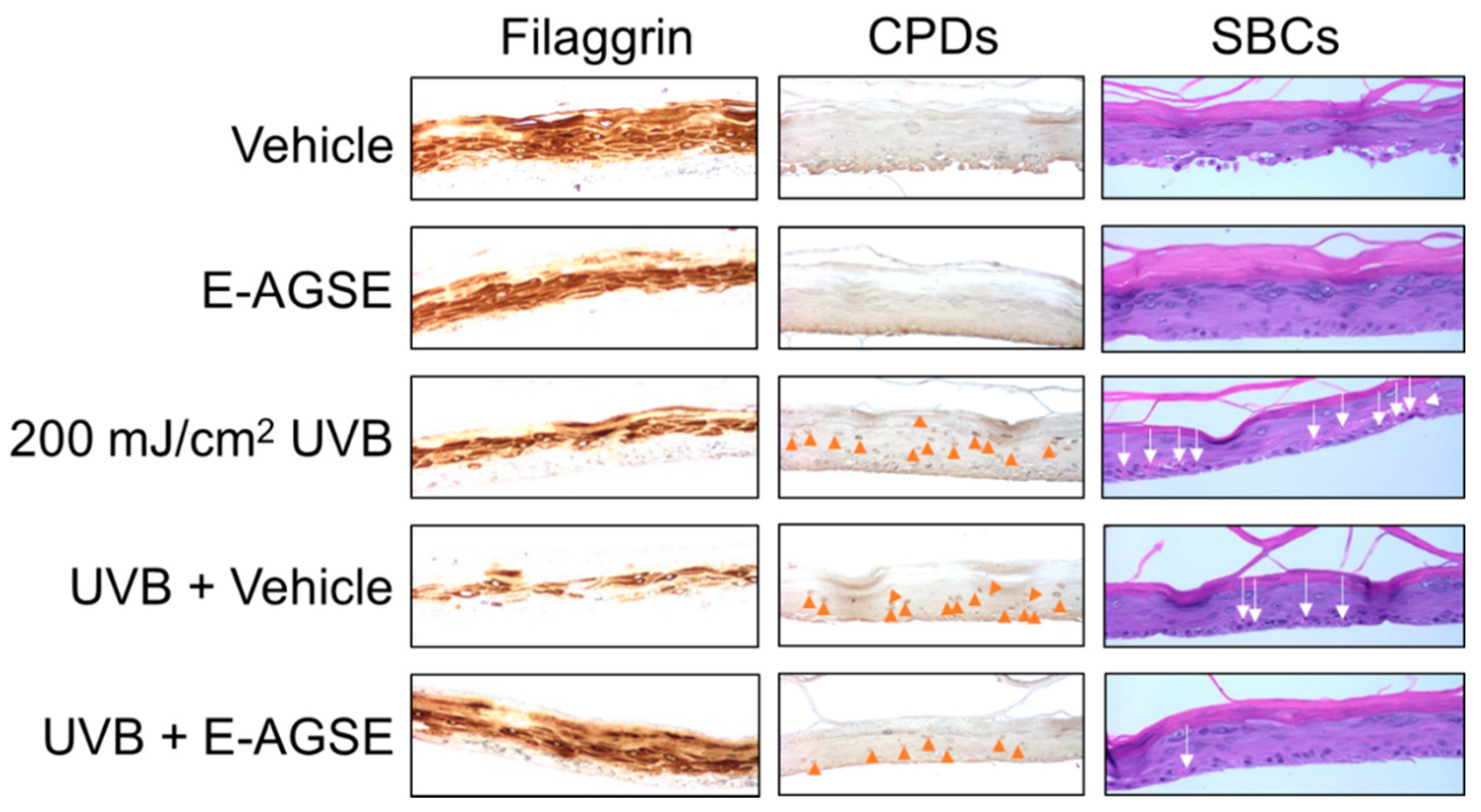
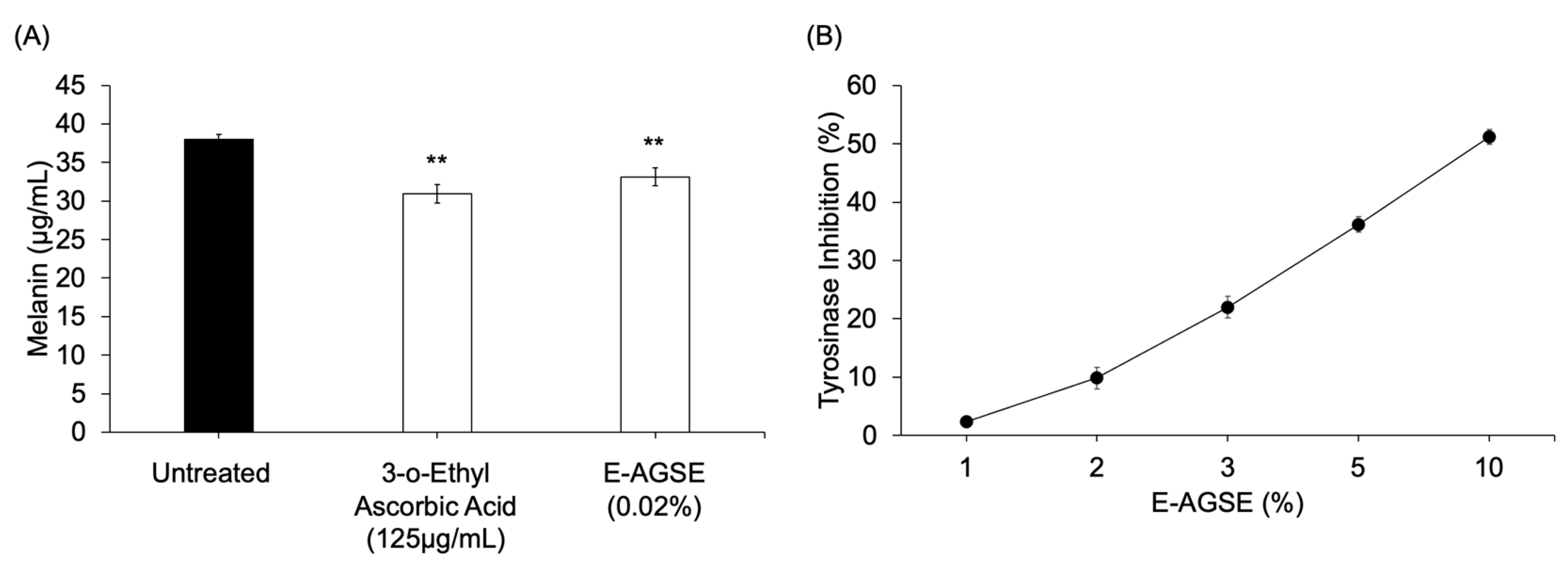
| Condition Group | Particle Size (Week 4) | Uniformity | Particle Size (Week 12) | Uniformity |
|---|---|---|---|---|
| room temperature | 93 nm | 0.314 | 94 nm | 0.326 |
| 4 °C | 95 nm | 0.336 | 91 nm | 0.316 |
| −18 °C | 82 nm | 0.396 | 93 nm | 0.325 |
| 48 °C | 96 nm | 0.369 | 119 nm | 0.367 |
| lighting | 89 nm | 0.301 | 93 nm | 0.304 |
| Test Group | Avg ± SD (% Change from Untreated) | ||
|---|---|---|---|
| Immunofluorescence, IOD/Cell | Gene (Fold Expression) | ||
| Collagen I * | Elastin * | Collagen III (COL3A1) ¥ | |
| Untreated | 1.00 ± 0.05 | 1.00 ± 0.1 | 1.02 ± 0.28 |
| TGF-β1 (100 ng/mL) | 2.32 ± 0.21 (132%) ** | ND | 1.93 ± 0.08 (89%) ** |
| E-AGSE (0.1%) | 3.32 ± 0.28 (232%) ** | 2.31 ± 0.32 (131%) ** | 2.07 ± 0.10 (102%) ** |
| Palmitoyl Tripeptide-5 (8 ppm) | 2.96 ± 0.73 (196%) ** | 0.99 ± 0.21 (0%) | 1.62 ± 0.41 (58%) |
| Test Group | Avg ± SD (% Change from Untreated) | |||
|---|---|---|---|---|
| Gene (Fold Expression) | ||||
| Occludin (OCLN) ¥ | Syndecan-1 (SDC1) ¥ | Heparan Sulfate Proteoglycan 2 (HSPG2) ¥ | Chondroitin Sulfate Proteoglycan 8 (CD44) ¥ | |
| Untreated | 1.00 ± 0.03 | 1.00 ± 0.02 | 1.00 ± 0.01 | 1.00 ± 0.02 |
| L-Rhamnose (1%) | 2.59 ± 1.27 (159%) ** | 1.41 ± 0.15 (41%) ** | 1.20 ± 0.21 (20%) * | 1.20 ± 0.09 (20%) ** |
| E-AGSE (0.1%) | 9.85 ± 3.87 (885%) ** | 2.03 ± 0.37 (103%) ** | 1.39 ± 0.22 (39%) ** | 1.47 ± 0.21 (47%) ** |
| GHK-Cu (0.05%) | 1.99 ± 0.81 (99%) ** | 1.42 ± 0.22 (42%) ** | 1.21 ± 0.14 (21%) ** | 1.15 ± 0.04 (15%) ** |
| Avg ± SD (% Inhibition from UVB + Vehicle) | |||
|---|---|---|---|
| Test Group | IL-8, pg/mL | CPDs (+Cells/Tissue) * | SBCs (+Cells/Tissue) * |
| Vehicle | 220 ± 24 | 0 ± 1 | 6 ± 1 |
| E-AGSE (0.1%) | 121 ± 6 | 1 ± 2 | 1 ± 0 |
| UVB (200 mJ/cm2) | 1051 ± 108 | 223 ± 1 | 24 ± 7 |
| UVB + Vehicle | 11321 ± 1850 (0 #) | 177 ± 33 (21% #) | 18 ± 3 (39% #) |
| UVB + E-AGSE (0.1%) | 339 ± 70 (98%) ‡ | 86 ± 21 (52%) ‡ | 7 ± 2 (93%) ‡ |
| Parameter (Instrument) | Time Point | Mean Change (Mean Change %) * | p Value ** |
|---|---|---|---|
| Skin wrinkle area (VISIA CR) | Week 2 | −11.20 | <0.05 |
| Week 4 | −13.66 | <0.05 | |
| Skin tone/brightening (Colorimeter® CL400) | Week 2 | +5.16 | <0.05 |
| Week 4 | +8.86 | <0.05 | |
| TEWL (Tewameter® TM300) | Week 2 | −7.15 | <0.05 |
| Week 4 | -10.10 | <0.05 | |
| Skin hydration (Corneometer® CM825) | Week 2 | +24.57 | <0.05 |
| Week 4 | +30.94 | <0.05 | |
| Lactic acid sting test | Week 2 | −11.59 | <0.05 |
| Week 4 | −19.81 | <0.001 |
| Self-Perception Questionnaire (SPQ) Parameter | Week 2 | Week 4 |
|---|---|---|
| Improved skin elasticity | 94% | 100% |
| Improved skin comfort | 100% | 100% |
| Improved skin brightness | 90% | 94% |
| Improved skin hydration | 97% | 100% |
| Improved skin firmness | 94% | 94% |
| Skin feels hydrated and brightened | 87% | 90% |
| Improved fine lines/nasolabial folds | 94% | 94% |
| Improved dry and fragile skin | 97% | 97% |
| Soothed skin tightness | 97% | 94% |
| Soothed facial redness | 90% | 94% |
| Alleviated dryness and sensitivity | 100% | 97% |
| Repaired/strengthened the skin barrier | 97% | 97% |
| Skin quality improvement | 100% | 90% |
| Mild and not irritating | 97% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, K.; Guo, L.; Hu, X.; Fitzgerald, C.; Rouzard, K.; Healy, J.; Tamura, M.; Stock, J.B.; Stock, M.; Pérez, E.; et al. Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties. Cosmetics 2022, 9, 4. https://doi.org/10.3390/cosmetics9010004
Tao K, Guo L, Hu X, Fitzgerald C, Rouzard K, Healy J, Tamura M, Stock JB, Stock M, Pérez E, et al. Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties. Cosmetics. 2022; 9(1):4. https://doi.org/10.3390/cosmetics9010004
Chicago/Turabian StyleTao, Kan, Lili Guo, Xincheng Hu, Corey Fitzgerald, Karl Rouzard, Jason Healy, Masanori Tamura, Jeffry B. Stock, Maxwell Stock, Eduardo Pérez, and et al. 2022. "Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties" Cosmetics 9, no. 1: 4. https://doi.org/10.3390/cosmetics9010004
APA StyleTao, K., Guo, L., Hu, X., Fitzgerald, C., Rouzard, K., Healy, J., Tamura, M., Stock, J. B., Stock, M., Pérez, E., & Fernández, J. R. (2022). Encapsulated Activated Grape Seed Extract: A Novel Formulation with Anti-Aging, Skin-Brightening, and Hydration Properties. Cosmetics, 9(1), 4. https://doi.org/10.3390/cosmetics9010004







