1. Introduction
The prevalence of allergic contact dermatitis (ACD) is increasing continuously in Europe and worldwide. A review of 28 studies published between 2007 and 2017 covering 20,107 patch test individuals in the general population found a pooled prevalence of around 20% in Europe, with 16.5% in children and adolescents, 27.9% in women and 13% in men [
1]. Dermatologists highlighted that allergen trends have clearly evolved over a defined period of time, sometimes in a short period with the emergence of new allergens [
2]. The frequency of occurrence of this skin pathology and the evolving context justify the need to assess skin sensitization potential for any ingredient intended for topical applications. Since the ban on animal testing in Europe, and its extension to many parts of the world, a battery of in vitro tests covering the key steps of the Adverse Outcome Pathway (AOP) for skin sensitization is recommended (OECD Series on Testing and Assessment No. 168 [
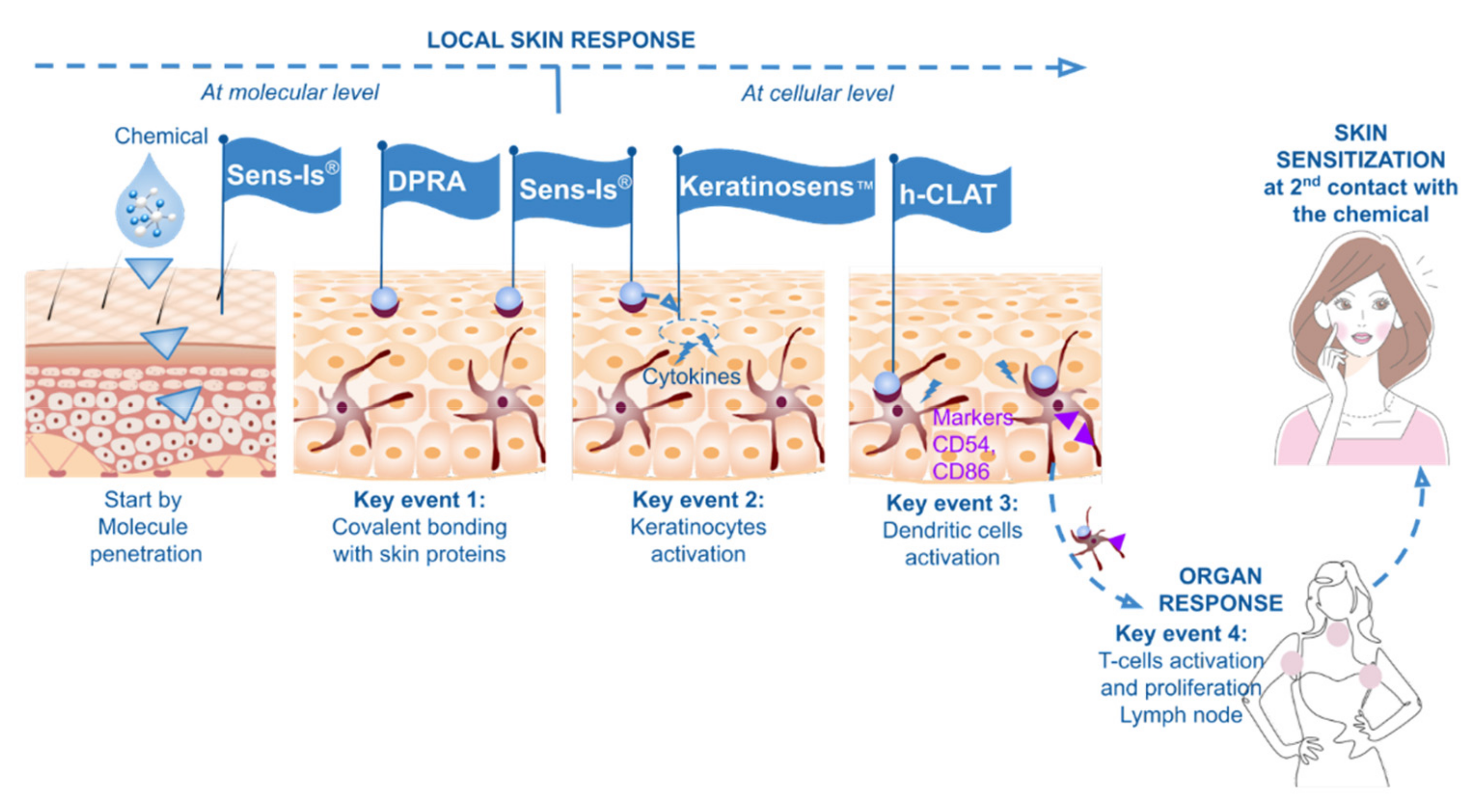
3]). The AOP concept describes a sequential chain of causally linked Key Events at different levels of biological organization that lead to an adverse health effect. In 2012, a document describing the skin sensitization AOP was published (OECD Series on Testing and Assessment No. 168). Four events are recognized as key, following the prerequisite of skin penetration: (1) molecular interaction with skin proteins; (2) response of activated keratinocytes with inflammatory and gene expression associated with cell signaling pathways (e.g., Antioxidant/electrophile Response Element-dependent pathways—ARE); (3) activation of dendritic cells associated with specific cell surface markers expression, chemokines and cytokines; and (4) T-cell activation and proliferation (
Figure 1).
This concept, combined with international in vitro method guidelines, has been taken up and integrated into a number of guidance documents in order to characterize the sensitizing potential of a substance (e.g., emitted at the worldwide level by OECD; at the European level by ECHA: European Chemicals Agency). To date, three in vitro methods are validated and described in the OECD guidelines (442C, 442D, 442E), and many others are under validation by governmental organizations on alternative test methods. Recently, defined assessment approaches were published (OECD 497 [
4]) to predict the skin sensitization hazard, but they were based mainly on the testing of simple and “easy to test” substances. In this context, an internal testing strategy [
5] focused on “difficult-to -test” ingredients was developed. “Difficult-to-test” ingredients included poorly water-soluble (<60 mg/L), surfactants and/or complex substances (Unknown or variable composition, complex reaction products or biological materials called UVCB). This ingredient category represents a big part of the cosmetic formulation components. Based on the skin sensitization AOP, the approach consisted in combining complementary in vitro models. The first part of this article will briefly describe this methodology.
Nowadays, sustainability is essential in the creation of a cosmetic product. Although sustainable development can be approached through different perspectives, the naturality of ingredients often plays a central role for consumers. Among plant-based materials, botanical extracts are key for the efficacy of cosmetics. Some of them were reported as potential causes of contact allergy dermatitis [
6]. Moreover, botanical extracts are known to be complex and complicated compounds to be tested using in vitro test models.
Considering this perspective, the purpose of this work was to assess the skin sensitization of botanical ingredients with, among them, experimental extracts currently under development.
2. Materials and Methods
2.1. Materials
Two well-known sensitizing botanical extracts: Evernia prunastri absolute and Styrax tonkinensis resin extract were included as positive references [
7].
Thirteen examples of botanical extracts, with complex chemical composition, were selected. These examples illustrate the diversity in the design that can be encountered during the development of botanical active ingredients (
Table 1). First, the data set included both terrestrial and marine biomass origin from different families. Secondly, diverse preparations were carried out from plant leaves, roots or fruits, mainly by conventional extraction process and, for one of the extracts, by biotechnology. Some botanicals were prepared as dried or oil extracts (100% active substance). Others were prepared in solvents of various polarity (hydrophilic solvents such as glycerin, or glycols, or lipophilic solvents such as Caprylic/Capric-C8/C10-triglyceride) at lower active substance concentration (i.e., <5%). Thirdly, the ingredients covered the range of solubility from not soluble in water to freely soluble. The final hydrophilic or lipophilic character is an important parameter with regard to the delivery of the active ingredient and the selection of the vehicle (formulation with aqueous or oily continuous phase). The selected botanical extracts were designed to target various biological efficacies (moisturizing, anti-ageing, soothing, skin brightening, skin barrier protection). Regarding expectations of skin sensitization, two experimental extracts,
Hippophae rhamnoides and
Commiphora myrrha, were already detected as sensitizers. The skin sensitization profiles of the other eleven botanicals were unknown.
The research projects on botanicals followed the principles of the Convention on Biodiversity. The samples of natural origin used in this work were accessed and utilized in compliance with the Nagoya Protocol and relevant national laws on accessing genetic resources and sharing the benefits arising from their use. The status of plant and marine resources were constantly monitored with regard to the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and to the International Union for Conservation of Nature (IUCN lists).
2.2. Methods
Two methods with OECD guidelines were used: 442D (ARE-Nrf2 Luciferase Test Method- KeratinoSens), 442E (human Cell Line Activation Test h-CLAT) and the Sens-Is assay [
8], currently in the work plan of the OECD, chosen for its ability to overcome solubility issues and to discriminate between irritants and sensitizers.
2.2.1. KeratinoSens™
KeratinoSens (Givaudan SA, Switzerland) (
Figure 2) is an in vitro assay focusing on the activation of the Keap1-Nrf2-ARE (Kelch-like ECH-associated protein 1—Nuclear factor erythroid—Related Factor 2-Antioxidant Response Element) pathway in transfected keratinocytes containing a luciferase gene, exposed to a test chemical. A concentration series of test chemicals based on the maximum achievable dilution, prepared in either aqueous media or Dimethyl Sulfoxide (DMSO), were applied to the cells for 48 h. Viability was then measured using the MTT test ((3-(four, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide: MTT; 27 μL of a solution at 5 mg/mL in Dulbecco’s phosphate-buffered saline was added to each well). Keratinocyte activation was assessed by luciferase measurement after addition of luciferin in the wells (50 μL in each well). Negative control was defined as the culture medium with or without the solvent used as a vehicle for test item preparation. The positive control was the Cinnamic Aldehyde (CAS No. 14371-10-9) tested at concentrations ranging from 4 to 64 μM. A test chemical was considered positive when the luciferase expression is higher by a factor of at least 1.5 compared to control wells with a dose-effect relationship. Furthermore, the cytotoxicity level must be below 30%.
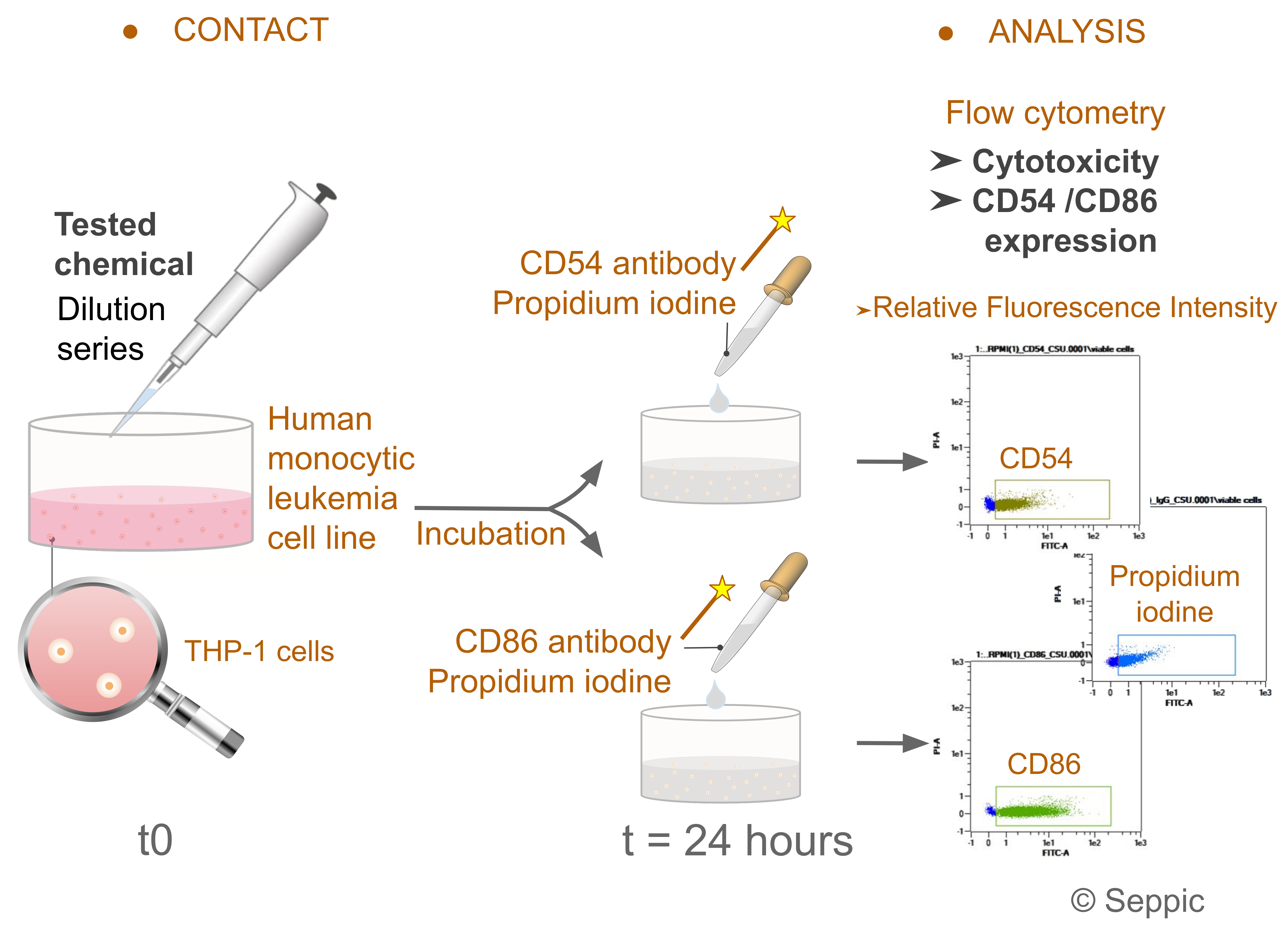
2.2.2. Human Cell Line Activation Test (h-CLAT)
h-CLAT (
Figure 3) is an in vitro assay quantifying changes in the expression of the CD86 and CD54 (Cluster of Differentiation 86 and 54) membrane phenotypic protein markers in a human monocytic leukemia cell line (THP-1 cells) exposed to a test chemical. Based on the CV75 value (i.e., concentration inducing 25% cytotoxicity), THP-1 cells were incubated with a series of eight concentrations (in either aqueous media or DMSO) for a period of 24 h. Cells were then labeled with fluorochrome-coupled anti-CD86 and anti-CD54 antibodies (50 μL antibody solution; 6µL anti-CD86 in 44 µL staining buffer and 3 µL of anti-CD54 in 47 µL of staining buffer) and analyzed by flow cytometry in order to determine the Relative Fluorescence Intensity (RFI). Propidium iodine (50 μg/mL diluted in PBS) assessed cell viability. Negative control was defined as Lactic acid (CAS No.: 50-21-5) tested at 1000 µg/mL. Positive control was 1-chloro-2,4-dinitrobenzen (DNCB; CAS No.: 97-00-7) tested at 4.0 µg/mL. A test chemical was considered positive as long as the expression of at least one of the two cell surface markers was reproducible and notably increased (i.e., CD86 RFI ≥ 150% and/or CD54 RFI ≥ 200% in at least one tested concentration). These results should be obtained in two independent runs and in the absence of strong cytotoxicity (i.e., viability ≥ 50%).
2.2.3. Sens-Is®
Sens-Is (Grasse, France) (
Figure 4) is an in vitro assay analyzing the expression of a panel of genes relevant to the biological processes [
8] in a reconstructed human epidermis model (Episkin
® (Lyon, France)) exposed to a test chemical. Two sets of genes involved in skin sensitization mechanisms are followed; (1) called ‘REDOX’ focuses on the Keap1-Nrf2-ARE dependent pathway, (2) named ‘SENS-IS’ is linked to signals leading to the activation of dendritic cells. Chemicals binding to cysteine activate the ‘REDOX’ group of genes, whereas chemicals binding only to lysine activate genes within the ‘SENS-IS’ group. The Sens-Is assay discriminates between irritants and sensitizers using a third group of dedicated genes. The experimental protocol follows the Immunosearch Standard Operating Protocol (SOP). Briefly, human 3D reconstructed epidermis were exposed for 15 min to the test chemical (30 µL), pure when possible or dissolved in Phosphate Buffered Saline (PBS), olive oil, DMSO or Dipropylene Glycol. After washing and 6 h post-incubation, tissues were prepared for complementary Deoxyribonucleic Acid (cDNA) quantification by Reverse Transcriptase—Polymerase Chain Reaction (RT-PCR). For each analysis, three negative controls (PBS, olive oil and DMSO), a positive irritation control Sodium Lauryl Sulfate (CAS No. 151-21-3) at 5%, and a positive sensitization control 2,4,6-trinitrobenzene sulfonic acid (TNBS; CAS No. 2508-19-2) at 1%, were performed. A test item was considered a sensitizer when at least seven genes in either group of genes are overexpressed (at least 1.25-fold control value).
3. Description of the Methodology Used to Assess Skin Sensitization
During the methodology development, previously published [
5], the results of “difficult to test” ingredients (i.e., poorly water-soluble components, complex substances, etc.), well-known as sensitizers and non-sensitizers, were firstly analyzed on each model. Sens-Is assay showed the highest accuracy and ability to detect true sensitizers. h-CLAT and KeratinoSens showed a lower ability to properly classify potential sensitizers. Finally, Direct Peptide Reactivity Assay (DPRA; OECD 442C), initially included in the experimental models, appeared unsuitable. The statistical analysis of the results highlighted the need to combine different methodologies to increase the reliability of the prediction.
The Sens-Is model was chosen as a pivotal test for additional benefits [
9,
10]:
Possibility of analyzing any substance whatever its solubility, physical state, or surfactants that could induce cytotoxicity on 2D cell culture models;
Ability to discriminate between irritants and sensitizers;
Proper prediction of the pre- or pro-haptens [
11];
Evaluation of the two first Key Events of the skin sensitization AOP and taking the dermal penetration of the compounds into account.
KeratinoSens enabled assessment of Key Event 2 in a complementary way of cell exposure. h-CLAT was an essential validated method to evaluate Key Event 3.
A sequential strategy was defined (
Figure 5): Sens-Is was the entry test, followed by h-CLAT and KeratinoSens. In a conservative approach, a Sens-Is positive result was considered sufficient to classify the tested ingredient as a skin sensitizer and implied that no other test needs to be performed. In the case of a negative result, this figure must be confirmed by another in vitro test, the h-CLAT. Hence, two concordant results covering the first three KE of the AOP allowed us to conclude on the absence of sensitizing potential. In the case of non-concordant results, a third test (KeratinoSens) was necessary to conclude.
Statistical analysis of the results compared to in vivo data showed an accuracy of 96% (24/25), with a sensitivity of 100% (11/11) and a specificity of 93% (13/14). These percentages were higher than 80% and higher than the individual tests were. The testing strategy was able to minimize the risk of a false negative conclusion.
4. Results of Botanical Ingredients
Results obtained with experimental models are presented in
Table 2. Well-known skin sensitizers,
Styrax tonkinensis resin extract,
Evernia prunastri absolute, provided a positive response in Sens-Is, leading to their categorization as sensitizers according to the testing strategy.
For weakly concentrated plant extracts (≤5% Active Substance) and in the absence of a phytochemical alert, only Sens-Is was performed. All of these Sens-Is tests were found to be negative, allowing further confirmation of good tolerance by clinical trials.
The other candidates in concentrated form came from current ingredient development projects for which the strategy was used to assess their skin sensitizing potential. Among them, Hippophae rhamnoides, Commiphora myrrha crude extracts, and Isatis tinctoria extract in propanediol provided positive Sens-Is responses leading to the conclusion that they are skin sensitizers. Virgin experimental plant oil showed a negative response with Sens-Is assay, while it induced the activation of dendritic cells in the h-CLAT assay. Keratinocyte response was also identified with the KeratinoSens, leading to the conclusion of sensitization potential. The discrepancy in the response could be explained by the model’s design. The h-CLAT and KeratinoSens can detect the presence of a sensitizer at very low concentrations. On the contrary, the Sens-Is, similarly to the LLNA, gives a positive response when the concentration of a sensitizing molecule is over the Effective Concentration for a stimulation index of 3 (EC3) limit. Orbignya phalerata and Aphloia theiformis extract did not induce a positive response in Sens-Is nor in h-CLAT assays and were therefore considered non-sensitizers.
5. Discussion
The in vitro results on the two references,
Styrax tonkinensis resin extract and
Evernia prunastri absolute (
Table 3), were in line with their well-known hazard classification for skin sensitization.
Hippophae rhamnoides and
Commiphora myrrha crude extracts, also identified as skin sensitizers with in vivo studies (unpublished data [
12,
13]), were properly detected in vitro. Moreover, recent literature on
Commiphora myrrha [
14] reported the identification of two reactive compounds (6-oxofuranodienones and methoxyfuranogermacrenones) susceptible to trigger the molecular initiating event of skin sensitization. These four results completed the statistical analysis of the testing strategy, resulting in an accuracy of 97% (28/29), a sensitivity of 100% (15/15) and a specificity of 93% (13/14). The other twelve botanicals, evaluated during the development of new ingredients, were therefore not considered for statistics, as their sensitizing profiles were unknown at the beginning of this work.
Isatis tinctoria extract was detected as a sensitizer. A deeper phytochemical analysis revealed traces of suspected sensitizing compounds, which strengthened confidence in the result. One of the compounds, isatin [
16], was specifically questioned.
Following the identification of virgin plant oil sensitization potential, further phytochemical composition was investigated. CPG analysis highlighted the presence of residual volatile organic components (VOC) with more than 30 terpene molecules, representing 3.3% in the oil composition. Among them, linalool was quantified as the main compound (i.e., around 1.4% of the total virgin oil) and was suspected to be responsible for the effect observed. Therefore, a purification step, dedicated to reduce VOCs, was added to the manufacturing process. The refined plant oil showed a residual VOC content of 30 ppm, in which one third is composed of linalool (i.e., 10 ppm). The absence of sensitization potential of this refined oil supported the previous hypothesis and made it possible to confirm the local tolerance at a clinical level.
Other botanical extracts with in vitro non-sensitizing responses, Orbignya phalerata, Aphloia theiformis, Ulva lactuca, Beta vulgaris, Hedychium coronarium, Asparagopsis armata, Arctium lappa, and Helichrysum stoechas culture lysate consistently demonstrated the absence of any allergic reaction during clinical trials at use levels.
6. Conclusions
The in vitro testing strategy previously developed for “difficult to test” ingredients was appropriate for the tested botanical ingredients. It is important to remember that some uncertainties still exist, mostly linked to the limits of the in vitro tests.
The high accuracy of 97% combined with 100% sensitivity removed the risk of false negative conclusions. Implementation of this approach in the development process of new ingredients guarantees the absence of skin sensitization hazards with high confidence in view of clinical steps. The essential contribution of phytochemistry investigations was highlighted for the development of non-sensitizing ingredients. Bearing in mind the strong consumer demand for natural products, the unexpected effects observed with some botanicals reinforces the need for an accurate safety assessment.
The development and availability of new approach methodologies (NAMs) could participate in determining the skin sensitization categories according to the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS).
Author Contributions
Conceptualization, M.P.; methodology, M.P., H.G., C.G. and F.C.; validation, M.P., H.G. and C.G.; formal analysis, M.P., H.G. and C.G.; investigation, H.G. and C.G.; data curation, H.G. and C.G.; writing—original draft preparation, M.P. and A.R.; writing—review and editing, M.P. and A.R.; visualization, A.R.; supervision, M.P.; project administration, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All the clinical trials were performed by clinical test specialist Contract Research Organization under the general principles of medical ethics in clinical research coming from the Declaration of Helsinki (June 1964) and its successive amendments, the international recommendations relating to Good Clinical Practices for conducting clinical trials for drugs ICH E6(R1) of 10/06/1996 (CPMP/ICH/135/95), the Directive of the European Parliament and Council 2001/20/EC concerning the harmonization of legislative, statutory and administrative provisions of the member States relating to the application of good clinical practices when conducting clinical trials for drugs for human use—OJ/EC of 01/05/2001, the recommendations of Colipa August 1997: “guidelines for the assessment of human skin compatibility”, and was in accordance with regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies through the responsibility of the Contract Research Organization.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank our phytochemists, Rémi Laville, Julien Fouilland and Virginie Anchartechahar for their valuable support in the characterization of botanical ingredients.
Conflicts of Interest
Hervé Groux and Françoise Cottrez have filed a patent for the SENS-IS assay. Mickael Puginier, Mathilde Bergal, Alicia Roso are employees of Seppic company. Cédric Gerbeix declares no conflict of interest.
References
- Farzad, A.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Derm. 2019, 80, 77–85. [Google Scholar] [CrossRef]
- Sukakul, T.; Chaweekulrat, P.; Limphoka, P.; Boonchai, W. Changing trends of contact allergens in Thailand: A 12-year retrospective study. Contact Derm. 2019, 81, 124–129. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins Part 1: Scientific Evidence; OECD Series on Testing and Assessment No.168; ENV/JM/MONO(2012)10/PART1; OECD: Paris, France, 2012. [Google Scholar]
- OECD. Defined Approaches on Skin Sensitisation; OECD Guidance No. 497; OECD: Paris, France, 2021. [Google Scholar]
- Bergal, M.; Puginier, M.; Gerbeix, C.; Groux, H.; Roso, A.; Cottrez, F.; Milius, A. In vitro testing strategy for assessing the skin sensitizing potential of “difficult to test” cosmetic ingredients. Toxicol. Vitro 2020, 65, 104781. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.R.; Norris, P.L.; Storrs, F.J. Allergic contact dermatitis to plant extracts in cosmetics. Semin. Cutan. Med. Surg. 2013, 32, 140–146. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Fragrance Allergens in Cosmetic Products; EU SCCS/1459/11; Scientific Committee on Consumer Safety: Luxembourg, 2012. [Google Scholar]
- Cottrez, F.; Boitel, E.; Ourlin, J.C.; Peiffer, J.L.; Fabre, I.; Henaoui, I.S.; Mari, B.; Vallauri, A.; Paquet, A.; Barbry, P.; et al. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicol. Vitro 2016, 32, 248–260. [Google Scholar] [CrossRef]
- Petry, T.; Bosch, A.; Koraïchi-Emeriau, F.; Eigler, D.; Germain, P.; Seidel, S. Assessment of the skin sensitisation hazard of functional polysiloxanes and silanes in the SENS-IS assay. Regul Toxicol Pharmacol. 2018, 98, 209–214. [Google Scholar] [CrossRef]
- Clouet, E.; Kerdine-Römer, S.; Ferret, P.J. Comparison and validation of an in vitro skin sensitization strategy using a data set of 33 chemical references. Toxicol. Vitro 2017, 45, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Kleinstreuer, N.; Alépée, N.; Allen, D.; Api, A.M.; Ashikaga, T.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit. Rev. Toxicol. 2018, 48, 344–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richeux, F. (Phycher Bio Dévelopement, Martillac, Gironde, France). OECD 406, Skin Sensitization, Guinea Pig Maximization Test Method; OECD: Paris, France, 2007; Unpublished work. [Google Scholar]
- Donny, E. (Envigo, Rossdorf, Germany). OECD 429, Skin Sensitization, Local Lymph Node Assay; OECD: Paris, France, 2008; Unpublished work. [Google Scholar]
- Zhou, Q.; Liu, Y.; Tang, Y.; Shokoohinia, Y.; Chittiboyina, A.G.; Wang, M.; Avonto, C. Identification of Potential Skin Sensitizers in Myrrh. Cosmetics 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT), Marzulli & Maybach Method; OECD: Paris, France, Unpublished work, 2015 to 2021.
- Scientific Committee on Consumer Products (SCCP) Opinion on Isatin-COLIPA N A129-SCCP/0876/05-3rd Plenary Meeting, 2005. Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_002.pdf (accessed on 30 December 2005).
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).