Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentations
2.2. Sample Preparation and Identification
2.3. Small-Scale Plant Extraction
2.4. Large-Scale Plant Extraction
2.5. Qualitative Phytochemical Analysis
2.5.1. Flavonoids
2.5.2. Tannin
2.5.3. Terpenoid and Steroid
2.5.4. Saponin
2.5.5. Alkaloid
2.6. Sample Preparation of Stability Test
2.7. Physical Stability Test
2.8. Determination of Total Flavonoid Content
2.9. DPPH Radical Scavenging Activity Assay
2.10. Chemical Constituent Analysis
3. Results and Discussion
3.1. Small-Scale Plant Extraction
3.2. Large-Scale Plant Extraction
3.3. Stability Test of Cinnamon Extract
3.3.1. Physical Stability Test
3.3.2. Total Flavonoid Content and Antioxidant Activity
3.3.3. Chemical Constituent Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batubara, I.; Prastya, M.E. Potential use of Indonesian medicinal plants for cosmetic and oral health: A review. Jurnal Kimia Valensi 2020, 6, 118–132. [Google Scholar]
- Amberg, N.; Fogarassy, C. Green Consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Dewettinck, K. Cinnamon and its derivatives as potential ingredient in functional food—A review. Int. J. Food Prop. 2017, 20, 2237–2263. [Google Scholar] [CrossRef]
- Menggala, S.R.; Vanhove, W.; Muhammad, D.R.A.; Hendri, J.; Speelman, S.; Van Damme, P. Sustainable harvesting of Cinnamomum burmannii (Nees & T. Nees) blume in kerinci regency, Indonesia. Sustainability 2019, 11, 6709. [Google Scholar] [CrossRef]
- Błaszczyk, N.; Rosiak, A.; Kałużna-Czaplińska, J. The potential role of cinnamon in human health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Sun, A.; Liu, X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci. Nutr. 2019, 7, 2186–2193. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Plumeriastuti, H.; Budiastuti; Effendi, M.E.; Budiarto. Identification of bioactive compound of the essential oils of Cinnamomum burmannii from several areas in Indonesia by gas chromatography–mass spectrometry method for antidiabetic potential. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 279–283. [Google Scholar] [CrossRef]
- Wahba, H.E.; Sarhan, A.Z.; Salama, A.B.; Sharaf-Eldin, M.A.; Gad, H.M. Effect of Seasonal variation on the growth and chemical composition of Cynara cardunculus L. plants. J. Mater. Environ. Sci. 2017, 8, 318–323. [Google Scholar]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Oladele, J.O.; Ajayi, E.I.; Oyeleke, O.M.; Oladele, O.T.; Olowookere, B.D.; Adeniyi, B.M.; Oyewole, O.I.; Oladiji, A.T. A systematic review on COVID-19 pandemic with special emphasis on curative potentials of nigeria based medicinal plants. Heliyon 2020, 6, e04897. [Google Scholar] [CrossRef] [PubMed]
- Khanal, A.; Devkota, H.P.; Kaundinnyayana, S.; Gyawali, P.; Ananda, R.; Adhikari, R. Culinary herbs and spices in Nepal: A review of their traditional uses, chemical constituents, and pharmacological activities. Ethnobot. Res. Appl. 2021, 21, 40. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.D.A.G.; De Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Ainane, T.; Khammour, F.; Merghoub, N.; Elabboubi, M.; Charaf, S.; Ainane, A.; Elkouali, M.H.; Talbi, M.; Abba, E.H.; Cherroud, S. Cosmetic bio-product based on cinnamon essential oil “Cinnamomum verum” for the treatment of mycoses: Preparation, chemical analysis and antimicrobial activity. MOJ Toxicol. 2019, 5, 5–8. [Google Scholar] [CrossRef]
- Suliman, R.S.; Ali, H.; Nurulain, I.; Shamiha, N.N.; Nizam, M.; Budiasih, S.; Suliman, R.; Al-Gebaly, A. cinnamon bark extract for the formulation and characterisation of antimicrobial cream. Int. J. Res. Ayurveda Pharm. 2017, 8, 200–206. [Google Scholar] [CrossRef]
- Rohmani, S.; Kuncoro, M.A.A. Stabilization and activity test handsanitizer gel of kemangi leaves extract. J. Pharm. Sci. Clin. Res. 2019, 1, 16–28. [Google Scholar] [CrossRef]
- Vieira, R.P.; Fernandes, A.R.; Kaneko, T.M.; Consiglieri, V.O.; Pinto, C.A.S.D.O.; Pereira, C.S.C.; Baby, A.R.; Velasco, M.V.R. Physical and physicochemical stability evaluation of cosmetic formulations containing soybean extract fermented by Bifidobacterium animalis. Braz. J. Pharm. Sci. 2009, 45, 515–525. [Google Scholar] [CrossRef][Green Version]
- Jeon, S.M.; Ahn, J.Y.; Park, S.N. A Study on the stability test for the cream containing Suaeda asparagoides Extract. J. Soc. Cosmet. 2007, 33, 231–238. [Google Scholar]
- Xu, J.; Zhou, L.; Miao, J.; Yu, W.; Zou, L.; Zhou, W.; Liu, C.; Liu, W. Effect of cinnamon essential oil nanoemulsion combined with ascorbic acid on enzymatic browning of cloudy apple juice. Food Bioproc. Tech. 2020, 13, 860–870. [Google Scholar] [CrossRef]
- Kurniawanti, D.D.A.; Dianhar, H.; Rahayu, D.U.C.; Sugita, P. Phytochemical screening and preliminary evaluation of antioxidant activity of three indonesian araucaria leaves extracts. Sci. Arch. 2021, 2, 250–254. [Google Scholar] [CrossRef]
- Bhandari, L.; Rajbhandari, M. Isolation of quercetin from flower petals, estimation of total phenolic, total flavonoid and antioxidant activity of the different parts of Rhododendron arboreum smith. Sci. World 2015, 12, 34–40. [Google Scholar] [CrossRef]
- Rahayu, D.U.C.; Al-Laily, R.S.; Khalwani, D.A.; Anjani, A.; Handayani, S.; Saepudin, E.; Dianhar, H.; Sugita, P. Microwave-assisted synthesis of 4-methyl coumarins, their antioxidant and antibacterial activities. Rasayan J. Chem. 2022, 15, 1053–1062. [Google Scholar] [CrossRef]
- Razali, M.; Didaskalou, C.; Kim, J.F.; Babaei, M.; Drioli, E.; Lee, Y.M.; Szekely, G. Exploring and exploiting the effect of solvent treatment in membrane separations. ACS Appl. Mater. Interfaces 2017, 9, 11279–11289. [Google Scholar] [CrossRef] [PubMed]
- FDA. Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act. Available online: https://www.fda.gov/media/94164/download (accessed on 27 March 2022).
- FDA. Alcohol Free. Available online: https://www.fda.gov/cosmetics/cosmetics-labeling-claims/alcohol-free (accessed on 27 March 2022).
- FDA. Toxicological Data for Class 3 Solvents. Available online: https://www.fda.gov/media/71194/download (accessed on 27 March 2022).
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophytic fungi isolated from garcinia plants. FEMS Immunol. Med. Microbiol. 2007, 51, 517–525. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of extraction conditions on the phenolic composition and antioxidant capacity of grape stem extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Leu, Y.-L.; Hung, Y.-Y.; Fang, J.-Y. Coumarin derivatives, but not coumarin itself, cause skin irritation via topical delivery. Toxicol. Lett. 2014, 226, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural flavones and flavonols: Relationships among antioxidant activity, glycation, and metalloproteinase inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; Van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Enko, J.; Gliszczyńska-Świgło, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addit. Contam. Part A 2015, 32, 1234–1242. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical stability of ascorbic acid integrated into commercial products: A review on bioactivity and delivery technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Herbig, A.-L.; Renard, C.M.G.C. Factors that impact the stability of vitamin c at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Pérez-Lamela, C.; Franco, I.; Falqué, E. Impact of high-pressure processing on antioxidant activity during storage of fruits and fruit products: A review. Molecules 2021, 26, 5265. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Niklasson, I.B.; Delaine, T.; Islam, M.N.; Karlsson, R.; Luthman, K.; Karlberg, A.-T. Cinnamyl alcohol oxidizes rapidly upon air exposure. Contact Derm. 2013, 68, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.; Cindrić, I.; Molnar, M. Coumarins in food and methods of their determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Voges, M.J.E.E.E.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. 2019, 116, 12558–12565. [Google Scholar] [CrossRef]
- De Taeye, C.; Cibaka, M.-L.K.; Collin, S. Occurrence and antioxidant activity of C1 degradation products in cocoa. Foods 2017, 6, 18. [Google Scholar] [CrossRef]
- Ashaari, N.S.; Rahim, M.H.A.; Sabri, S.; Lai, K.S.; Song, A.A.-L.; Rahim, R.A.; Abdullah, J.O. Kinetic studies and homology modeling of a dual-substrate linalool/nerolidol synthase from Plectranthus amboinicus. Sci. Rep. 2021, 11, 17094. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
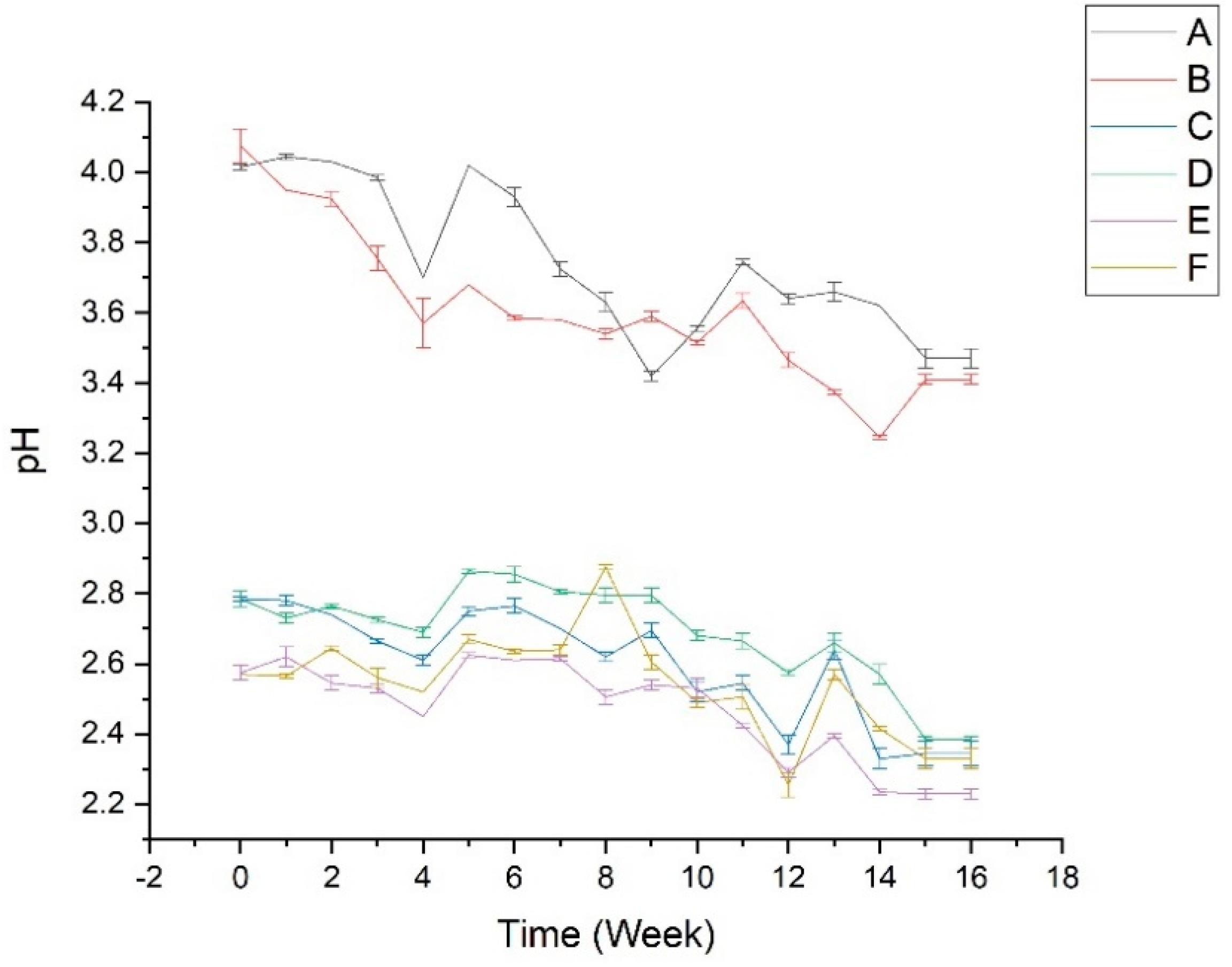


| Sample (Extract) | Addition of Ascorbic Acid | Stored Temperature |
|---|---|---|
| A | - | RT (27.0 + 1.0 °C) |
| B | - | 40 + 0.5 °C |
| C | 10%, w/w | RT (27.0 + 1.0 °C) |
| D | 10%, w/w | 40 + 0.5 °C |
| E | 15%, w/w | RT (27.0 + 1.0 °C) |
| F | 15%, w/w | 40 + 0.5 °C |
| Cinnamon Extract | Extract Weight (g) | Yield (%) a | Qualitative Flavonoid Test b | Quantitative Flavonoid Content (mg QE/g Extract) c | IC50 Antioxidant (mg/mL) d | Remarks of Antioxidant Activity e | |
|---|---|---|---|---|---|---|---|
| Shinoda | Acetophenone | ||||||
| EtOH | 10.75 | 21.50 | +++ | +++ | 0.0179 + 7.9 × 10−5 | 0.0162 + 7.5 × 10−4 | Strong |
| EtOAc | 1.04 | 2.08 | ++ | ++ | 0.0177 + 2.1 × 10−4 | 0.1069 + 1.7 × 10−3 | Weak |
| Acetone | 6.91 | 13.82 | + | + | 0.0066 + 3.1 × 10−4 | 0.0397 + 7.0 × 10−4 | Strong |
| Cinnamon Extract | Extract Weight (g) | Yield (%) a | Quantitative Flavonoid Content (mg QE/g Extract) | IC50 Antioxidant (mg/mL) b | Remarks of Antioxidant Activity c |
|---|---|---|---|---|---|
| EtOH (large-scale) | 787.984 | 13.13 | 9.852 + 0.309 | 0.0259 + 1.8 × 10−3 | Strong |
| Analysis | Compound Group | Compound | Relative Abundance (%) | m/z | Retention Time (min) |
|---|---|---|---|---|---|
| GC-MS | Phenylpropanoid | (E)-Cinnamaldehyde | 36.71 + 4.67 | 132 | 9.38 |
| Cinnamyl alcohol | 56.09 + 5.31 | 134 | 9.83 | ||
| Coumarin | 10.63 + 5.00 | 146 | 11.38 | ||
| 3,4-Dihydrocoumarin | 12.08 + 0.59 | 148 | 15.70 | ||
| LC-MS | Phenylpropanoid | (E)-Cinnamaldehyde | 21.24 + 0.64 | 132 | 18.10 |
| Cinnamyl alcohol | 10.55 + 1.87 | 134 | 10.44 | ||
| Coumarin | 9.43 + 3.44 | 146 | 6.77 | ||
| 3,4-Dihydrocoumarin | 1.00 + 0.52 | 148 | 13.34 | ||
| Flavonoids | Kaempferol | 8.37 + 0.89 | 286 | 8.79 | |
| Procyanidin dimer | 16.05 + 3.20 | 578 | 14.60 | ||
| Procyanidin trimer | 31.18 + 3.78 | 864 | 4.47 | ||
| Terpenoid | Linalool | 4.21 + 0.57 | 154 | 21.32 |
| Sample (Extract) | Week 0–7 | Week 8–16 | ||||
|---|---|---|---|---|---|---|
| Odor | Texture | Color | Odor | Texture | Color | |
| A | Strong | Viscous | Dark brown | Strong | Hard, thick | Dark brown |
| B | Strong | Viscous | Dark brown | Strong | Hard, thick | Dark brown |
| C | Slightly strong | Slightly viscous | Reddish brown | Slightly strong | Viscous | Reddish brown |
| D | Slightly strong | Slightly viscous | Reddish brown | Slightly strong | Viscous | Reddish brown |
| E | Slightly strong | Slightly viscous | Reddish brown | Slightly strong | Viscous | Reddish brown |
| F | Slightly strong | Slightly viscous | Reddish brown | Slightly strong | Viscous | Reddish brown |
| Compound | Relative Abundance (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample E | Sample F | |||||||||||||
| W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | |
| (E)-Cinnamaldehyde | 33.4 | 13.5 | 12.7 | 40.0 | 4.2 | 3.0 | 29.6 | 10.1 | 9.1 | 37.4 | 27.4 | 24.7 | 24.2 | 23.1 | 14.5 | 28.8 | 21.8 | 16.4 |
| Cinnamyl alcohol | 11.7 | - | - | 12.5 | - | - | 14.1 | - | - | 13.0 | - | - | 12.2 | - | - | 12.9 | - | - |
| Coumarin | 59.8 | 25.0 | 24.3 | 52.3 | 10.0 | 7.7 | 60.8 | 37.6 | 36.9 | 57.0 | 35.3 | 21.3 | 46.1 | 21.5 | 11.3 | 45.0 | 41.6 | 25.4 |
| 3,4-Dihydrocoumarin | 7.1 | 61.5 | 64.7 | 14.2 | 50.7 | 90.3 | 8.5 | 52.3 | 54.0 | 8.7 | 37.3 | 54.0 | 27.5 | 55.2 | 74.2 | 24.4 | 36.6 | 58.2 |
| (E)-Cinnamic acid | - | - | - | - | 35.1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Compound | Relative Abundance (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample E | Sample F | |||||||||||||
| W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | W0 | W8 | W16 | |
| (E)-Cinnamaldehyde | 21.3 | 18.1 | 15.4 | 21.6 | 15.3 | 10.2 | 18.2 | 10.8 | 9.9 | 18.2 | 12.5 | 11.3 | 14.2 | 14.4 | 9.3 | 17.7 | 8.7 | 4.3 |
| Cinnamyl alcohol | 9.1 | 4.4 | 1.2 | 12.0 | 4.1 | 3.9 | 9.9 | 7.2 | 1.7 | 12.6 | 9.0 | 1.0 | 8.2 | 4.0 | 3.7 | 10.2 | 3.6 | 1.5 |
| Coumarin | 11.1 | 7.7 | 1.9 | 6.6 | 5.8 | 3.7 | 12.7 | 7.8 | 5.0 | 16.8 | 14.0 | 3.1 | 11.9 | 6.9 | 4.2 | 11.9 | 7.5 | 4.3 |
| 3,4-Dihydrocoumarin | 1.2 | 5.5 | 6.3 | 1.3 | 7.5 | 8.3 | 2.6 | 10.8 | 11.6 | 1.1 | 7.6 | 7.5 | 3.2 | 10.3 | 12.6 | 1.5 | 9.9 | 10.6 |
| Kaempferol | 9.1 | 4.9 | - | 8.5 | 7.7 | - | 8.2 | 7.2 | - | 10.6 | 1.2 | - | 6.6 | 1.2 | - | 14.3 | 2.5 | - |
| Procyanidin dimer | 18.0 | 30.2 | 45.1 | 14.4 | 21.8 | 25.4 | 12.7 | 25.0 | 25.4 | 16.8 | 31.3 | 64.8 | 23.4 | 40.5 | 53.0 | 18.4 | 32.2 | 35.6 |
| Procyanidin trimer | 34.0 | 19.8 | 1.3 | 28.7 | 17.7 | 3.3 | 27.2 | 16.3 | 2.5 | 25.9 | 18.9 | 1.2 | 31.0 | 15.7 | 1.5 | 30.8 | 14.4 | 1.3 |
| Linalool | 5.1 | 4.9 | 5.5 | 4.8 | 4.1 | 18.4 | 8.2 | 11.2 | 15.1 | 7.4 | 8.8 | 7.5 | 7.2 | 11.4 | 12.3 | 4.4 | 10.6 | 12.6 |
| Ascorbic acid | - | - | - | - | - | - | 11.2 | 4.3 | 3.7 | 11.1 | 4.4 | 1.2 | 12.8 | 4.0 | 3.4 | 13.3 | 3.6 | 1.4 |
| (E)-Cinnamic acid | - | 1.3 | 2.3 | - | 1.3 | 4.2 | - | 1.2 | 5.0 | - | 1.3 | 3.1 | - | 1.4 | 4.3 | - | 1.7 | 4.3 |
| Dehydroascorbic acid | - | - | - | - | - | - | - | 5.4 | 1.6 | - | 5.8 | 1.1 | - | 3.7 | 1.0 | - | 3.5 | 1.5 |
| 2,3-Diketogulonic acid | - | - | - | - | - | - | - | 1.5 | 1.3 | - | 1.2 | 1.1 | - | 2.6 | 1.0 | - | 1.4 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, D.U.C.; Hakim, R.A.; Mawarni, S.A.; Satriani, A.R. Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid. Cosmetics 2022, 9, 57. https://doi.org/10.3390/cosmetics9030057
Rahayu DUC, Hakim RA, Mawarni SA, Satriani AR. Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid. Cosmetics. 2022; 9(3):57. https://doi.org/10.3390/cosmetics9030057
Chicago/Turabian StyleRahayu, Dyah Utami Cahyaning, Regina Ainunnisa Hakim, Shofi Airiza Mawarni, and Andhina Rizkya Satriani. 2022. "Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid" Cosmetics 9, no. 3: 57. https://doi.org/10.3390/cosmetics9030057
APA StyleRahayu, D. U. C., Hakim, R. A., Mawarni, S. A., & Satriani, A. R. (2022). Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid. Cosmetics, 9(3), 57. https://doi.org/10.3390/cosmetics9030057







