Application of Electrochemically Reduced Water for New No-Rinse Shampoo: Design and Optimization Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Preparation Method
2.3. Measurement of Vesicle Size, Size Distribution, Zeta Potential, and Conductivity
2.4. pH Measurement
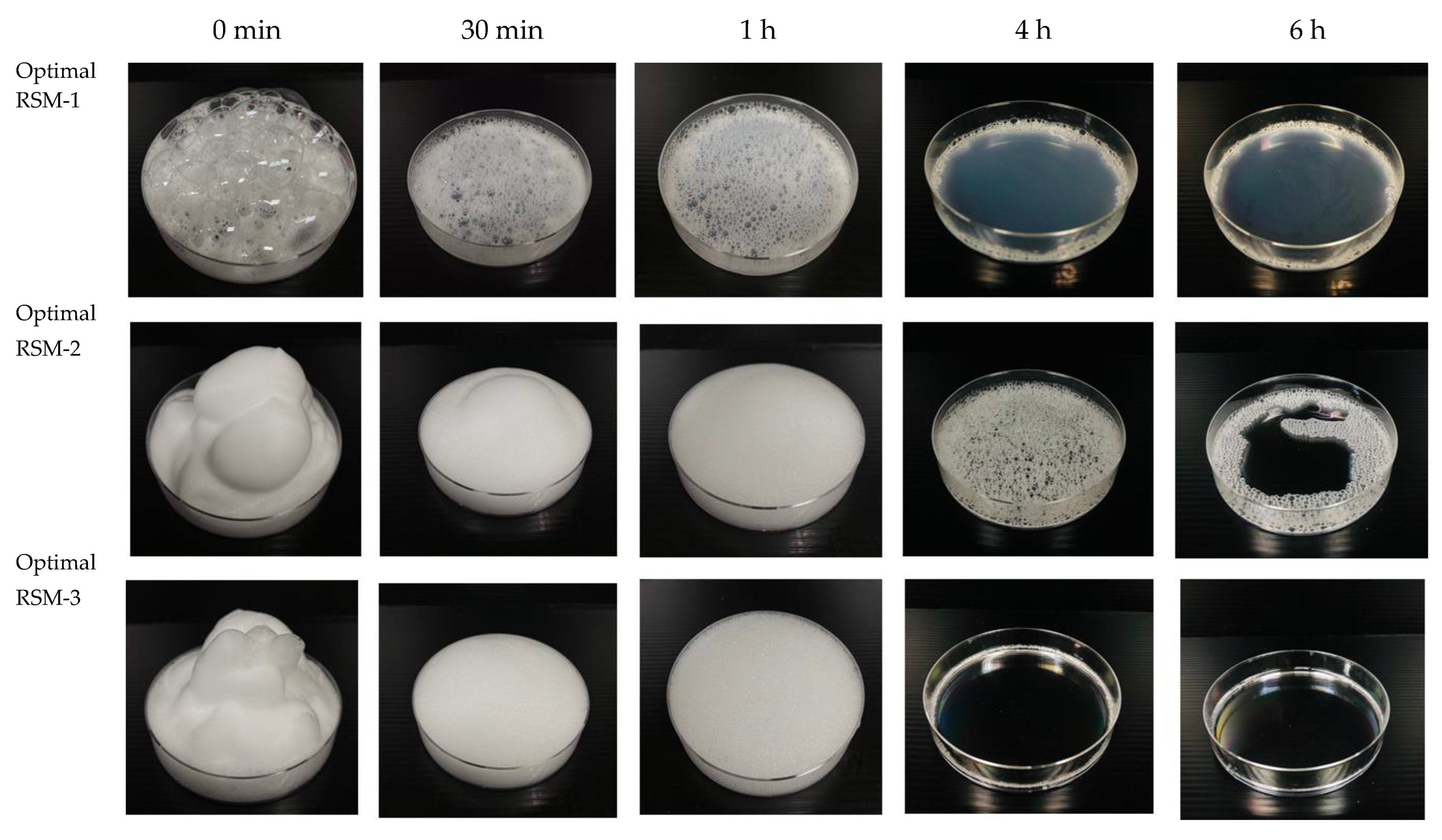
2.5. Foamability Measurement
2.6. Wetting Time Measurement
2.7. Dirt Dispersion Measurement
2.8. Turbidity Measurement
2.9. Stability Testing
2.10. Antibacterial and Antifungal Test
2.11. Determination of Optimal Formulation
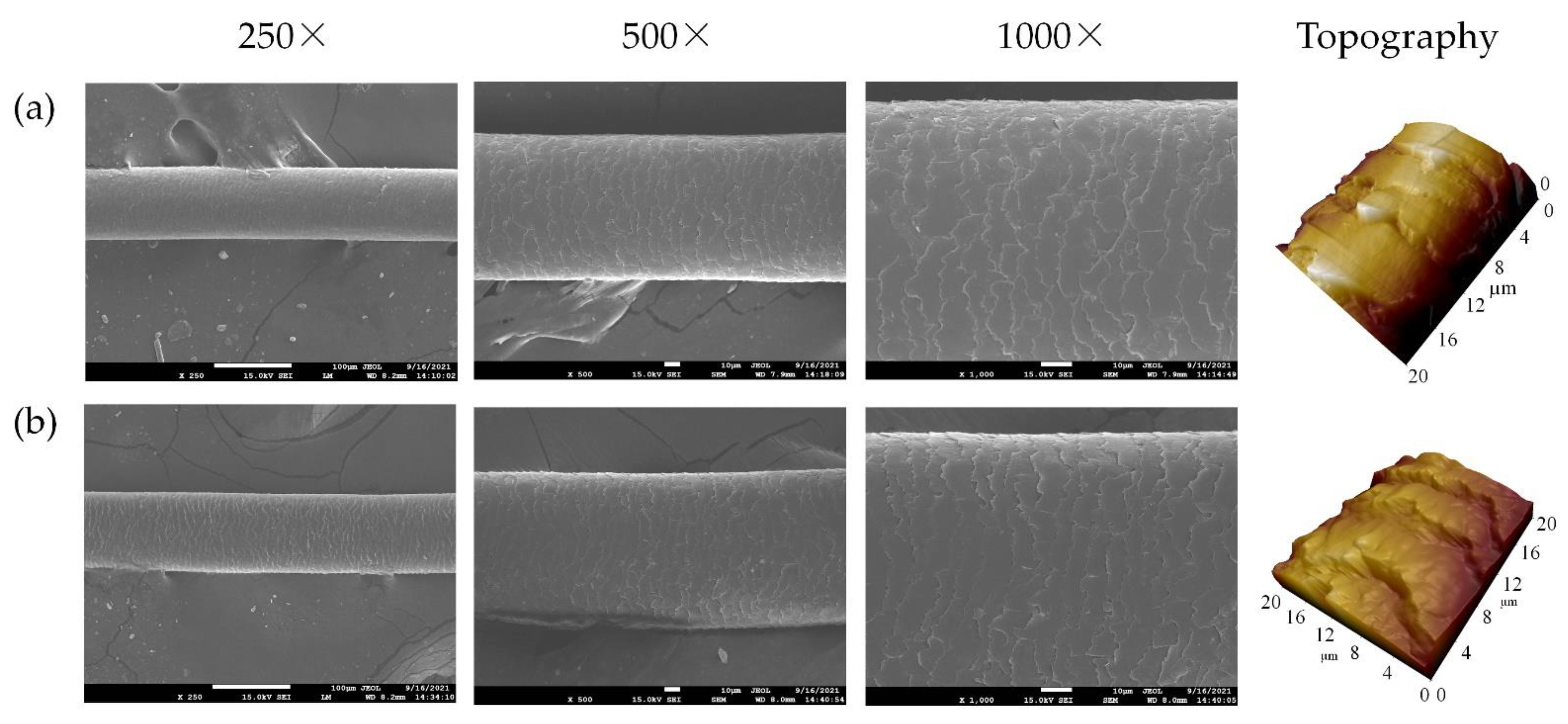
2.12. Scanning Electron Microscopy (SEM) Analysis
2.13. Atomic Force Microscopy (AFM)
2.14. Ethics in the Animal Study
2.15. Computer Programs
3. Results
3.1. Preliminary Study
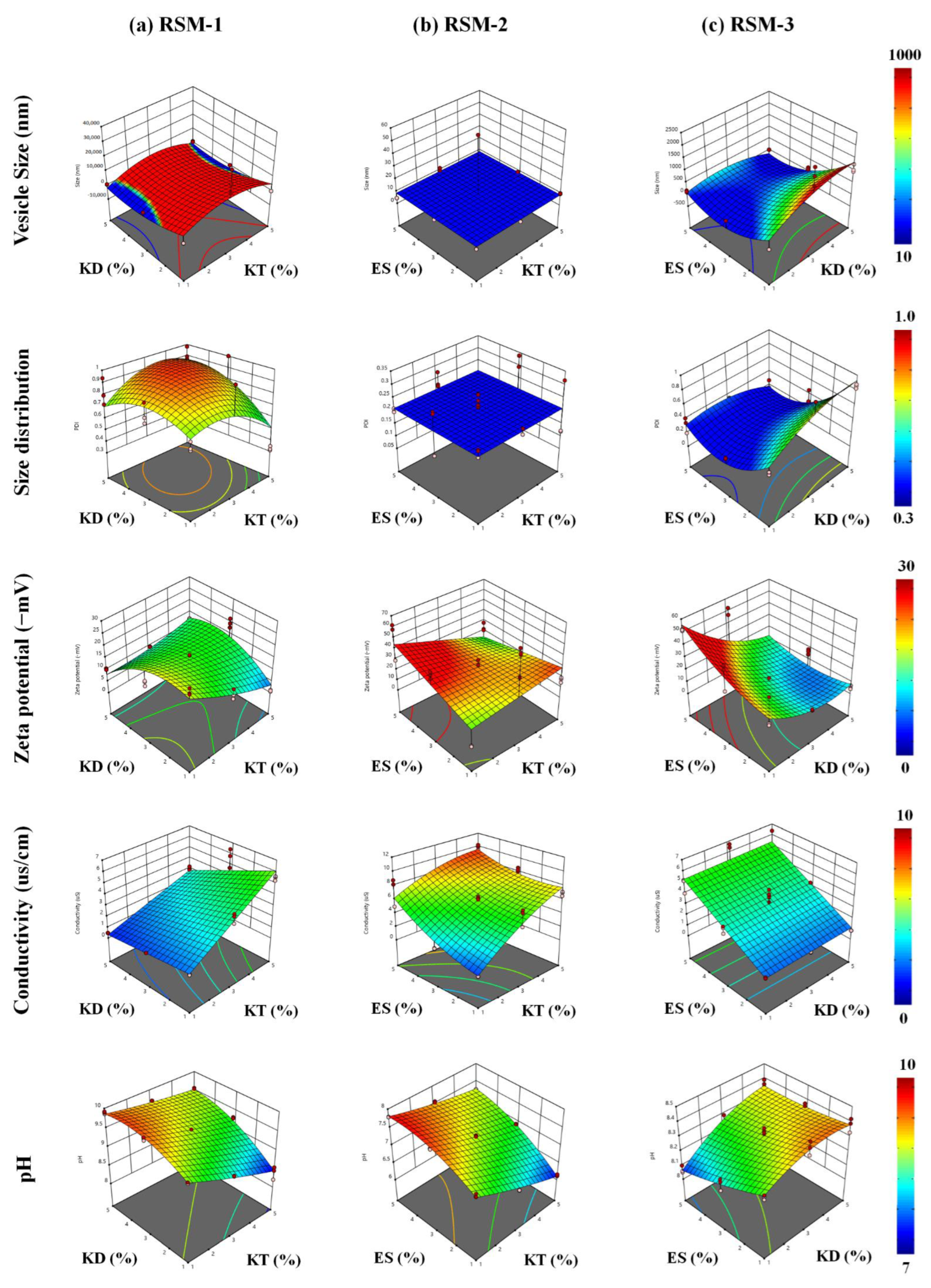
3.2. Identification of the Response Surface by RSM
3.3. Formulation Optimization Using RSM
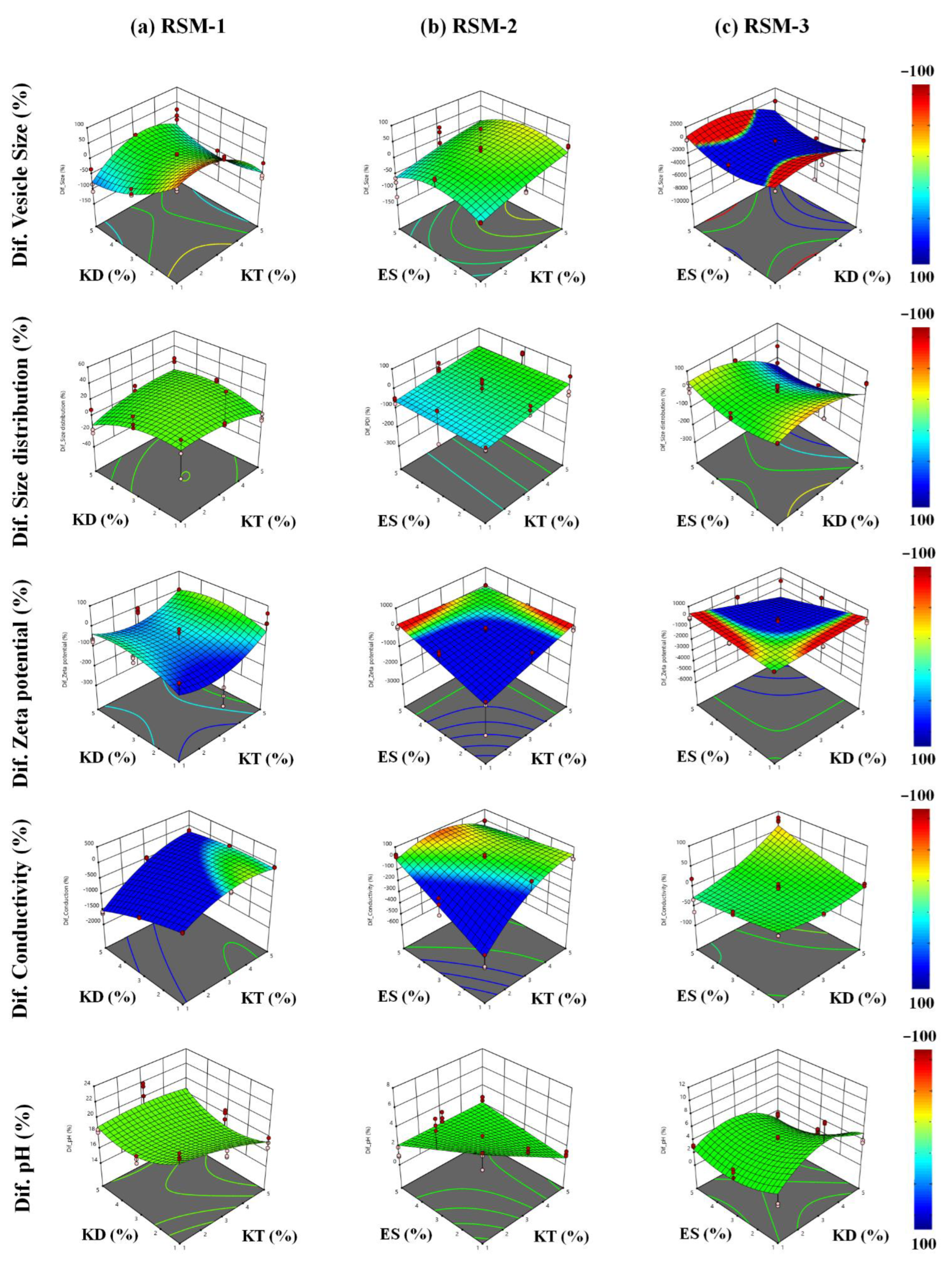
3.4. Comparative Study
4. Discussion
4.1. Preliminary Study
4.2. Identification of the Response Surface by RSM
4.3. Formulation Optimization Using RSM
4.4. Comparative Study
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
Appendix C
Appendix D
References
- World Health Organization. Critical Preparedness, Readiness and Response Actions for COVID-19-7. Available online: https://www.who.int/publications-detail/critical-preparedness-readiness-and-response-actions-for-covid-19 (accessed on 6 April 2020).
- Jakhar, D.; Kaur, I.; Kandhari, R.; Kaul, S.; Garg, P.; Bansal, S. Hair care during COVID-19: Practical tips for health care workers. Indian J. Med. Sci. 2020, 72, 114–115. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Badali, H.; Khodavaisy, S. Opportunistic Fungal Infections in the Epidemic Area of COVID-19: A Clinical and Diagnostic Perspective from Iran. Mycopathologia 2020, 185, 607–611. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Pohl, C.H. Opportunistic pathogenic fungal co-infections are prevalent in critically ill COVID-19 patients: Are they risk factors for disease severity? South Afr. Med. J. 2020, 110, 1081. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo, A Review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, K.; Machado, J.; Carvalho, V.; Soares, F.; Matos, D.; Castro, M.; Pereira, F.; Lopes, H. A New Methodology for Use by a Single Caregiver to Bathe Bedridden Elderly Persons Using Advanced Mechatronic Systems. Healthcare 2019, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Daliri, E.B.-M.; Oh, D.-H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Yan, H.; Hamasaki, T.; Kinjo, T.; Nakamichi, N.; Teruya, K.; Kabayama, S.; Shirahata, S. Electrochemically Reduced Water Protects Neural Cells from Oxidative Damage. Oxidative Med. Cell. Longev. 2014, 2014, 869121. [Google Scholar] [CrossRef]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef]
- Tomás, M.; Agonia, A.S.; Borges, L.; de Oliveira, A.P.; de Oliveira, R.P. Isothiazolinones Quantification in Shampoo Matrices: A Matter of Method Optimization or Stability Driven by Interactions? Cosmetics 2020, 7, 4. [Google Scholar] [CrossRef]
- Rele, A.S.; Mohile, R.B. Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. J. Cosmet. Sci. 2003, 54, 175–192. [Google Scholar] [PubMed]
- Cosmetic Ingredient Review (CIR). Final report on the safety assessment of cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA. J. Am. Coll. Toxicol. 1986, 5, 415–454. [Google Scholar] [CrossRef]
- Gholami, A.; Golestaneh, M.; Andalib, Z. A new method for determination of cocamidopropyl betaine synthesized from coconut oil through spectral shift of Eriochrome Black T. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 192, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Cocamidopropyl betaine (CAPB). Int. J. Toxicol. 2012, 31, 77S–111S. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Amino Acid Alkyl Amides as Used in Cosmetics. Int. J. Toxicol. 2017, 36, 17S–56S. [Google Scholar] [CrossRef]
- Cui, X.; Jiang, Y.; Yang, C.; Lu, X.; Chen, H.; Mao, S.; Liu, M.; Yuan, H.; Luo, P.; Du, Y. Mechanism of the Mixed Surfactant Micelle Formation. J. Phys. Chem. B 2010, 114, 7808–7816. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modified magnetic nanoadsorbents (sMNP)—An endeavor to diminish probable cancer risk. Sci. Rep. 2019, 9, 18339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Gama, R.M.; Baby, A.R.; Velasco, M.V.R. In Vitro Methodologies to Evaluate the Effects of Hair Care Products on Hair Fiber. Cosmetics 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Deeksha; Malviya, R.; Sharma, P.K. Advancement in Shampoo (A Dermal Care Product): Preparation Methods, Patents and Commercial Utility. Recent Patents Inflamm. Allergy Drug Discov. 2014, 8, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Kaushal, B.; Ghode, S. Formulations and evaluation of herbal shampoo and comparative studies with herbal marketed shampoo. Int. J. Pharma. Bio. Sci. 2012, 3, P638–P645. [Google Scholar]
- Rehan, F.; Ahemad, N.; Islam, R.A.; Gupta, M.; Gan, S.H.; Chowdhury, E.H. Optimization and Formulation of Nanostructured and Self-Assembled Caseinate Micelles for Enhanced Cytotoxic Effects of Paclitaxel on Breast Cancer Cells. Pharmaceutics 2020, 12, 984. [Google Scholar] [CrossRef]
- Okajima, M. The new technology and practical application of special electrolyzed deoxidized and ionized water with negative ion. Negat. Ion Pract. Soc. Jpn. 2002, 2, 1–14. [Google Scholar]
- Takayama, K.; Obata, Y.; Morishita, M.; Nagai, T. Multivariate spline interpolation as a novel method to optimize pharmaceutical formulations. Die Pharm. 2004, 59, 392–395. [Google Scholar]
- Kikuchi, S.; Takayama, K. Reliability assessment for the optimal formulations of pharmaceutical products predicted by a nonlinear response surface method. Int. J. Pharm. 2009, 374, 5–11. [Google Scholar] [CrossRef]
- Kikuchi, S.; Takayama, K. Multivariate statistical approach to optimizing sustained-release tablet formulations containing diltiazem hydrochloride as a model highly water-soluble drug. Int. J. Pharm. 2010, 386, 149–155. [Google Scholar] [CrossRef]
- Dos Santos, J.D.; Edwards, H.G.; de Oliveira, L.F.C. Raman spectroscopy and electronic microscopy structural studies of Caucasian and Afro human hair. Heliyon 2019, 5, e01582. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-H.; Lim, J.H.; Kil Son, S.; Choi, J.; Kang, N.-G.; Lee, S.-M. Prevention of lipid loss from hair by surface and internal modification. Sci. Rep. 2019, 9, 9834. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pal, R. Investigation of Surfactant-Polymer Interactions Using Rheology and Surface Tension Measurements. Polymers 2020, 12, 2302. [Google Scholar] [CrossRef]
- Mainkar, A.R.; Jolly, C.I. Evaluation of commercial herbal shampoos. Int. J. Cosmet. Sci. 2000, 22, 385–391. [Google Scholar] [CrossRef]
- Duangjit, S.; Nimcharoenwan, T.; Chomya, N.; Locharoenrat, N.; Ngawhirunpat, T. Computational design strategy: An approach to enhancing the transdermal delivery of optimal capsaicin-loaded transinvasomes. Drug Dev. Ind. Pharm. 2016, 43, 98–107. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, B.-K. Antibacterial effect of electrolyzed water on oral bacteria. J. Microbiol. 2006, 44, 417–422. [Google Scholar] [PubMed]
- Willems, N.; Houwers, D.J.; Schlotter, Y.M.; Theelen, B.; Boekhout, T. Disseminated Candidiasis in a Young, Previously Healthy, Dog and Review of Literature. Mycopathologia 2017, 182, 591–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Carpena, P.; Aguiar, J.; Bernaola-Galván, A.P.; Ruiz, C.C. Problems Associated with the Treatment of Conductivity−Concentration Data in Surfactant Solutions: Simulations and Experiments. Langmuir 2002, 18, 6054–6058. [Google Scholar] [CrossRef]
- Inoue, T.; Ebina, H.; Dong, B.; Zheng, L. Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J. Colloid Interface Sci. 2007, 314, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Berriche, L.; Badache, L.; Benhariz, S.H.; Gharbi, A.; Talhi, W. Mixed micellization and surface properties of non-ionic/cationic surfactants. J. Dispers. Sci. Technol. 2018, 40, 378–389. [Google Scholar] [CrossRef]
- Xue, Y.; Qian, C.; Wang, Z.; Xu, J.-H.; Yang, R.; Qi, H. Investigation of extractive microbial transformation in nonionic surfactant micelle aqueous solution using response surface methodology. Appl. Microbiol. Biotechnol. 2009, 85, 517–524. [Google Scholar] [CrossRef]
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric micelles for delivery of poorly soluble drugs: Preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target. 2005, 13, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vakilinezhad, M.A.; Tanha, S.; Montaseri, H.; Dinarvand, R.; Azadi, A.; Javar, H.A. Application of Response Surface Method for Preparation, Optimization, and Characterization of Nicotinamide Loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2018, 8, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Abismaıl, B.; Canselier, J.; Wilhelm, A.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Al-Qahtani, H.; Al-Sharafi, A.; Bahattab, S.; Hassan, G.; Al-Aqeeli, N.; Kassas, M. Environmental Dust Particles Repelling from A Hydrophobic Surface under Electrostatic Influence. Sci. Rep. 2019, 9, 8703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Vonarbourg, A.; Passirani, C.; Desigaux, L.; Allard, E.; Saulnier, P.; Lambert, O.; Benoit, J.-P.; Pitard, B. The encapsulation of DNA molecules within biomimetic lipid nanocapsules. Biomaterials 2009, 30, 3197–3204. [Google Scholar] [CrossRef]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Development of Determining the Critical Micelle Concentration of Surfactants by a Simple and Fast Titration Method. Anal. Chem. 2019, 92, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.F.; Costa, C.C.; Gomes, A.C.; Matamá, T.; Cavaco-Paulo, A. Human Hair and the Impact of Cosmetic Procedures: A Review on Cleansing and Shape-Modulating Cosmetics. Cosmetics 2016, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.F.R.G.; Pichler, J.; Adriano, A.R.; Cecato, P.; de Almeida, A.M. The shampoo pH can affect the hair: Myth or Reality? Int. J. Trichology 2014, 6, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, K.; Muthukrishnan, N.; Hariprasad, K.S. Surface roughness parameters optimization in machining A356/SiC/20p metal matrix composites by PCD tool using response surface methodology and desirability function. Mach. Sci. Technol. 2008, 12, 529–545. [Google Scholar] [CrossRef]
- Lorza, R.L.; Calvo, M.Á.M.; Labari, C.B.; Fuente, P.J.R. Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals 2018, 8, 711. [Google Scholar] [CrossRef] [Green Version]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; El Hamri, R.; Taitai, A. Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using Box–Behnken design and desirability function. Appl. Surf. Sci. 2012, 258, 4402–4410. [Google Scholar] [CrossRef]
- Khodadoust, S.; Hadjmohammadi, M. Determination of N-methylcarbamate insecticides in water samples using dispersive liquid–liquid microextraction and HPLC with the aid of experimental design and desirability function. Anal. Chim. Acta 2011, 699, 113–119. [Google Scholar] [CrossRef]
- Krogh, H.V.; Kristensen, S. A study of skin diseases in dogs and cats. II. Microflora of the normal skin of dogs and cats. Nord. Vet. Med. 1976, 28, 459–463. [Google Scholar]
- Santin, R.; Mattei, A.S.; Waller, S.B.; Madrid, I.M.; Cleff, M.B.; Xavier, M.O.; de Oliveira Nobre, M.; Nascente, P.D.S.; De Mello, J.R.B.; Meireles, M.C.A. Clinical and mycological analysis of dog’s oral cavity. Braz. J. Microbiol. 2013, 44, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Krasowska, A.; Biegalska, A.; Lukaszewicz, M.; Krasowska, A.; Łukaszewicz, M. Comparison of antimicrobial activity of three commercially used quaternary ammonium surfactants. Sepsis 2012, 5, 170–174. [Google Scholar] [CrossRef]
- Do, E.; Lee, H.G.; Park, M.; Cho, Y.-J.; Kim, D.H.; Park, S.-H.; Eun, D.; Park, T.; An, S.; Jung, W.H. Antifungal Mechanism of Action of Lauryl Betaine Against Skin-Associated Fungus. Malassezia Restricta. Mycobiol. 2019, 47, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Chan, J. Anti-Microbial Properties of Amino Acid-Based Surfactants. Available online: https://www.linkedin.com/pulse/anti-microbial-properties-amino-acid-based-surfactants-joseph-chan (accessed on 27 July 2021).
- Okajima, M.; Kanzaki, M.; Ishibashi, Y.; Wada, Y.; Hino, F.; Kitahara, Y.; Shimokawa, K.-I.; Ishii, F. In vitro bactericidal activity against periodontopathic bacteria by electrolyzed ion-reduced water. Drug Discov. Ther. 2011, 5, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef]

| Form. | Code | RSM-1 | RSM-2 | RSM-3 | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | KT (%) [+/−] | KD (%) [0] | KT (%) [+/−] | ES (%) [−] | KD (%) [0] | ES (%) [−] | |
| F1 | −1 | −1 | 1 | 1 | 1 | 1 | 1 | 1 |
| F2 | −1 | 0 | 1 | 3 | 1 | 3 | 1 | 3 |
| F3 | −1 | 1 | 1 | 5 | 1 | 5 | 1 | 5 |
| F4 | 0 | −1 | 3 | 1 | 3 | 1 | 3 | 1 |
| F5 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| F6 | 0 | 1 | 3 | 5 | 3 | 5 | 3 | 5 |
| F7 | 1 | −1 | 5 | 1 | 5 | 1 | 5 | 1 |
| F8 | 1 | 0 | 5 | 3 | 5 | 3 | 5 | 3 |
| F9 | 1 | 1 | 5 | 5 | 5 | 5 | 5 | 5 |
| F10 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| F11 | 0 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| Optimal Formulation | KD (%) [0] | KT (%) [+/−] | ES (%) [−] | Size (nm) | PDI | Zeta Potential (−mV) | Conductivity (µS/cm) | pH | Foamability | Wetting Time (min) | Desirability |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RSM-1 | 2.2 | 1.0 | - | 61.7 | 0.8 | 22.5 | 0.9 | 9.7 | 0.98 | 16.4 | 0.69 |
| RSM-2 | - | 2.3 | 3.7 | 8.6 | 0.2 | 30.2 | 5.7 | 7.5 | 0.98 | 13.3 | 0.66 |
| RSM-3 | 1.7 | - | 4.1 | 3.6 | 0.2 | 32.6 | 4.3 | 8.2 | 0.98 | 18.9 | 0.79 |
| Optimal Formulation | Size (nm) | PDI | Zeta Potential (−mV) | Conductivity (µS/cm) | pH | Foamability | Wetting Time (min) | Turbidity | Dirt Dispersion (cm) |
|---|---|---|---|---|---|---|---|---|---|
| RSM-1 | 407.17 ± 11.55 # | 0.65 ± 0.06 | 20.73 ± 0.93 * | 1.01 ± 0.03 * | 9.6 ± 0.01 * | 0.98 * | 0.11 ± 0.02 | 0.788 ± 0.00 | 0.45 ± 0.05 |
| RSM-2 | 4.38 ± 0.05 | 0.13 ± 0.02 | 32.90 ± 0.45 * | 6.01 ± 0.22 * | 7.5 ± 0.02 * | 0.98 * | 5.75 ± 1.12 | 0.012 ± 0.00 | 0.60 ± 0.10 |
| RSM-3 | 9.06 ± 0.25 | 0.38 ± 0.01 | 34.87 ± 14.81 * | 4.64 ± 0.98 * | 8.4 ± 0.00 * | 0.98 * | 3.14 ± 1.49 | 0.004 ± 0.00 | 0.77 ± 0.06 |
| Optimal Formulation | KT (%) | ES (%) | Lavender Oil (%) | Preservative (%) | ERW (%) | Aqua qs to (%) |
|---|---|---|---|---|---|---|
| DS1 | 2.3 | 3.7 | 0.1 | 1 | - | 100 |
| DS2 | 3.8 | 1.0 | 0.1 | 1 | - | 100 |
| DS3 | 0.5 | 3.0 | 0.1 | 1 | - | 100 |
| DS4 | 0.5 | 3.0 | 0.1 | 1 | 50 | 100 |
| DS5 | n/a | n/a | n/a | n/a | - | 100 |
| E. coli | (CFU/mL) | |||
|---|---|---|---|---|
| Groups | 0 h | 2 h | 6 h | 24 h |
| DS1 | 0 | 0 | 0 | 0 |
| DS2 | 0 | 0 | 0 | 0 |
| DS3 | 2.98 ± 0.5 × 10⁶ | 1.20 ± 0.33 × 10⁶ | 9.3 ± 3.2 × 104 | 0 |
| DS4 | 0 | 0 | 0 | 0 |
| DS5 | 3.10 ± 0.14 × 10⁶ | 8.50 ± 0.70 × 102 | 0 | 0 |
| 125 ug/mL Ampicillin | >3.0 × 10⁶ | 1.31 ± 0.42 × 104 | 1.95 ± 0.95 × 104 | 1.00 ± 0.02 × 103 |
| 250 ug/mL Ampicillin | >3.0 × 10⁶ | 4.3 ± 0.28 × 105 | 2.55 ± 0.84 × 105 | 3.20 ± 0.85 × 103 |
| Negative | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ |
| S. epidermidis | ||||
| DS1 | 0 | 0 | 0 | 0 |
| DS2 | 0 | 0 | 0 | 0 |
| DS3 | >3.0 × 10⁶ | 2.30 ± 0.04 × 106 | 2.93 ± 1.51 × 105 | 1.19 ± 1.03 × 103 |
| DS4 | 0 | 0 | 0 | 0 |
| DS5 | 3.65 ± 0.06 x105 | 0 | 0 | 0 |
| 125 ug/mL Ampicillin | >3.0 × 10⁶ | 6.60 ± 1.98 × 105 | 6.35 ± 1.20 × 104 | 2.01 ± 1.83 × 103 |
| 250 ug/mL Ampicillin | >3.0 × 10⁶ | 1.08 ± 0.34 × 105 | 3.82 ± 0.21 × 104 | 1.05 ± 0.07 × 103 |
| Negative | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ |
| S. aureus | ||||
| DS1 | 3.86 ± 0.40 × 105 | 1.00 × 103 | 0 | 0 |
| DS2 | 1.13 ± 0.71 × 104 | 2.00 ±1.41 × 103 | 0 | 0 |
| DS3 | 2.52 ± 0.67 × 106 | 2.26 ± 0.91 × 106 | 8.70 ± 2.88 × 105 | 2.79 ± 2.12 × 105 |
| DS4 | >3.0 × 10⁶ | 3.72 ± 0.62 × 105 | 2.21 ± 0.70 × 105 | 1.25 ± 1.89 × 101 |
| DS5 | 1.07 ± 0.09 × 106 | 1.45 ± 0.78 × 103 | 1.25 ± 0.21 × 102 | 0 |
| 125 ug/mL Ampicillin | 6.91 ± 0.57 × 106 | 5.11 ± 0.85 × 106 | 3.78 ± 0.49 × 106 | 4.00 × 104 |
| 250 ug/mL Ampicillin | >3.0 × 10⁶ | 4.86 ± 0.76 × 106 | >3.0 × 10⁶ | 2.17 ± 0.85 × 105 |
| 250 ug/mL Tetracycline | >3.0 × 10⁶ | 2.30 ± 0.21 × 105 | 0 | 0 |
| Negative | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ | >3.0 × 10⁶ |
| C. albicans | ||||
| DS1 | 7.65 ± 1.91 × 104 | 0 | 0 | 0 |
| DS2 | 1.21 ± 0.64 × 104 | 0 | 0 | 1.31 ± 1.53 × 103 |
| DS3 | 1.84 × 105 | 4.30 × 105 | 7.82 × 104 | 7.75 × 104 |
| DS4 | 1.70 ± 0.55 × 105 | 2.04 ± 0.70 × 104 | 1.15 ± 1.32 × 104 | 3.28 ± 3.27 × 103 |
| DS5 | 1.52 ± 0.28 × 104 | 3.26 ± 0.17 x104 | 0 | 0 |
| 2 ug/mL amphotericin B | 1.49 ± 0.23 × 105 | 2.01 ± 0.31 × 103 | 3.05 ± 1.07 × 102 | 1.73 ± 0.16 × 104 |
| Negative | 1.57 ± 0.29 × 105 | 2.00 ± 0.08 × 105 | >3.0 × 10⁶ | >3.0 × 10⁶ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangjit, S.; Sritananuwat, P.; Bumrungthai, S.; Ngawhirunpat, T.; Takayama, K. Application of Electrochemically Reduced Water for New No-Rinse Shampoo: Design and Optimization Using Response Surface Methodology. Cosmetics 2022, 9, 104. https://doi.org/10.3390/cosmetics9050104
Duangjit S, Sritananuwat P, Bumrungthai S, Ngawhirunpat T, Takayama K. Application of Electrochemically Reduced Water for New No-Rinse Shampoo: Design and Optimization Using Response Surface Methodology. Cosmetics. 2022; 9(5):104. https://doi.org/10.3390/cosmetics9050104
Chicago/Turabian StyleDuangjit, Sureewan, Phaijit Sritananuwat, Sureewan Bumrungthai, Tanasait Ngawhirunpat, and Kozo Takayama. 2022. "Application of Electrochemically Reduced Water for New No-Rinse Shampoo: Design and Optimization Using Response Surface Methodology" Cosmetics 9, no. 5: 104. https://doi.org/10.3390/cosmetics9050104
APA StyleDuangjit, S., Sritananuwat, P., Bumrungthai, S., Ngawhirunpat, T., & Takayama, K. (2022). Application of Electrochemically Reduced Water for New No-Rinse Shampoo: Design and Optimization Using Response Surface Methodology. Cosmetics, 9(5), 104. https://doi.org/10.3390/cosmetics9050104






