Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care
Abstract
:1. Introduction
2. Chitosan: Physicochemical and Functional Properties
2.1. Physicochemical Properties
2.2. Functional Properties
2.2.1. Antimicrobial Activity
2.2.2. Antioxidant Activity
2.2.3. Mucoadhesive Properties
2.2.4. Penetration Enhancement
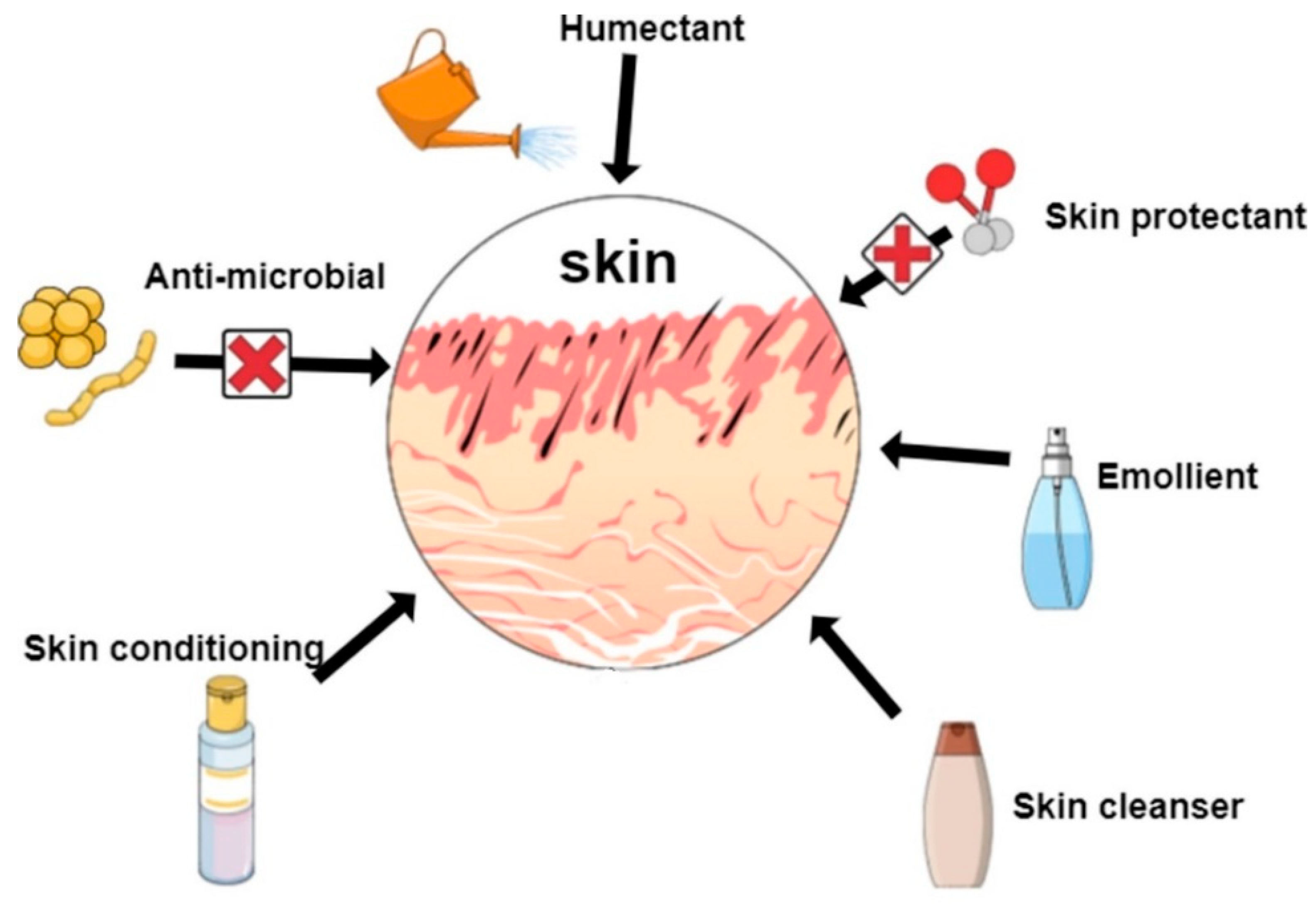
3. Chitosan in Skin and Hair Care
3.1. Chitosan as Ingredient in Skin Care Products
3.1.1. Chitosan Applications as a Humectant and Moisturizing Agent
3.1.2. Skin Aging
3.1.3. UV Protection
3.1.4. Skin Cleansing
3.1.5. Antibacterial Role
3.2. Chitosan as an Ingredient in Hair Care Products
4. Chitosan as Delivery Systems
5. Toxicity Aspects of Chitosan
6. Prospects and Challenges of Chitosan as Cosmetic Ingredients
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Mahesh, S.K.; Fathima, J.; Veena, V.G. Cosmetic Potential of Natural Products: Industrial Applications. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Swamy, M.K., Akhtar, M.S., Eds.; Springer: Singapore, 2019; pp. 215–250. [Google Scholar]
- Luengo, G.S.; Fameau, A.-L.; Léonforte, F.; Greaves, A.J. Surface science of cosmetic substrates, cleansing actives and formulations. Adv. Colloid Interface Sci. 2021, 290, 102383. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of polyelectrolytes and polyelectrolytes-surfactant mixtures at surfaces: A physico-chemical approach to a cosmetic challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Leonforte, F.; Serrano-Pueyo, A.; Regulski, K.; Tournier-Couturier, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of molecular structure of eco-friendly glycolipid biosurfactants on the adsorption of hair-care conditioning polymers. Colloids Surf. B 2020, 185, 110578. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Bowman, D.M.; van Calster, G.; Friedrichs, S. Nanomaterials and regulation of cosmetics. Nat. Nanotechnol. 2010, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The Role of Polymers in Cosmetics: Recent Trends. In Cosmetic Nanotechnology. Polymers and Colloids in Cosmetics; Morgan, S.E., Havelka, K.O., Lochhead, R.Y., Eds.; American Chemical Society: Washington, DC, USA, 2007; pp. 3–56. [Google Scholar] [CrossRef]
- Ricapito, N.G.; Ghobril, C.; Zhang, H.; Grinstaff, M.W.; Putnam, D. Synthetic Biomaterials from Metabolically Derived Synthons. Chem. Rev. 2016, 116, 2664–2704. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; Silva, C.F.d.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Lautenschläger, H. (Poly)Saccharides in cosmetic products—From alginate to xanthan gum. Kosmet. Prax. 2009, 2009, 12–15. [Google Scholar]
- L’Haridon, J.; Martz, P.; Chenéble, J.C.; Campion, J.F.; Colombe, L. Ecodesign of cosmetic formulae: Methodology and application. Int. J. Cosmet. Sci. 2018, 40, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Gawade, R.P.; Chinke, S.L.; Alegaonkar, P.S. Polymers in cosmetics. In Polymer Science and Innovative Applications. Materials, Techniques, and Future Developments; AlMaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 545–565. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.P.; Giauffret, J.Y.; Martini, M.C.; Gonnet, J.F.; Soulé, G. Safety evaluation of gums and thickeners used in cosmetic formulations. Int. J. Cosmet. Sci. 1982, 4, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Schefer, L.; Adamcik, J.; Mezzenga, R. Unravelling secondary structure changes on individual anionic polysaccharide chains by atomic force microscopy. Angew. Chem. Int. Ed. 2014, 53, 5376–5379. [Google Scholar] [CrossRef]
- Joanna, K.; Michał, P.; Anna, P. Osmotic properties of polysaccharides solutions. Solubility of polysaccharides. In Solubility of Polysaccharides; Xu, Z., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Cumpstey, I. Chemical modification of polysaccharides. ISRN Org. Chem. 2013, 2013, 417672. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Martino, P.D. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced Changes in the Fabrication of Multilayers of Poly(acrylic acid) and Chitosan: Fabrication, Properties, and Tests as a Drug Storage and Delivery System. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef]
- Guzmán, E.; Chuliá-Jordán, R.; Ortega, F.; Rubio, R.G. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly(acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef] [PubMed]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T. Chitin and chitin-related compounds in plant-fungal interactions. Mycology. 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Tahriri, M.; Rasoulianboroujeni, M.; Koturi, H.; Tayebi, L. Preparation of natural chitosan from shrimp shell with different deacetylation degree. Mater. Res. Innov. 2018, 22, 177–181. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of n-acetylation degree in chitosan using raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 223–269. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Cheba, B.A. Chitin and chitosan: Marine biopolymers with unique properties and versatile applications. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Gomes, L.P.; Andrade, C.T.; Del Aguila, E.M.; Alexander, C.; Paschoalin, V.M. Assessing the antimicrobial activity of chitosan nanoparticles by fluorescence-labeling. Int. J. Biotechnol. Bioeng. 2018, 12, 111–117. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Xia, W. One-step procedure for enhancing the antibacterial and antioxidant properties of a polysaccharide polymer: Kojic acid grafted onto chitosan. Int. J. Biol. Macromol. 2018, 113, 1125–1133. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Casettari, L.; Ciandrini, E.; Illum, L.; Baffone, W. Chitosans inhibit the growth and the adhesion of Klebsiella pneumoniae and Escherichia coli clinical isolates on urinary catheters. Int. J. Antimicrob. Agents 2017, 50, 135–141. [Google Scholar] [CrossRef]
- Demetgül, C.; Beyazit, N. Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives. Carbohydr. Polym. 2018, 181, 812–817. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheerj, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.; Khutoryanskiy, V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Moreno, J.A.S.; Mendes, A.C.; Stephansen, K.; Engwer, C.; Goycoolea, F.M.; Boisen, A.; Nielsen, L.H.; Chronakis, I.S. Development of electrosprayed mucoadhesive chitosan microparticles. Carbohydr. Polym. 2018, 190, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Praputbut, S.; Kompella, U.B.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of mucoadhesive chitosan-dextran sulfate nanoparticles into the porcine cornea. Colloids Surf. B 2017, 149, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Contri, R.; Fiel, L.; Alnasif, N.; Pohlmann, A.; Guterres, S.; Schäfer-Korting, M. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Kojima, T.; Kitano, H.; Niwa, M.; Saito, K.; Matsushita, Y.; Fukushima, K. Imaging analysis of cosmetic ingredients interacted with human hair using TOF-SIMS. Surf. Interface Anal. 2011, 43, 562–565. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Abedin, N.; Bashar, R.; Jimmy, A.N.; Khan, N.A. Unraveling Consumer Decisions towards Animal Ingredients in Personal-care Items: The Case of Dhaka City Dwellers. Am. J. Mark. Res. 2020, 6, 19–27. [Google Scholar]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Nadda, A.K.; Husain, M.S.B.; Gupta, A. Biopolymers from waste biomass and its applications in the cosmetic industry: A review. Mat. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- EWG’s Skin Deep®. Your Guide to Safer Personal Care Products. Available online: https://www.ewg.org/skindeep/browse/ingredients/701308-CHITOSAN/?ingredient_id=701308-CHITOSAN&page=1 (accessed on 2 September 2022).
- Rejinold, N.S.; Choi, G.; Choy, J. Chitosan hybrids for cosmeceutical applications in skin, hair and dental care: An update. Emergent Mater. 2021, 4, 1125–1142. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 298, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Xiao, L.; Liu, Y.; Yu, H. Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J. Appl. Polym. Sci. 2002, 86, 1724–1730. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and Moisturizing Properties of Carboxymethyl Chitosan with Different Molecular Weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Mi, Y.; Miao, Q.; Cui, J.; Tan, W.; Guo, Z. Novel 2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives: Synthesis, Characterization, Moisture Absorption and Retention Properties. Molecules 2021, 26, 4238. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.D.; Kumar, I. Wound healing and skin regeneration. In Bionanomaterials for Skin Regeneration; Leonida, M.D., Kumar, I., Eds.; Springer: Charm, Switzerland, 2016; pp. 17–25. [Google Scholar]
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Z.; Li, D.-D.; Luo, H.; Li, W.-J.; Huang, Y.-M.; Li, J.-C.; Hu, Z.; Huang, N.; Guo, M.-H.; Chen, Y.; et al. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp. Gerontol. 2018, 103, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef]
- Chen, l.; Guo, B.; Luo, J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr. Polym. 2017, 173, 100–106. [Google Scholar] [CrossRef]
- Verma, M.; Gahlot, N.; Singh, S.S.J.; Rose, N.M. UV protection and antibacterial treatment of cellulosic fibre (cotton) using chitosan and onion skin dye. Carbohydr. Polym. 2021, 257, 117612. [Google Scholar] [CrossRef] [PubMed]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Tunku Mahmud, T.H.; Abdul-Aziz, A.; Muda, R. A review on the potential use of chitosan-based delivery system in mild facial cleansing formulation. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 432–437. [Google Scholar] [CrossRef]
- Theerawattanawit, C.; Phaiyarin, P.; Wanichwecharungruang, S.; Noppakun, N.; Asawanonda, P.; Kumtornrut, C. The Efficacy and Safety of Chitosan on Facial Skin Sebum. Skin Pharmacol. Physiol. 2022, 35, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tangkijngamvong, N.; Phaiyarin, P.; Wanichwecharungruang, S.; Kumtornrut, C. The anti-sebum property of chitosan particles. J. Cosmet. Dermatol. 2020, 19, 2135–2140. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, J.; Grządka, E.; Maciołek, U.; Godek, E.; Guzmán, E. The journey of tuning chitosan properties in colloidal systems: Interactions with surfactants in the bulk and on the alumina surface. Chem. Eng. J. 2022, 450, 138145. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Akanno, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Behavior of the water/vapor interface of chitosan solutions with an anionic surfactant: Effect of polymer–surfactant interactions. Phys. Chem. Chem. Phys. 2020, 22, 23360–23373. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Fernández-Pérez, C.; Barba-Nieto, I.; Ortega, F.; Leonforte, F.; Rubio, R.G.; Luengo, G.S. Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants. Polymers 2022, 14, 1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Grabska, S.; Sionkowska, A. The properties of hair covered by conditioners containing collagen, chitosan and hyaluronic acid. Eur. J. Med. Technol. 2019, 3, 11–17. [Google Scholar]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan nanoparticles making their way to clinical practice: A feasibility study on their topical use for acne treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahraze, O.; Gómez-Gómez, L.; Niza, E. Comparative evaluation of carvacrol and eugenol chitosan nanoparticles as eco-friendly preservative agents in cosmetics. Int. J. Biol. Macromol. 2022, 206, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahraze, O.; Gómez-Gómez, L.; Niza, E. Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. Int. J. Mol. Sci. 2022, 23, 898. [Google Scholar] [CrossRef] [PubMed]
- Zubareva, A.; Shagdarova, B.; Varlamov, V.; Kashirin, E.; Svirshchevskaya, E. Penetration and toxicity of chitosan and its derivatives. Eur. Polym. J. 2017, 93, 743–749. [Google Scholar] [CrossRef]
- Keana, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Kumar, M.; Vivekanand, V.; Pareek, N. Insect Chitin and Chitosan: Structure, Properties, Production, and Implementation Prospective. In Natural Materials and Products from Insects: Chemistry and Applications; Kumar, D., Shahid, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 51–66. [Google Scholar] [CrossRef]

| Type of Products | Forms | Applications |
|---|---|---|
| Toiletry Hygiene Personal care Skin care Oral care Dental care Hair care Cosmeceuticals | Solution Powder Film | Functional additives Moisturizers: maintain skin moisture, tone skin Thickening agent Hydrating and film-forming agent Role in surfactant stability; stabilize emulsion Antistatic effect Bacteriostatic Encapsulating agent Delivery systems Products: shampoos, creams, skin creams, creams for acne treatment, lotions, bath lotions, nail polish, fixtures, make-up powder, lacquers, nail lacquers, nail enamel, varnishes, hair sprays, hair colorants, and wave agents Cleaning products: cleansing milk, face peel, facial toner, soap, and bath agent Hair care: elastic film on hair, increase its softness and mechanical strength, improve suppleness of hair, remove oils and sebum from hairs, reduce static electricity in hair, retain moisture, and maintain hair’s style Oral care, dental care: toothpaste and chewing gum |
| Commercial Name | Manufacturer | Application |
|---|---|---|
| Hydamer™ | Chitinor AS (Tromsø, Norway) | Film-forming and fixative agent, deodorizing |
| Ritachitosan® | Rita Corporation (Crystal Lake, IL, USA) | Film-forming agent |
| Curasan™ | Chemisches Laboratorium Dr. Kurt Richter GmbH (Berlin, Germany) | Film-forming agent |
| Zenvivo™ | Clariant (Muttenz, Switzerland) | Film-forming agent, antimicrobial, deodorizing, moisturizer |
| KIOsmetine® | Kitozyme (Herstal, Belgium) | Film-forming agent, moisturizer |
| Chitosonic® Acid | Personal Care Products Council (Washington DC, USA) | Antimicrobial, moisturizer |
| ChitoClearTM | Primex Manufacturing Inc. (Langley, BC, Canada) | Film-forming agent |
| Everquat™ Q50H | Sino Lion (Florham Park, NJ, USA) | Shining agent, antidandruff agent, hair growth promoter, anti-hair-loss agent |
| Vinkocos p-6N | Vink Chemicals GmbH & Co. KG (Kakenstorf, Germany) | Film-forming and wetting agent, thickener, stabilizer |
| Jeen-Chitosan | Jeen International (Fairfield, NJ, USA) | Film-forming agent, moisturizer |
| Triozan | Ovensa Inc. (Aurora, ON, Canada) | Penetration enhancer |
| Product | Manufacturer | Application |
|---|---|---|
| Scalp Purifying Micellar Shampoo | Kristin Ess Hair (Los Angeles, CA, USA) | Shampoo |
| Brazilian Joia Strengthening + Smoothing Shampoo | Sol de Janeiro, Inc. (New York, NY, USA) | Shampoo |
| Extra Gentle Conditioner | Kristin Ess Hair (Los Angeles, CA, USA) | Hair conditioner |
| Herbal Essences Set Me Up Gel | Procter and Gamble (Cincinnati, OH, USA) | Hair-styling gel |
| Re Vamp Mid Length Repair Cream | Vernom Francois (Los Angeles, CA, USA) | Hair serum |
| Anti-Aging Moisture Lotion | Murad LLC (El Segundo, CA, USA) | Skin Care |
| Ultimate Miracle Worker Multi-Rejuvenating Cream | Philosophy (New York, NY, USA) | Skin Care |
| St. Yves Replenishing Mineral Therapy Body Lotion | Unilever (London, UK) | Skin moisturizer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. https://doi.org/10.3390/cosmetics9050099
Guzmán E, Ortega F, Rubio RG. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics. 2022; 9(5):99. https://doi.org/10.3390/cosmetics9050099
Chicago/Turabian StyleGuzmán, Eduardo, Francisco Ortega, and Ramón G. Rubio. 2022. "Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care" Cosmetics 9, no. 5: 99. https://doi.org/10.3390/cosmetics9050099
APA StyleGuzmán, E., Ortega, F., & Rubio, R. G. (2022). Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics, 9(5), 99. https://doi.org/10.3390/cosmetics9050099








