Identification of Prognostic Factors and Predicting the Therapeutic Effect of Laser Photocoagulation for DME Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Expert Evaluation of the Effectiveness of Diabetic Macular Edema (DME) Treatment
2.3. Identification of Prognostic Factors
- 1.
- NearestPoint—The closest point geometrically is selected for each point and the distance between them is calculated.
- 2.
- GenDelaunay—Delaunay triangulation is built for all points and the distances are calculated for the connected points.
- 3.
- LocDelaunay—The algorithm is similar to the previous one, but the Delaunay triangulation is not built over all the points, but over closed local areas. For this purpose, a preliminary clustering is performed.
- Arithmetic mean;
- Variance;
- Root-mean-square deviation (RMS);
- Median;
- Kurtosis;
- Asymmetry;
- Minimum value;
- Maximum value;
- Mode.
- The number of coagulates;
- The number of local areas.
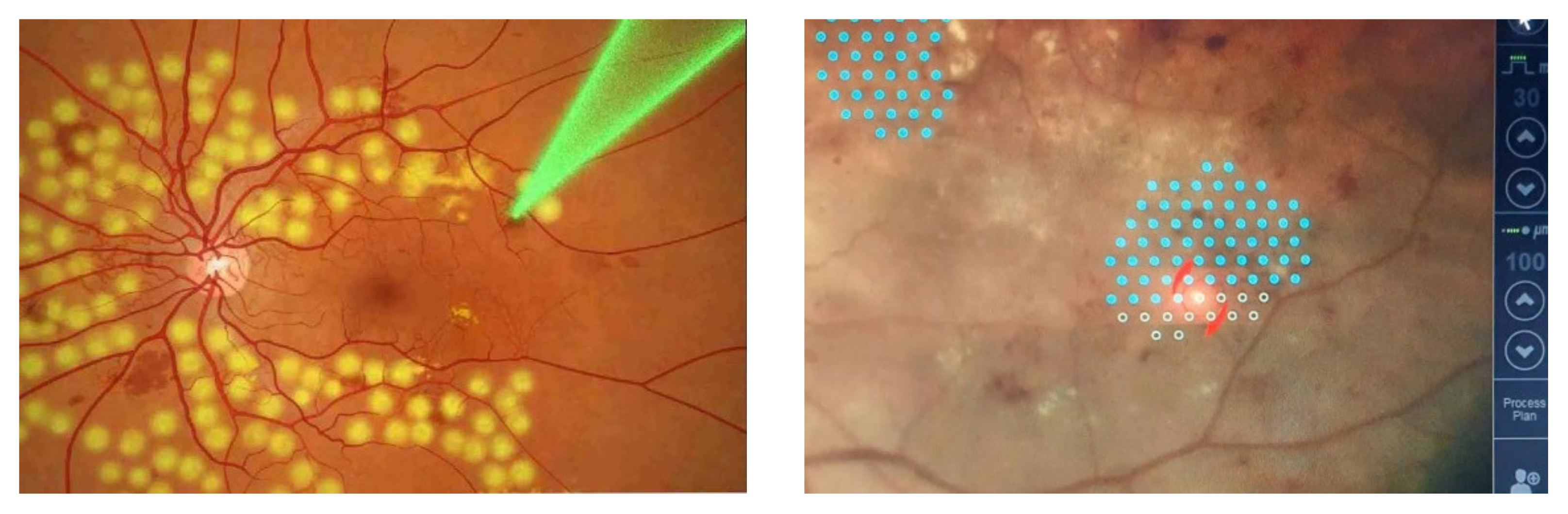
2.4. Technology for Intelligent Analysis of the Photocoagulation Plan
2.5. Algorithms for Automatic Arrangement of Coagulates

3. Results
3.1. Comparison of Manual and Semi-Automatic Arrangement of Coagulates
3.2. Investigation of the Technology of the Intelligent Analysis of the Preliminary Photocoagulation Plan
- Initial set—All the 26 selected features (Initial);
- Threshold features—The features with the weight of not less than 0.1 (Threshold);
- Combination of N features—A set formed as a result of complete enumeration of all combinations and selection of a set with the maximum separability criterion (Comb_5, Comb_6, Comb_7, Comb_8, Comb_9);
- Informative features—a set of features obtained as a result of applying the transformation matrix to the uncorrelated features (Informative).
3.3. Investigation of the Algorithms of Automatic Arrangement of Coagulates
- Informative—The features identified in Section 3.2;
- Strict—The features that have demonstrated good classification accuracy separately;
- Empirical—The features chosen by doctors.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vorobieva, I.V.; Merkushenkova, D.A. Diabetic retinopathy in patients with type 2 diabetes mellitus. Epidemiology, a modern view of pathogenesis. Ophthalmology 2012, 9, 18–21. [Google Scholar]
- Dedov, I.I.; Shestakova, M.V.; Vikulova, O.K. State Register of Diabetes Mellitus in the Russian Federation: Status of 2014 and Development Prospects. Diabetes Mellit. 2015, 18, 5–23. [Google Scholar]
- Dedov, I.I.; Shestakova, M.V.; Galstyan, G.R. Prevalence of type 2 diabetes mellitus in the adult population of Russia (NATION study). Diabetes Mellit. 2016, 19, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Saaddine, J.B.; Chou, C.F. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010, 304, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428. [Google Scholar] [PubMed]
- Ixcamey, M.; Palma, C. Diabetic macular edema. Dis. Mon. 2021, 67, 101138. [Google Scholar] [CrossRef]
- Tan, G.S.; Cheung, N.; Simo, R. Diabetic macular edema. Lancet Diab. Endoc. 2017, 5, 143–155. [Google Scholar] [CrossRef]
- Doga, A.V.; Kachalina, G.F.; Pedanova, E.K.; Buryakov, D.A. Modern diagnostic and treatment aspects of diabetic macular edema. Ophthalmol. Diabetes 2014, 4, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Bratko, G.V.; Chernykh, V.V.; Sazonova, O.V. On early diagnostics and the occurence rate of diabetic macular edema and identification of diabetes risk groups. Sib. Sci. Med. J. 2015, 35, 33–36. [Google Scholar]
- Wong, T.Y.; Liew, G.; Tapp, R.J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: Three population-based cross-sectional studies. Lancet 2008, 371, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.K.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy IV. Ophthalmology 1984, 91, 1464–1474. [Google Scholar] [CrossRef]
- Amirov, A.N.; Abdulaeva, E.A.; Minkhuzina, E.L. Diabetic macular edema: Epidemiology, pathogenesis, diagnosis, clinical presentation, and treatment. Kazan Med. J. 2015, 96, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, J.; Cho, S.; Song, J.; Lee, M.; Kim, S.H.; Lee, J.Y.; Shin, D.H.; Kim, J.M.; Bae, J.H.; et al. Development of Decision Support Software for Deep Learning-Based Automated Retinal Disease Screening Using Relatively Limited Fundus Photograph Data. Electronics 2021, 10, 163. [Google Scholar] [CrossRef]
- Ghani, A.; See, C.H.; Sudhakaran, V.; Ahmad, J.; Abd-Alhameed, R. Accelerating Retinal Fundus Image Classification Using Artificial Neural Networks (ANNs) and Reconfigurable Hardware (FPGA). Electronics 2019, 8, 1522. [Google Scholar] [CrossRef] [Green Version]
- Sri Durga Kameswari, S.; Krishna Kishore, K. Classification of Retinal Fundus Images Based on Alexnet and Transfer Learning. Int. J. Sci. Technol. Res. 2020, 9, 2004–2006. [Google Scholar]
- Luo, L.; Xue, D.; Feng, X. Automatic Diabetic Retinopathy Grading via Self-Knowledge Distillation. Electronics 2020, 9, 1337. [Google Scholar] [CrossRef]
- Bodapati, J.D.; Naralasetti, V.; Shareef, S.N.; Hakak, S.; Bilal, M.; Maddikunta, P.K.R.; Jo, O. Blended Multi-Modal Deep ConvNet Features for Diabetic Retinopathy Severity Prediction. Electronics 2020, 9, 914. [Google Scholar] [CrossRef]
- Acharya, U.R.; Ng, E.Y.; Tan, J.H.; Sree, S.V.; Ng, K.H. An integrated index for the identification of diabetic retinopathy stages using texture parameters. J. Med. Syst. 2012, 36, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Adelman, R.A. Recent developments in laser treatment of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2015, 22, 157–163. [Google Scholar] [CrossRef]
- Kozak, I.; Luttrull, J.K. Modern retinal laser therapy. Saudi J. Ophthalmol. 2015, 29, 137–146. [Google Scholar] [CrossRef]
- Astakhov, Y.S.; Shadrichev, F.E.; Krasavina, M.I.; Grigorieva, N.N. Modern approaches to the treatment of diabetic macular edema. Ophthalmol. Statements 2009, 4, 59–69. [Google Scholar]
- Zamytsky, E.A.; Zolotarev, A.V.; Karlova, E.V.; Zamytsky, P.A. Analysis of the coagulates intensity in laser treatment of diabetic macular edema in a Navilas robotic laser system. Saratov J. Med. Sci. Res. 2017, 13, 375–378. [Google Scholar]
- Park, Y.G.; Kim, E.Y.; Roh, Y.J. Laser-based strategies to treat diabetic macular edema: History and new promising therapies. J. Ophthalmol. 2014, 2014, 769213. [Google Scholar] [CrossRef] [Green Version]
- Moutray, T.; Evans, J.R.; Lois, N.; Armstrong, D.J.; Peto, T.; Azuara-Blanco, A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2018, 3, CD012314. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, A.; Rasta, S.H.; Banaei, T.; Asgharsharghi Bonab, A. Effects of Laser Physical Parameters on Lesion Size in Retinal Photocoagulation Surgery: Clinical OCT and Experimental Study. J. Biomed. Phys. Eng. 2017, 7, 355–364. [Google Scholar] [PubMed]
- Shirokanev, A.; Ilyasova, N.; Andriyanov, N.; Zamytskiy, E.; Zolotarev, A.; Kirsh, D. Modeling of Fundus Laser Exposure for Estimating Safe Laser Coagulation Parameters in the Treatment of Diabetic Retinopathy. Mathematics 2021, 9, 967. [Google Scholar] [CrossRef]
- Chhablani, J.; Kozak, I.; Barteselli, G.; El-Emam, S. A novel navigated laser system brings new efficacy to the treatment of reti-novascular disorders. Oman J. Ophthalmol. 2013, 6, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Mathai, A.; Rani, P.; Gupta, V.; Arevalo, J.F.; Kozak, I. Comparison of Conventional Pattern and Novel Navigated Panretinal Photocoagulation in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3432–3438. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, A.S.; Langer, J.; Liegl, R.; Haritoglou, C.; Wolf, A.; Kozak, I.; Seidensticker, F.; Ulbig, M.; Freeman, W.R.; Kampik, A.; et al. Navigated macular laser decreases retreatment rate for diabetic macular edema: A comparison with conventional macular laser. Clin. Ophthalmol. 2013, 7, 121–128. [Google Scholar]
- Ilyasova, N.; Kirsh, D.; Paringer, R.; Kupriyanov, A.; Shirokanev, A.; Zamycky, E. Coagulate map formation algorithms for laser eye treatment. In Proceedings of the 3rd International Conference on Frontiers of Signal Processing ICFSP, Paris, France, 6–8 September 2017; pp. 120–124. [Google Scholar]
- Shirokanev, A.S.; Kirsh, D.V.; Ilyasova, N.Y.; Kupriyanov, A.V. Investigation of algorithms for coagulate arrangement in fundus images. Comput. Opt. 2018, 42, 712–721. [Google Scholar] [CrossRef]
- Ilyasova, N.Y.; Demin, N.S.; Shirokanev, A.S.; Kupriyanov, A.V.; Zamytskiy, E.A. Method for selection macular edema region using optical coherence tomography data. Comput. Opt. 2020, 44, 250–258. [Google Scholar] [CrossRef]
- Shirokanev, A.S.; Kibitkina, A.S.; Ilyasova, N.Y. Information technology of laser coagulation strategy selection in diabetic retinopathy treatment. In Proceedings of the Computer Science & Information Technology (CS & IT), Barcelona, Spain, 10–12 June 2020; pp. 59–64. [Google Scholar]
- Claro, M.; Veras, R.; Santana, A.; Araújo, F.; Silva, R.; Almeida, J.; Leite, D. An hybrid feature space from texture information and transfer learning for glaucoma classification. J. Vis. Commun. Image Represent. 2019, 64, 102597. [Google Scholar] [CrossRef]
- Budai, A.; Bock, R.; Maier, A.; Hornegger, J.; Michelson, G. Robust Vessel Segmentation in Fundus Images. Int. J. Biomed. Imaging 2013, 2013, 154860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131. [Google Scholar] [CrossRef]
- Ilyasova, N.Y.; Shirokanev, A.S.; Kupriyanov, A.V.; Paringer, R.A. Technology of intellectual feature selection for a system of automatic formation of a coagulate plan on retina. Comput. Opt. 2019, 43, 304–315. [Google Scholar] [CrossRef]
- Huy, N.H.; Frenzel, S.; Bandt, C. Two-Step Linear Discriminant Analysis for Classification of EEG Data. In Data Analysis, Machine Learning and Knowledge Discovery; Springer: Cham, Switzerland, 2014; pp. 51–59. [Google Scholar]
- Smolle, J. Computer recognition of skin structures using discriminant and cluster analysis. Ski. Res. Technol. 2008, 6, 58–63. [Google Scholar] [CrossRef]
- Ghosh, J.; Shuvo, S.B. Improving Classification Model’s Performance Using Linear Discriminant Analysis on Linear Data. In Proceedings of the 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kanpur, India, 6–8 July 2019; pp. 1–5. [Google Scholar] [CrossRef]





| Comment | Score |
|---|---|
| Visual acuity improved | +1 |
| Visual acuity unchanged | 0 |
| Visual acuity decreased | −1 |
| Mean retinal thickness decreased | +1 |
| Mean retinal thickness unchanged | 0 |
| Mean retinal thickness increased | −1 |
| Initial Classes | Qualitative Classes | General Classes |
|---|---|---|
| The treatment was successful | The treatment led to positive effect | Safe treatment |
| Only visual acuity improved | ||
| Only retinal thickness improved | ||
| The treatment did not lead to positive effect | The treatment did not give any result | |
| Visual acuity improved, but retinal thickness worsened | Amphibolic effect of the treatment | Dangerous treatment |
| Retinal thickness improved, but visual acuity worsened | ||
| Visual acuity worsened | Negative effect of the treatment | |
| Retina condition worsened | ||
| The treatment led to negative effect |
| Prognostic Factor | Characteristics |
|---|---|
| Uniformity | Median |
| Coverage area | Sample dimension |
| Balance | Asymmetry, Kurtosis, Minimum distance, Maximum distance |
| Histogram uniformity | Arithmetic mean, Variance |
| Determinism | Variance, Arithmetic mean, Median, Minimum distance, Maximum distance |
| NearestPoint | GenDelaunay | LocDelaunay | General |
|---|---|---|---|
| Arithmetic mean RMS Kurtosis Asymmetry Minimum | Arithmetic mean RMS Kurtosis Median Minimum | Arithmetic mean RMS Kurtosis Maximum | The number of local areas The number of coagulates |
| Sets of Features | Classification Accuracy % | ||
|---|---|---|---|
| Bayes | Decision Trees | Random Forest | |
| Initial | 66.4 | 70.7 | 82.8 |
| Threshold | 69.7 | 74.9 | 86.0 |
| Comb_5 | 83.9 | 77.4 | 75.7 |
| Comb_6 | 86.9 | 73.9 | 76.5 |
| Comb_7 | 68.9 | 74.8 | 74.8 |
| Comb_8 | 83.4 | 80.8 | 82.5 |
| Comb_9 | 81.8 | 80.0 | 78.3 |
| Informative | 86.2 | 87.9 | 87.9 |
| Map | Forecast % | ||
|---|---|---|---|
| Informative Features | Strict Features | Empirical Features | |
| Random | 62.2 | 91.9 | 87.7 |
| Square | 86.2 | 95.8 | 85.5 |
| Hexagonal | 84.7 | 98.9 | 95.0 |
| Wave | 88.0 | 99.1 | 97.0 |
| Boundary | 94.4 | 99.3 | 97.6 |
| Adaptive boundary | 95.4 | 99.5 | 98.1 |
| Ordered | 89.3 | 99.2 | 97.0 |
| Square island | 88.0 | 96.4 | 86.3 |
| Hexagonal island | 88.2 | 99.1 | 96.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyasova, N.; Shirokanev, A.; Kirsh, D.; Demin, N.; Zamytskiy, E.; Paringer, R.; Antonov, A. Identification of Prognostic Factors and Predicting the Therapeutic Effect of Laser Photocoagulation for DME Treatment. Electronics 2021, 10, 1420. https://doi.org/10.3390/electronics10121420
Ilyasova N, Shirokanev A, Kirsh D, Demin N, Zamytskiy E, Paringer R, Antonov A. Identification of Prognostic Factors and Predicting the Therapeutic Effect of Laser Photocoagulation for DME Treatment. Electronics. 2021; 10(12):1420. https://doi.org/10.3390/electronics10121420
Chicago/Turabian StyleIlyasova, Nataly, Aleksandr Shirokanev, Dmitriy Kirsh, Nikita Demin, Evgeniy Zamytskiy, Rustam Paringer, and Alexey Antonov. 2021. "Identification of Prognostic Factors and Predicting the Therapeutic Effect of Laser Photocoagulation for DME Treatment" Electronics 10, no. 12: 1420. https://doi.org/10.3390/electronics10121420







