Active Sense: Early Staging of Non-Insulin Dependent Diabetes Mellitus (NIDDM) Hinges upon Recognizing Daily Activity Pattern
Abstract
:1. Introduction
2. Related Work
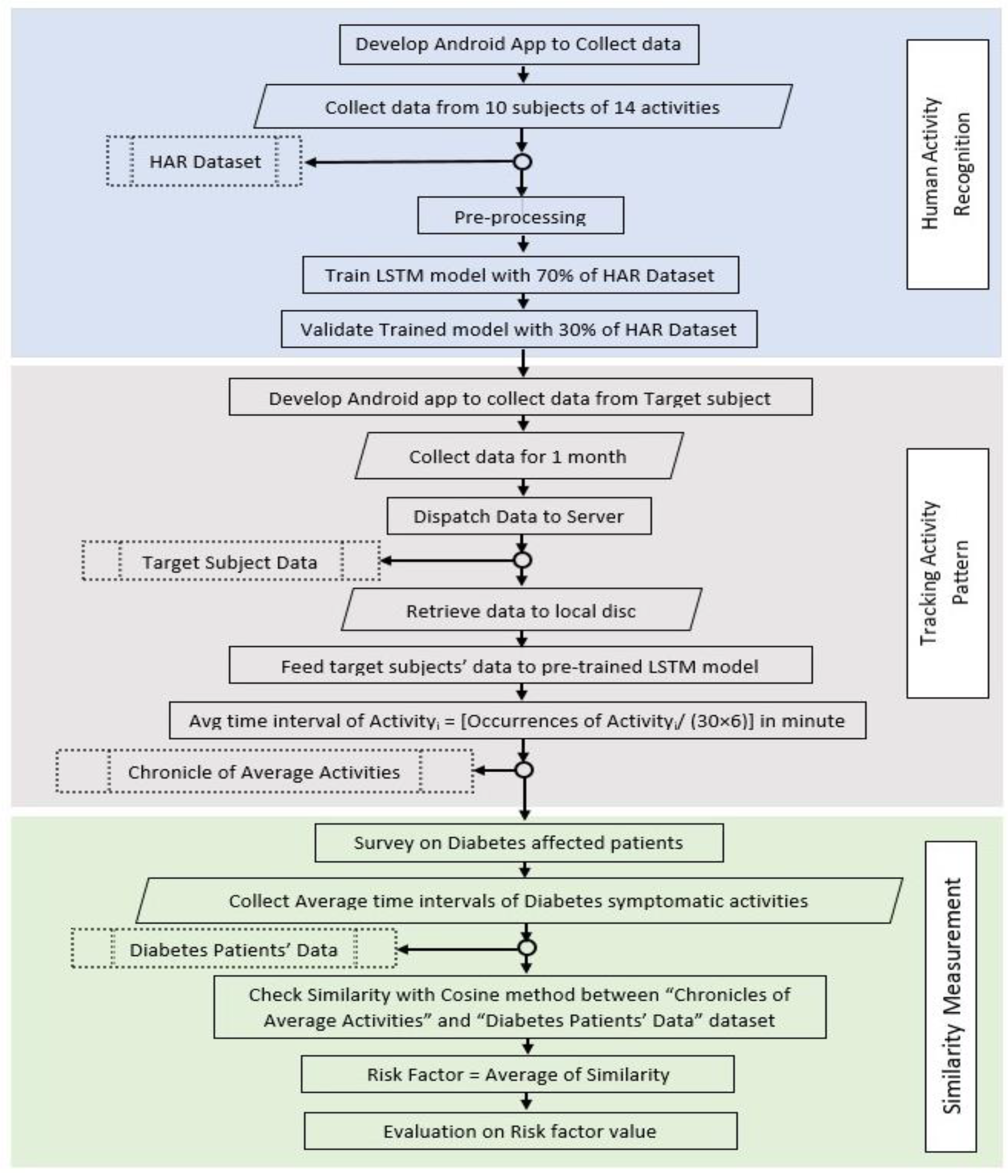
3. Materials and Methods
3.1. Human Activity Recognition
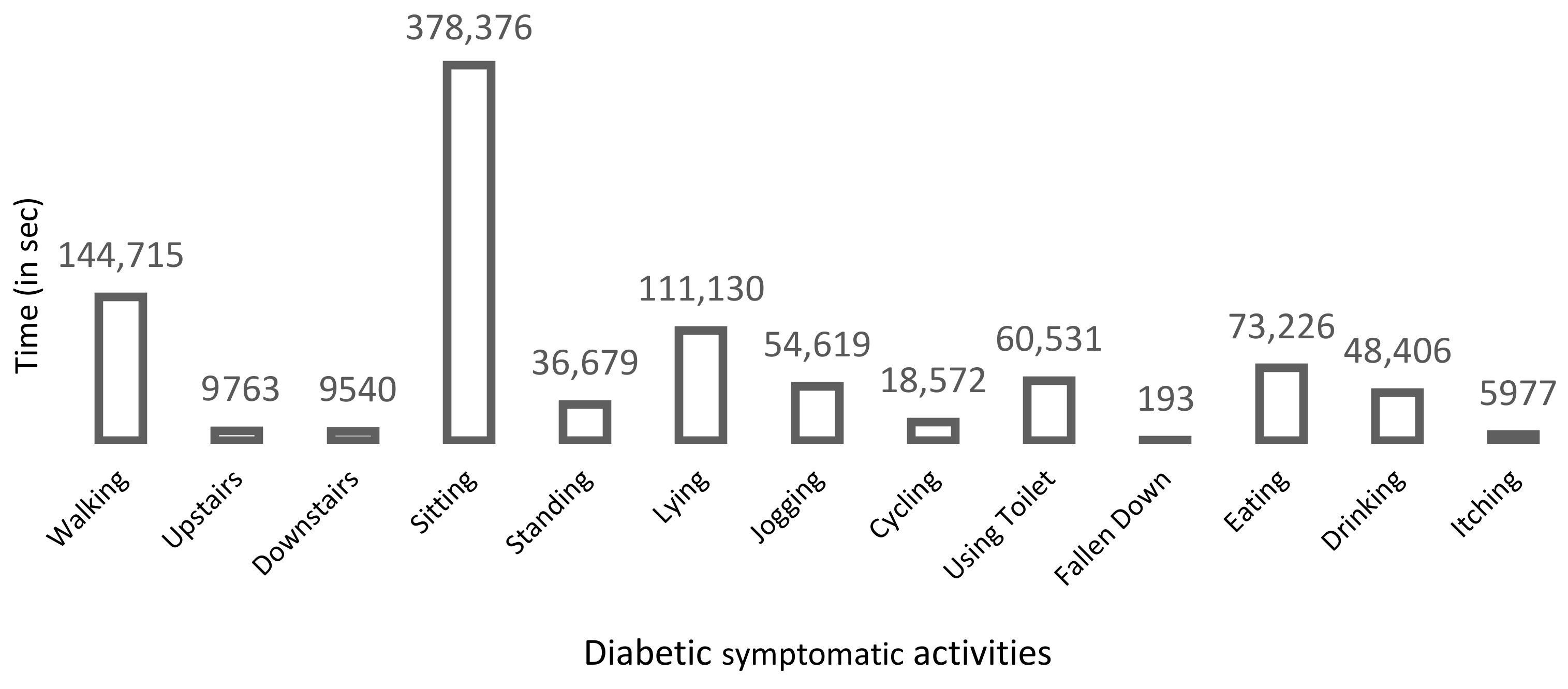
3.1.1. Symptomatic Activities
3.1.2. Sensors’ Data Collection
3.1.3. Data Pre-Processing
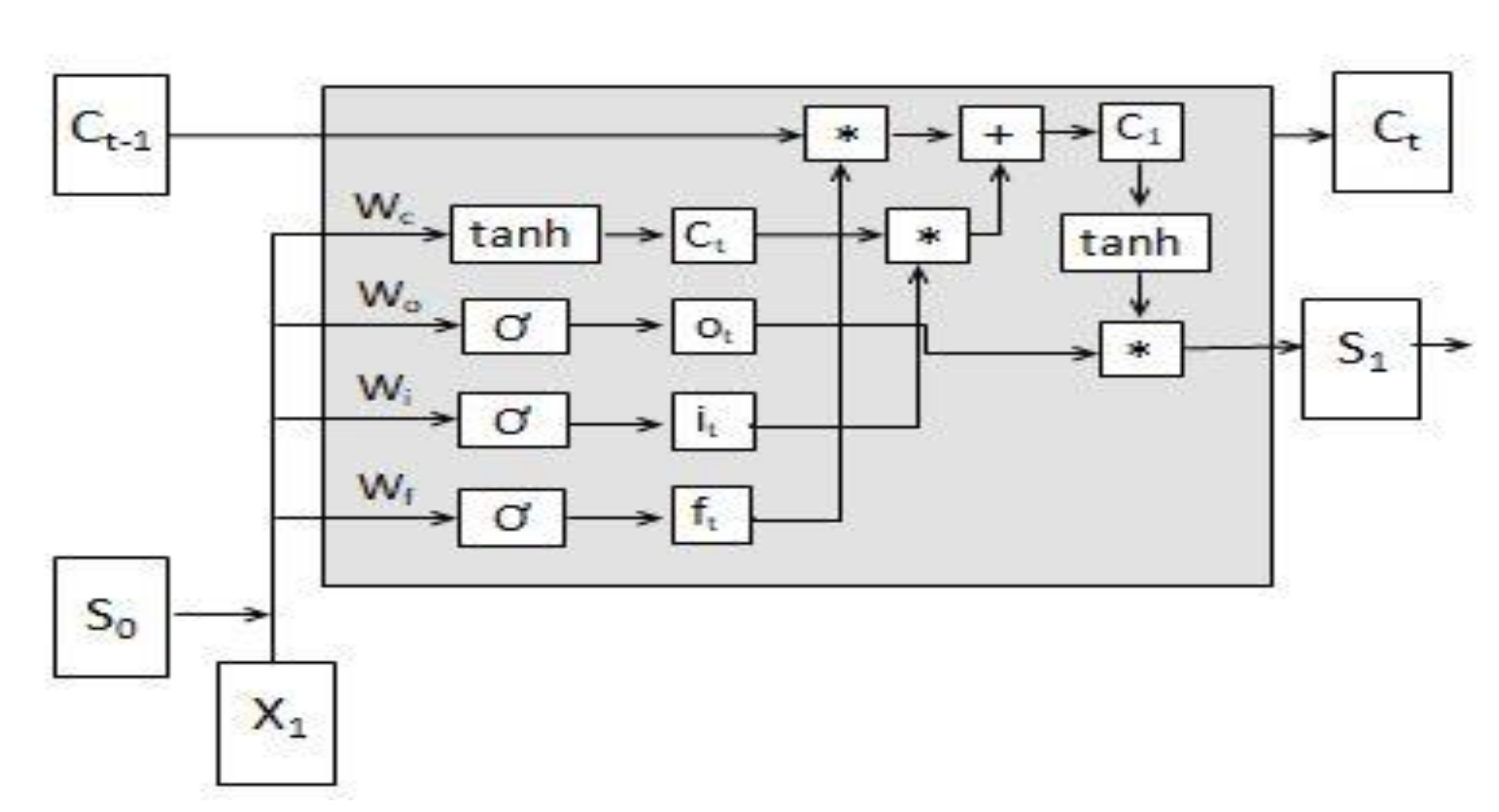
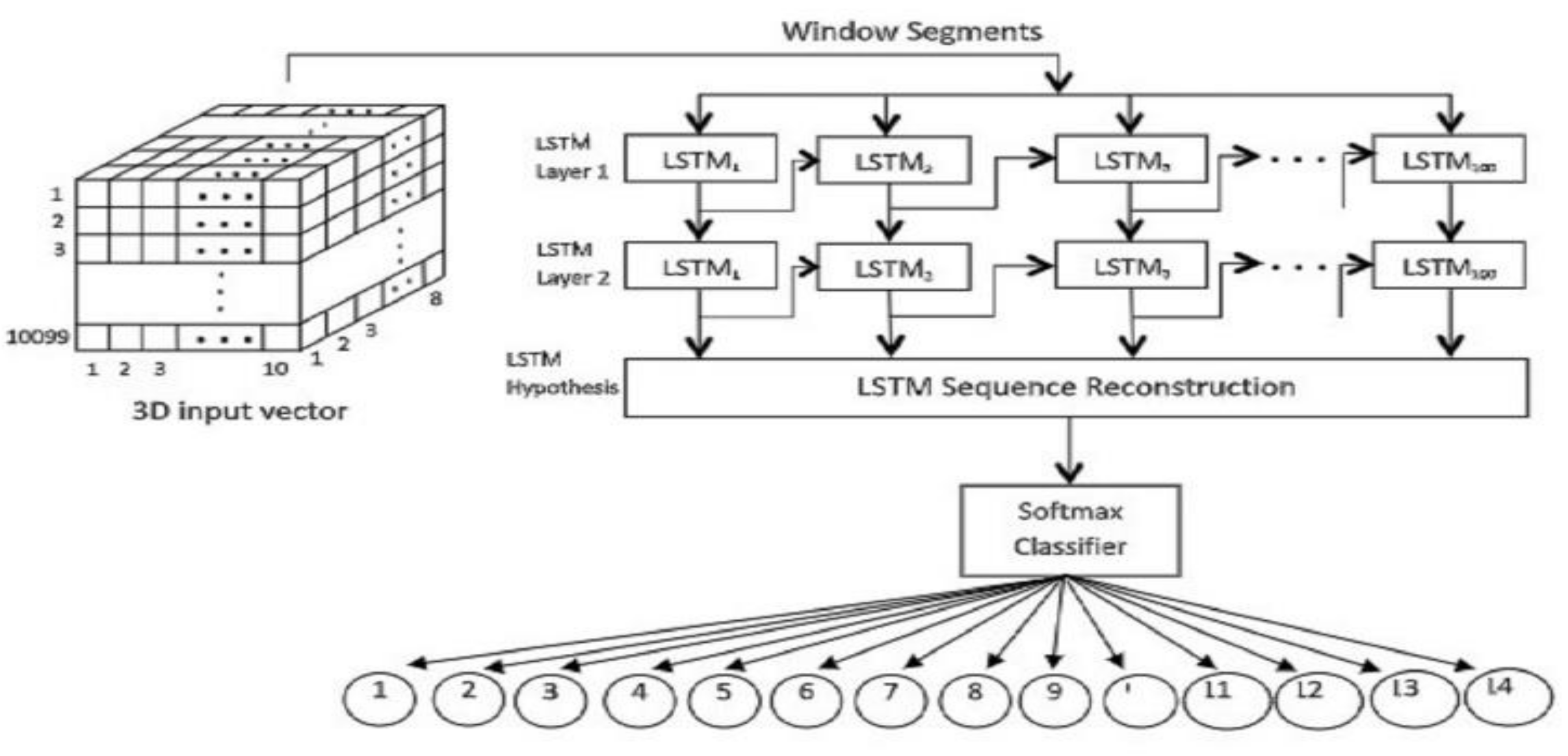
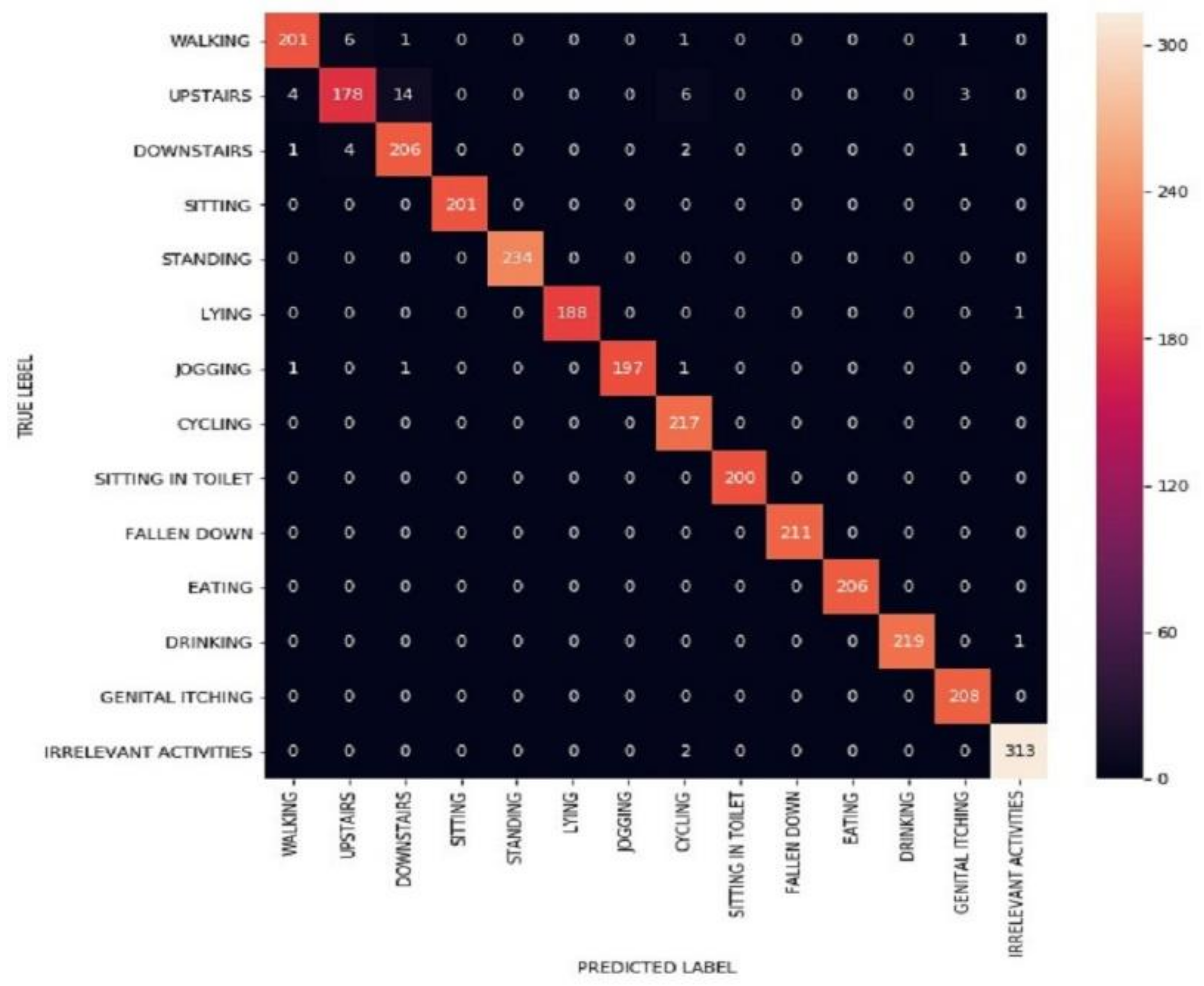
3.1.4. LSTM Model Assessment
3.1.5. Fusing LSTM and Evolution
3.2. Tracking Activities of Experimental Subject
3.2.1. Data Collection from Experimental Subject
3.2.2. Fusing Pre-Trained LSTM Model on Experimental Subject’s Dataset
3.3. Similarity Measurement
4. Assessment of Risk Factor
5. Conclusions and Future Scopes
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Narayan, K.V. How generally applicable is a simple diabetes detection questionnaire? Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 196–197. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of A WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Li, R.; Zhang, P.; Barker, L.E.; Chowdhury, F.M.; Zhang, X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: A systematic review. Diabetes Care 2010, 33, 1872–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association, A.D. Economic costs of Diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Pei, J.H.; Kuang, J.; Chen, H.M.; Chen, Z.; Li, Z.W.; Yang, H.Z. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metabolism 2015, 64, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Helmrich, S.P.; Ragland, D.R.; Leung, R.W.; Paffenbarger, R.S., Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1991, 325, 147–152. [Google Scholar] [CrossRef]
- Duong, T.V.; Bui, H.H.; Phung, D.Q.; Venkatesh, S. Activity Recognition and Abnormality Detection with the Switching Hidden Semi-Markov Model. In Proceedings of the 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05), San Diego, CA, USA, 20–25 June 2005. [Google Scholar]
- Bachlin, M.; Plotnik, M.; Roggen, D.; Maidan, I.; Hausdorff, J.M.; Giladi, N.; Troster, G. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans. Inf. Technol. Biomed. 2009, 14, 436–446. [Google Scholar] [CrossRef]
- Barna, A.; Masum, A.K.M.; Hossain, M.E.; Bahadur, E.H.; Alam, M.S. A Study on Human Activity Recognition Using Gyroscope, Accelerometer, Temperature and Humidity Data. In Proceedings of the 2019 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’sBazar, Bangladesh, 7–9 February 2019. [Google Scholar]
- Bahadur, E.H.; Masum, A.K.M.; Barua, A.; Alam, M.G.R.; Chowdhury, M.A.U.Z.; Alam, M.R. LSTM Based Approach for Diabetic Symptomatic Activity Recognition Using Smartphone Sensors. In Proceedings of the 2019 22nd International Conference on Computer and Information Technology (ICCIT), Dhaka, Bangladesh, 18–20 December 2019. [Google Scholar]
- Masum, A.K.M.; Bahadur, E.H.; Ruhi, F.A. Scrutiny of Mental Depression through Smartphone Sensors Using Machine Learning Approaches. Int. J. Innov. Comput. 2020, 10. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Bahadur, E.H.; Shan-A-Alahi, A.; Chowdhury, M.A.U.Z.; Uddin, M.R.; Al Noman, A. Human Activity Recognition Using Accelerometer, Gyroscope and Magnetometer Sensors: Deep Neural Network Approaches. In Proceedings of the 2019 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kanpur, India, 6–8 July 2019. [Google Scholar]
- Masum, A.K.M.; Barua, A.; Bahadur, E.H.; Alam, M.R.; Chowdhury, M.A.U.Z.; Alam, M.S. Human Activity Recognition Using Multiple Smartphone Sensors. In Proceedings of the 2018 International Conference on Innovations in Science, Engineering and Technology (ICISET), Chittagong, Bangladesh, 27–28 October 2018. [Google Scholar]
- Masum, A.K.M.; Jannat, S.; Bahadur, E.H.; Alam, M.G.R.; Khan, S.I.; Alam, M.R. Human Activity Recognition Using Smartphone Sensors: A Dense Neural Network Approach. In Proceedings of the 2019 1st International Conference on Advances in Science, Engineering and Robotics Technology (ICASERT), Dhaka, Bangladesh, 3–5 May 2019. [Google Scholar]
- Bodor, R.; Jackson, B.; Papanikolopoulos, N. Vision-BASED human Tracking and Activity Recognition. In Proceedings of the 11th Mediterranean Conf. on Control and Automation, Rhodes, Greece, 18–20 June 2003. [Google Scholar]
- Ni, B.; Wang, G.; Moulin, P. Rgbd-Hudaact: A Color-Depth Video Database for Human Daily Activity Recognition. In Proceedings of the 2011 IEEE International Conference on Computer Vision Workshops (ICCV Workshops), Barcelona, Spain, 6–13 November 2011. [Google Scholar]
- Weinland, D.; Ronfard, R.; Boyer, E. A survey of vision-based methods for action representation, segmentation and recognition. Comput. Vis. Image Underst. 2011, 115, 224–241. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Aggarwal, J. Spatio-temporal Depth Cuboid Similarity Feature for Activity Recognition Using Depth Camera. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013. [Google Scholar]
- Aggarwal, J.K.; Xia, L. Human activity recognition from 3d data: A review. Pattern Recognit. Lett. 2014, 48, 70–80. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C. Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 2014, 15, 1321–1330. [Google Scholar] [CrossRef]
- Attal, F.; Mohammed, S.; Dedabrishvili, M.; Chamroukhi, F.; Oukhellou, L.; Amirat, Y. Physical human activity recognition using wearable sensors. Sensors 2015, 15, 31314–31338. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Yin, Z. Human Activity Recognition Using Wearable Sensors by Deep Convolutional Neural Networks. In Proceedings of the Proceedings of the 23rd ACM International Conference on Multimedia, New York, NY, USA, 26–30 October 2015. [Google Scholar]
- Hammerla, N.Y.; Halloran, S.; Plötz, T. Deep, convolutional, and recurrent models for human activity recognition using wearables. arXiv 2016, arXiv:1604.08880. [Google Scholar]
- Ronao, A.C.; Cho, S.-B. Deep convolutional neural networks for human activity recognition with smartphone sensors. In International Conference on Neural Information Processing; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Reyes-Ortiz, J.-L.; Oneto, L.; Sama, A.; Parra, X.; Anguita, D. Transition-aware human activity recognition using smartphones. Neurocomputing 2016, 171, 754–767. [Google Scholar] [CrossRef] [Green Version]
- Ronao, A.C.; Cho, S.-B. Human activity recognition with smartphone sensors using deep learning neural networks. Expert Syst. Appl. 2016, 59, 235–244. [Google Scholar] [CrossRef]
- Hassan, M.M.; Uddin, M.Z.; Mohamed, A.; Almogren, A. A robust human activity recognition system using smartphone sensors and deep learning. Future Gener. Comput. Syst. 2018, 81, 307–313. [Google Scholar] [CrossRef]
- Ha, S.; Choi, S. Convolutional Neural Networks for Human Activity Recognition Using Multiple Accelerometer and Gyroscope Sensors. In Proceedings of the 2016 International Joint Conference on Neural Networks (IJCNN), Vancouver, BC, Canada, 24–29 July 2016. [Google Scholar]
- Ignatov, A. Real-time human activity recognition from accelerometer data using Convolutional Neural Networks. Appl. Soft Comput. 2018, 62, 915–922. [Google Scholar] [CrossRef]
- Alsheikh, M.A.; Selim, A.; Niyato, D.; Doyle, L.; Lin, S.; Tan, H.P. Deep Activity Recognition Models with Triaxial Accelerometers. In Proceedings of the Workshops at the Thirtieth AAAI Conference on Artificial Intelligence, Phoenix, AZ, USA, 12–17 February 2016. [Google Scholar]
- Chen, Y.; Zhong, K.; Zhang, J.; Sun, Q.; Zhao, X. Lstm Networks for Mobile Human Activity Recognition. In Proceedings of the 2016 International Conference on Artificial Intelligence: Technologies and Applications, Bangkok, Thailand, 24–25 January 2016. [Google Scholar]
- Ordóñez, J.F.; Roggen, D. Deep convolutional and lstm recurrent neural networks for multimodal wearable activity recognition. Sensors 2016, 16, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, F.; Suárez, L.F.; Villamizar, J.; Altuve, M. Human Activity Recognition on Smartphones Using a Bidirectional LSTM Network. In Proceedings of the 2019 XXII Symposium on Image, Signal Processing and Artificial Vision (STSIVA), Bucaramanga, Colombia, 24–26 April 2019. [Google Scholar]
- Zhao, Y.; Yang, R.; Chevalier, G.; Xu, X.; Zhang, Z. Deep residual bidir-LSTM for human activity recognition using wearable sensors. Math. Probl. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Rosner, B.; Hennekens, C.H.; Speizer, F.E.; Rimm, E.B.; Krolewski, A.S. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991, 338, 774–778. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Schwarz, P.; Lindström, J. Long-term benefits from lifestyle interventions for type 2 diabetes prevention: Time to expand the efforts. Diabetes Care 2011, 34 (Suppl. 2), S210–S214. [Google Scholar] [CrossRef] [Green Version]
- Helmrich, S.P.; Ragland, D.R.; Paffenbarger, J.R. Prevention of non-insulin-dependent diabetes mellitus with physical activity. Med. Sci. Sports Exerc. 1994, 26, 824–830. [Google Scholar] [CrossRef]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B.; Leitzmann, M.F.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Rimm, E.B. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch. Intern. Med. 2001, 161, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Kriska, M.A.; Bennett, P.H. An epidemiological perspective of the relationship between physical activity and NIDDM: From activity assessment to intervention. Diabetes Metab. Rev. 1992, 8, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Ruderman, N.; Campaigne, B.N.; Devlin, J.T.; Schneider, S.H. Physical activity/exercise and Diabetes mellitus. Diabetes Care 2003, 26, S73. [Google Scholar] [PubMed] [Green Version]
- Lee, J.; Kim, J. Energy-efficient real-time human activity recognition on smart mobile devices. Mob. Inf. Syst. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Niu, D.; Jiang, B.; Zuo, X.; Yang, J. Meta-HAR: Federated Representation Learning for Human Activity Recognition. In Proceedings of the Web Conference 2021, Ljubljana, Slovenia, 12–23 April 2021. [Google Scholar]
- Association, A.D. 3. Foundations of care and comprehensive medical evaluation. Diabetes Care 2016, 39 (Suppl. 1), S23–S35. [Google Scholar]
- Coombes, B.; Tucker, K.; Hug, F.; Scott, A.; Geytenbeek, M.; Cox, E.R.; Gajanand, T.; Coombes, J.S. Relationships between cardiovascular disease risk factors and Achilles tendon structural and mechanical properties in people with Type 2 Diabetes. Muscles Ligaments Tendons J. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Sun, H.; Xiao, L.; Zhou, Y.; Yin, W.; Xu, T.; Yuan, J. Combined effect of urinary monohydroxylated polycyclic aromatic hydrocarbons and impaired lung function on Diabetes. Environ. Res. 2016, 148, 467–474. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia-newly emerging and high impact complications of diabetes. J. Diabetes Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef]
- Davies, T.T.; Graue, M.; Igland, J.; Tell, G.S.; Birkeland, K.I.; Peyrot, M.; Haltbakk, J. Diabetes prevalence among older people receiving care at home: Associations with symptoms, health status and psychological well-being. Diabet. Med. 2019, 36, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, S.; Li, W.; Qin, S.J. Selection of the number of principal components: The variance of the reconstruction error criterion with a comparison to other methods. Ind. Eng. Chem. Res. 1999, 38, 4389–4401. [Google Scholar] [CrossRef]
- Gers, F.A.; Schmidhuber, J. Recurrent Nets That Time and Count. In Proceedings of the IEEE-INNS-ENNS International Joint Conference on Neural Networks. IJCNN 2000. Neural Computing: New Challenges and Perspectives for the New Millennium, Como, Italy, 27 July 2000. [Google Scholar]
- Lu, Y.; Salem, F.M. Simplified Gating in Long Short-Term Memory (Lstm) Recurrent Neural Networks. In Proceedings of the 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Boston, MA, USA, 6–9 August 2017. [Google Scholar]
- Cheng, X.; Huang, B.; Zong, J. Device-free Human Activity Recognition Based on GMM-HMM using Channel State Information. IEEE Access 2021, 9, 76592–76601. [Google Scholar] [CrossRef]
- Nurwulan, R.N.; Selamaj, G. A Comparative Evaluation of Acceleration and Jerk in Human Activity Recognition Using Machine Learning Techniques. In Proceedings of the 1st International Conference on Electronics, Biomedical Engineering, and Health Informatics, Surabaya, Indonesia, 8–9 October 2020. [Google Scholar]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bener, A.; Yousafzai, M.T.; Al-Hamaq, A.O.; Mohammad, A.G.; DeFronzo, R.A. Parental transmission of type 2 diabetes mellitus in a highly endogamous population. World J. Diabetes 2013, 4, 40. [Google Scholar] [CrossRef]
- Pell, S.; D’Alonzo, C.A. Some aspects of hypertension in Diabetes mellitus. JAMA 1967, 202, 10–16. [Google Scholar] [CrossRef]
- Committee, I.E. International Expert Committee report on the role of the A1C assay in the diagnosis of Diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- WHO. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Zhou, X.H.; Ji, L.N.; Luo, Y.Y.; Zhang, X.Y.; Han, X.Y.; Qiao, Q. Performance of HbA1c for detecting newly diagnosed Diabetes and prediabetes in Chinese communities living in Beijing. Diabet. Med. 2009, 26, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.M.; Haggerty, S.; Herman, W.H. HbA1c for the Diagnosis of Diabetes and Prediabetes: Is It Time for a Mid-Course Correction? Oxford University Press: Oxford, UK, 2010. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadur, E.H.; Masum, A.K.M.; Barua, A.; Uddin, M.Z. Active Sense: Early Staging of Non-Insulin Dependent Diabetes Mellitus (NIDDM) Hinges upon Recognizing Daily Activity Pattern. Electronics 2021, 10, 2194. https://doi.org/10.3390/electronics10182194
Bahadur EH, Masum AKM, Barua A, Uddin MZ. Active Sense: Early Staging of Non-Insulin Dependent Diabetes Mellitus (NIDDM) Hinges upon Recognizing Daily Activity Pattern. Electronics. 2021; 10(18):2194. https://doi.org/10.3390/electronics10182194
Chicago/Turabian StyleBahadur, Erfanul Hoque, Abdul Kadar Muhammad Masum, Arnab Barua, and Md Zia Uddin. 2021. "Active Sense: Early Staging of Non-Insulin Dependent Diabetes Mellitus (NIDDM) Hinges upon Recognizing Daily Activity Pattern" Electronics 10, no. 18: 2194. https://doi.org/10.3390/electronics10182194
APA StyleBahadur, E. H., Masum, A. K. M., Barua, A., & Uddin, M. Z. (2021). Active Sense: Early Staging of Non-Insulin Dependent Diabetes Mellitus (NIDDM) Hinges upon Recognizing Daily Activity Pattern. Electronics, 10(18), 2194. https://doi.org/10.3390/electronics10182194






