Offset Compensation in Resistive Stretch Sensors Using Low-Frequency Feedback Topology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Topologies of Signal Conditioning Circuits for Resistive Bands
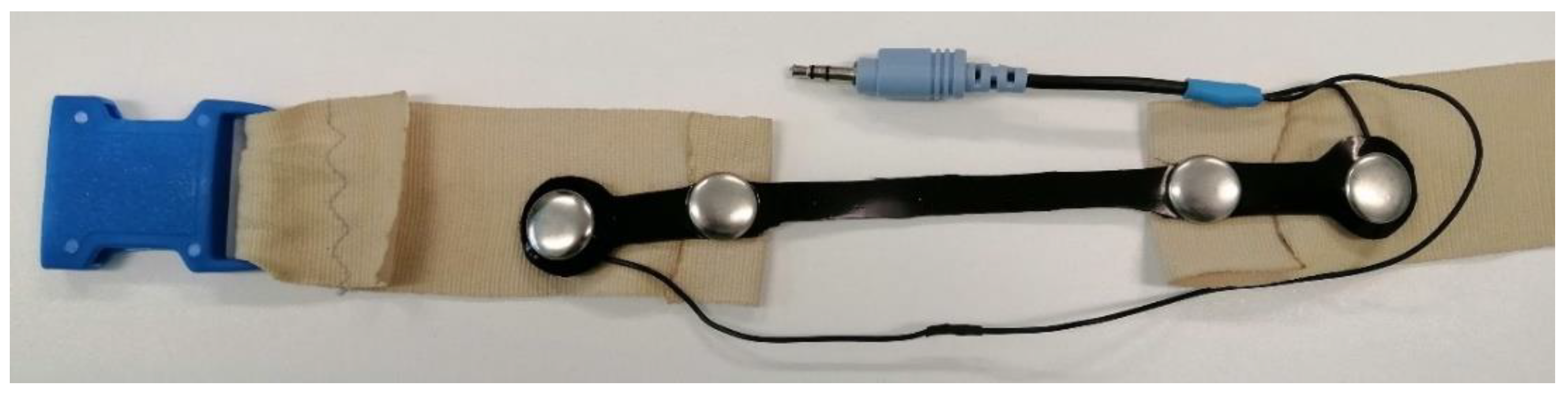
2.1.1. The Prototype of a Resistive Belt
2.1.2. Standard Differential Amplifier
2.1.3. Conventional Methods of Increasing the Dynamic Range of the Signal
2.1.4. Proposed Low-Frequency Feedback Topology
3. Results
3.1. Simulation Results of Different Circuits
3.1.1. Simple Differential Amplifier
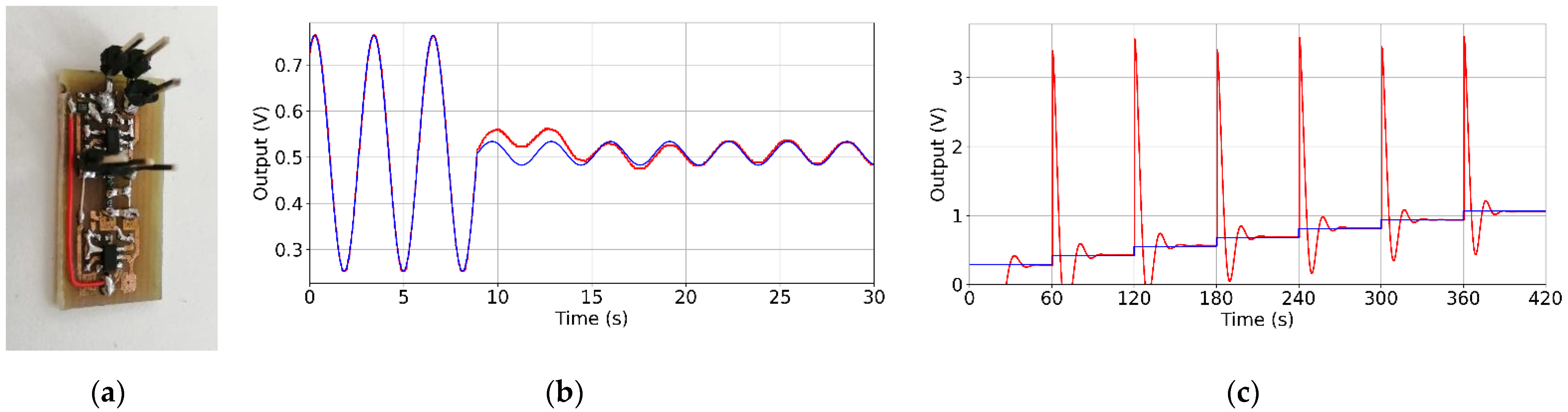
- An initial offset of 200 Ohm.
- A 0.3 Hz sine wave multiplied by a sawtooth signal. Sawtooth amplitude was 10 Ohm and its period was 10 s.
- A 100 Ohm step every 60 s, which was added to the initial offset.
- An 800 Ohm spike at the beginning and after 30 s of the simulation.
3.1.2. Differential Amplifier with a High-Pass Filter
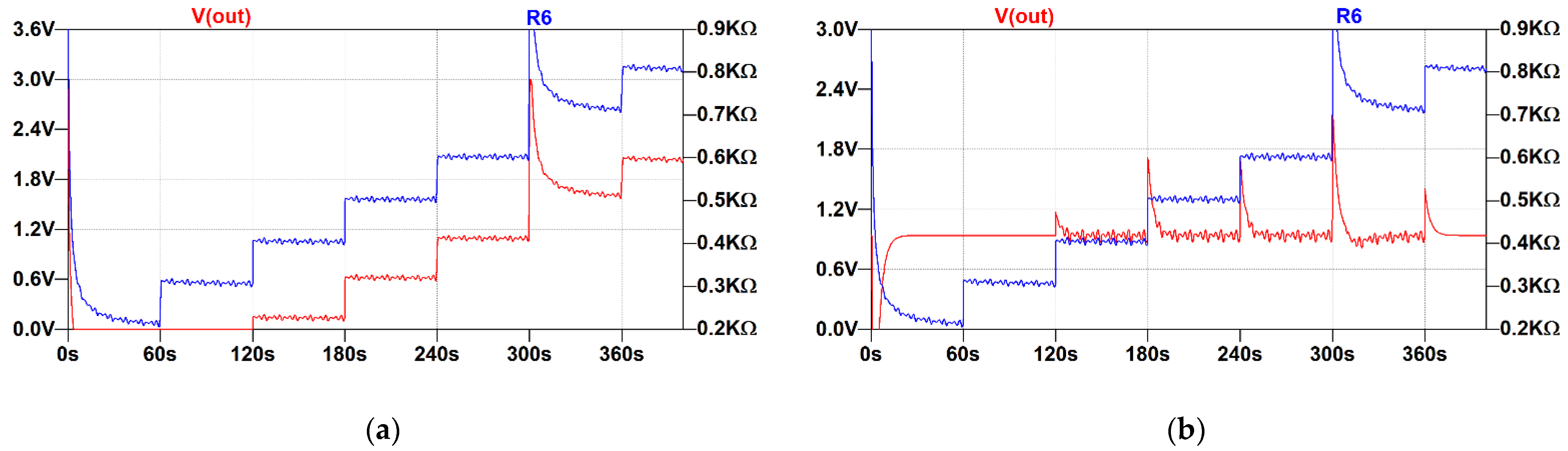
3.1.3. Proposed Low-Frequency Feedback Topology
- The improved architecture can correctly represent small changes in the resistance in presence of the offset ranging from 200 Ohm to 800 Ohm and possibly above. In such limits, the output stays well below 1.8 V, thus allowing measurement by an internal ADC of an MCU. This does not account for artifacts generated by rapid movement, and, in this case, the limits of the ADC must be observed.
- The offset is not completely removed from the signal, but it is significantly attenuated compared with the signal of interest. This feature can be used to extract information about the strain of the respiratory belt if such data are needed.
- The circuit does not exhibit parasitic oscillations or positive feedback under such conditions.
- When a rapid change in resistance occurs, the circuit needs a few tens of seconds to converge and establish a new operating point. Compared to the duration of the entire examination (a few hours), these artifacts are negligible. Furthermore, the occurrence of the apnea event during position change is unlikely.
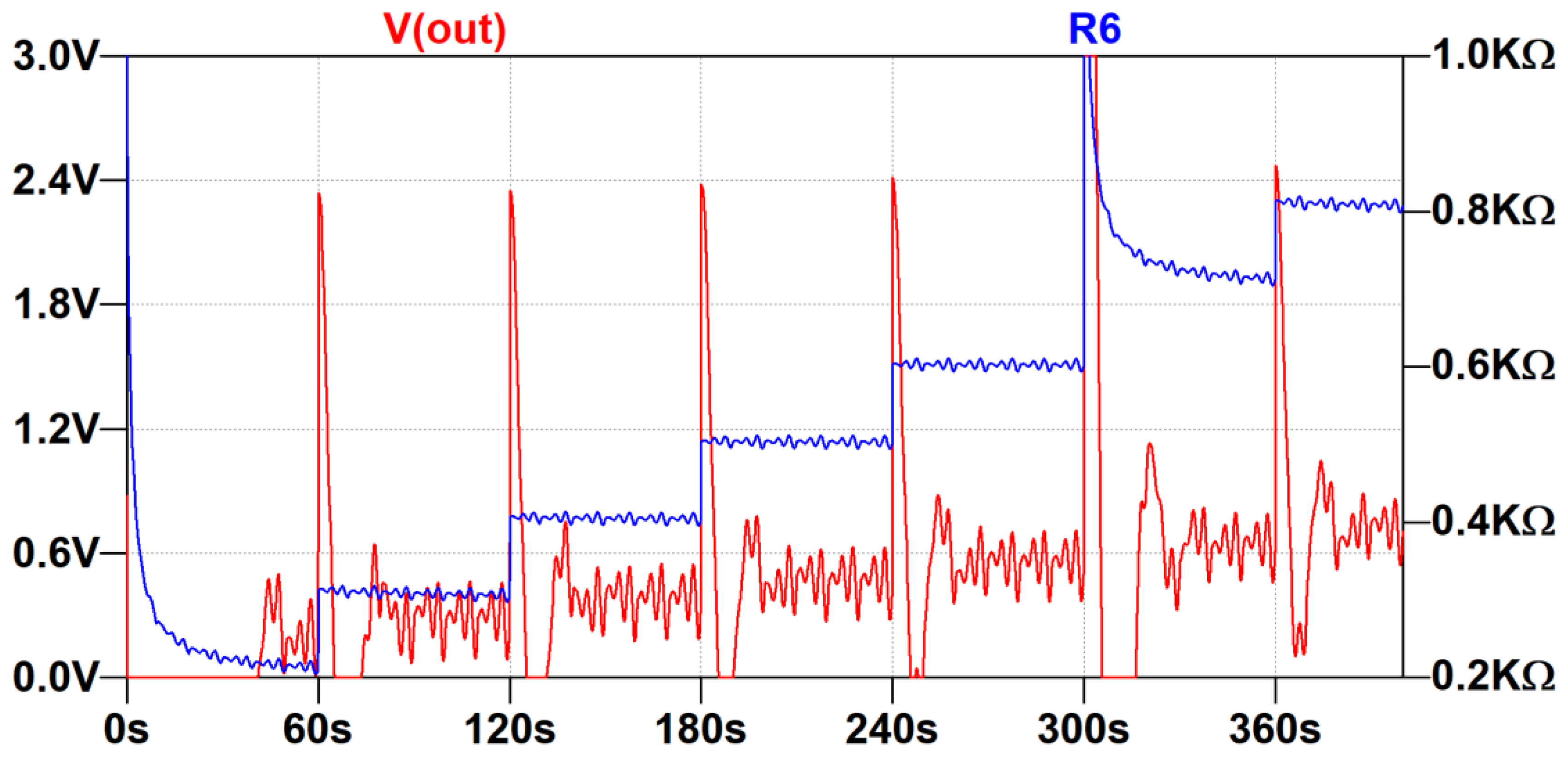
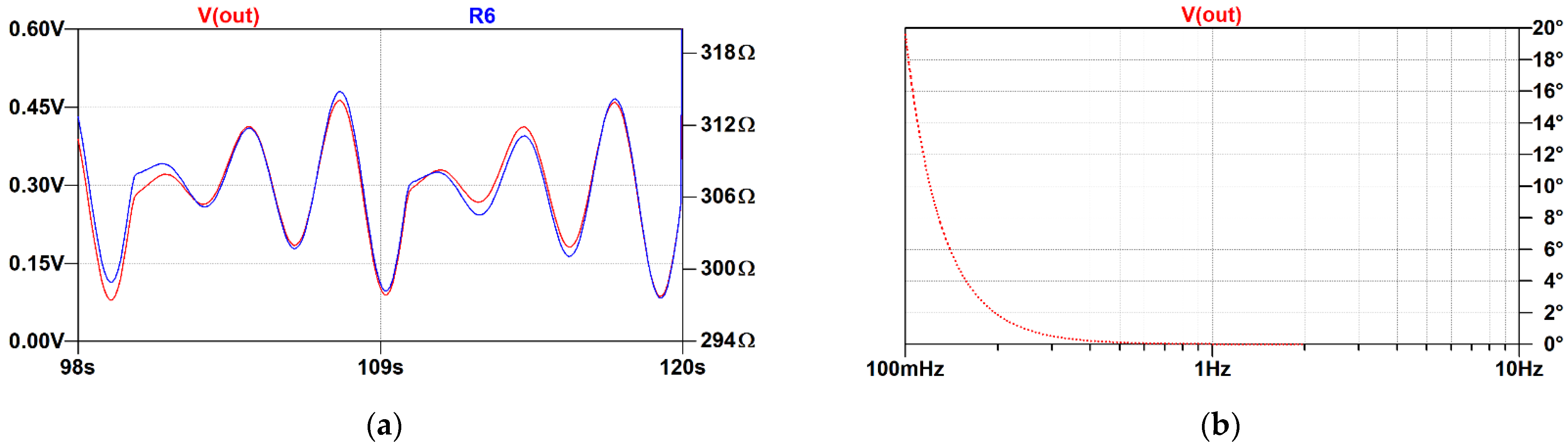
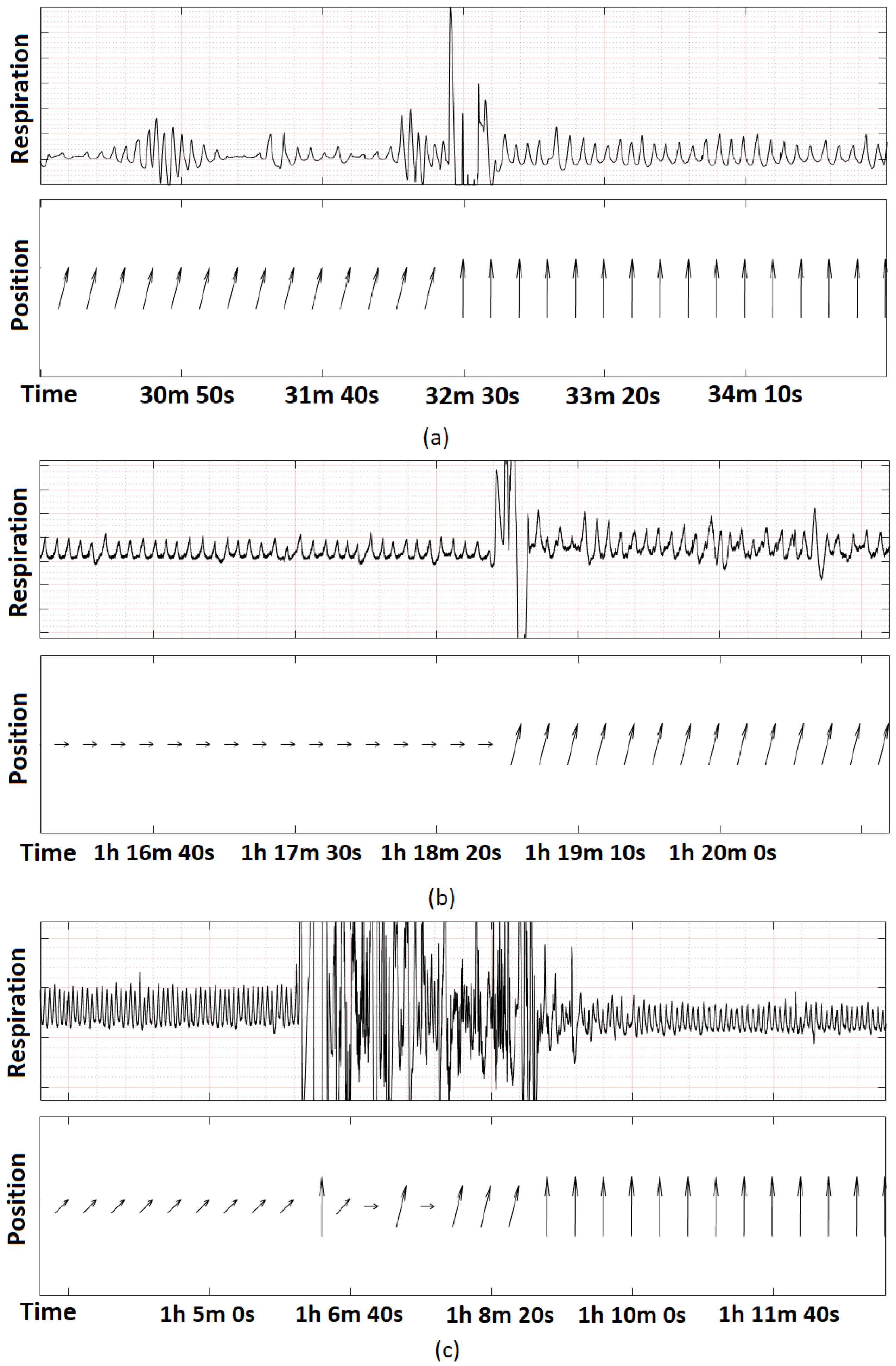
3.2. Evaluation of the Low-Frequency Feedback Prototype
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, J.; Cyganek, B. An LSTM Network for Apnea and Hypopnea Episodes Detection in Respiratory Signals. Sensors 2021, 21, 5858. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Nagubadi, S.; Kryger, M.H.; Mokhlesi, B. Epidemiology of obstructive sleep apnea: A population-based perspective. Expert Rev. Respir. Med. 2008, 2, 349–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, S.A.; Penz, E.; Fenton, M.; Skomro, R. Investigating Cost Implications of Incorporating Level III At-Home Testing into a Polysomnography Based Sleep Medicine Program Using Administrative Data. Can. Respir. J. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Lu, Q.; Li, S.; Pu, Z.; Gao, F.; Zhou, B. Risk factors associated with the severity of obstructive sleep apnea syndrome among adults. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Kushida, A.C.; Giacomini, A.; Lee, M.K.; Guilleminault, C.; Dement, W.C. Technical protocol for the use of esophageal manometry in the diagnosis of sleep-related breathing disorders. Sleep Med. 2002, 3, 163–173. [Google Scholar] [CrossRef]
- Berry, R.B.; Quan, S.F.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Mao, M.; Plante, D.T.; Pressman, M.R.; Troester, M.M.; et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; V2.6; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Koo, B.B.; Drummond, C.; Surovec, S.; Johnson, N.; Marvin, S.A.; Redline, S. Validation of a Polyvinylidene Fluoride Impedance Sensor for Respiratory Event Classification during Polysomnography. J. Clin. Sleep Med. 2011, 7, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zheng, J.; Wu, H.; Wang, W.; Wang, B.; Liu, H. Development of a Respiratory Inductive Plethysmography Module Supporting Multiple Sensors for Wearable Systems. Sensors 2012, 12, 13167–13184. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K. Respiration Rate Measurement Based on Impedance Pneumography; Texas Instruments: Dallas, TX, USA, 2011; Available online: https://www.ti.com/lit/an/sbaa181/sbaa181.pdf (accessed on 25 July 2022).
- Berry, R.B.; Ryals, S.; Girdhar, A.; Wagner, M.H. Use of Chest Wall Electromyography to Detect Respiratory Effort during Polysomnography. J. Clin. Sleep Med. 2016, 12, 1239–1244. [Google Scholar] [CrossRef] [Green Version]
- Gubbi, J.; Khandoker, A.; Palaniswami, M.S. Classification of sleep apnea types using wavelet packet analysis of short-term ECG signals. Int. J. Clin. Monit. Comput. 2011, 26, 1–11. [Google Scholar] [CrossRef]
- Hummel, R.; Bradley, T.D.; Packer, D.; Alshaer, H. Distinguishing obstructive from central sleep apneas and hypopneas using linear SVM and acoustic features. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2236–2240. [Google Scholar] [CrossRef]
- Bhandari, A.; Krahmer, F.; Raskar, R. On unlimited sampling. In Proceedings of the 2017 International Conference on Sampling Theory and Applications (SampTA), Tallinn, Estonia, 3–7 July 2017; pp. 31–35. [Google Scholar] [CrossRef] [Green Version]
- McKennoch, S.; Wilson, D.M. Autoranging compensation for variable baseline chemical sensors. In Proceedings of the SPIE-The International Society for Optical Engineering, Boston, MA, USA, 22 February 2002; pp. 96–107. [Google Scholar] [CrossRef]
- Mantenuto, P.; De Marcellis, A.; Ferri, G. Uncalibrated Analog Bridge-Based Interface for Wide-Range Resistive Sensor Estimation. IEEE Sens. J. 2011, 12, 1413–1414. [Google Scholar] [CrossRef]
- Kishore, K.; Malik, S.; Baghini, M.S.; Akbar, S.A. A Dual-Differential Subtractor-Based Auto-Nulling Signal Conditioning Circuit for Wide-Range Resistive Sensors. IEEE Sens. J. 2020, 20, 3047–3056. [Google Scholar] [CrossRef]
- De Marcellis, A.; Ferri, G.; Mantenuto, P. A novel 6-decades fully-analog uncalibrated Wheatstone bridge-based resistive sensor interface. Sens. Actuators B Chem. 2013, 189, 130–140. [Google Scholar] [CrossRef]
- Das, M.; Sivakami, V.; Pal, A.; Vasuki, B. Analog-digital conditioning circuit for RTD temperature measurement. In Proceedings of the 2018 15th IEEE India Council International Conference (INDICON), Coimbatore, India, 16–18 December 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakagawa, S.; Ishikuro, H. High-Resolution Auto-Balancing Wheatstone-Bridge with Successive Approximation of ΔΣ-Modulated Digitally Controlled Variable Resistor. In Proceedings of the 2019 26th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Genoa, Italy, 27–29 November 2019; pp. 474–477. [Google Scholar] [CrossRef]
- Leinonen, M.; Juuti, J.; Jantunen, H. Interface circuit for resistive sensors utilizing digital potentiometers. Sens. Actuators A Phys. 2007, 138, 97–104. [Google Scholar] [CrossRef]
- Johnson, C.; Chen, C. Bridge-to-computer data acquisition system with feedback nulling. IEEE Trans. Instrum. Meas. 1990, 39, 531–534. [Google Scholar] [CrossRef]
- Vallerian, M.; Hutu, F.; Miscopein, B.; Villemaud, G.; Risset, T. Additive companding implementation to reduce ADC constraints for multiple signals digitization. In Proceedings of the 2015 IEEE 13th International New Circuits and Systems Conference (NEWCAS), Grenoble, France, 7–10 June 2015; pp. 1–4. [Google Scholar] [CrossRef] [Green Version]
- De Marcellis, A.; Reig, C.; Cubells-Beltran, M.-D. Current-Based Measurement Technique for High Sensitivity Detection of Resistive Bridges With External Balancing Through Control Voltages. IEEE Sens. J. 2016, 17, 404–411. [Google Scholar] [CrossRef]
- Jain, V.; George, B. Self-balancing digitizer for resistive half-bridge. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Van Der Goes, F.; Meijer, G. A simple accurate bridge-transducer interface with continuous autocalibration. IEEE Trans. Instrum. Meas. 1997, 46, 704–710. [Google Scholar] [CrossRef] [Green Version]
- De Marcellis, A.; Ferri, G.; Mantenuto, P. Uncalibrated operational amplifier-based sensor interface for capacitive/resistive sensor applications. IET Circuits Devices Syst. 2015, 9, 249–255. [Google Scholar] [CrossRef]
- Mochizuki, K.; Watanabe, K. A high-resolution, linear resistance-to-frequency converter. IEEE Trans. Instrum. Meas. 1996, 45, 761–764. [Google Scholar] [CrossRef] [Green Version]
- Elangovan, K.; Anoop, C.S. Simple and Efficient Relaxation-Oscillator-Based Digital Techniques for Resistive Sensors—Design and Performance Evaluation. IEEE Trans. Instrum. Meas. 2020, 69, 6070–6079. [Google Scholar] [CrossRef]
- Jiang, P.; Zhu, R. Elimination of Drifts in Long-Duration Monitoring for Apnea-Hypopnea of Human Respiration. Sensors 2016, 16, 1779. [Google Scholar] [CrossRef] [Green Version]
- Sosna, C.; Buchner, R.; Lang, W. A Temperature Compensation Circuit for Thermal Flow Sensors Operated in Constant-Temperature-Difference Mode. IEEE Trans. Instrum. Meas. 2010, 59, 1715–1721. [Google Scholar] [CrossRef]
- J-Flex Electrically Conductive Silicone Sheet. Available online: https://www.j-flex.com/wp-content/uploads/2019/09/J-Flex-Datasheet_Electrically-Conductive-Silicone-Sheet-65-Shore-A.pdf (accessed on 28 August 2022).
- Karki, J. Application Note: Signal Conditioning Wheatstone Resistive Bridge Sensors; Texas Instruments: Dallas, TX, USA, 1999; Available online: http://www.ti.com/lit/an/sloa034/sloa034.pdf (accessed on 25 July 2022).
- ST Microelectronics. STM32F401xB STM32F401xC Datasheet. Available online: https://www.st.com/resource/en/datasheet/stm32f401cb.pdf (accessed on 25 July 2022).
- Kim, K.A.; Lee, I.K.; Choi, S.S.; Kim, S.S.; Lee, T.S.; Cha, E.J. Wearable transducer to monitor respiration in a wireless way. In Proceedings of the 2007 6th International Special Topic Conference on Information Technology Applications in Biomedicine, Tokyo, Japan, 8–11 November 2007; pp. 174–176. [Google Scholar] [CrossRef]
- Analog Devices. Datasheet: AD8402: 2-Channel Digital Potentiometer. 2010. Available online: http://www.analog.com/static/imported-files/data_sheets/AD8400_8402_8403.pdf (accessed on 25 July 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzazga, J.; Cyganek, B. Offset Compensation in Resistive Stretch Sensors Using Low-Frequency Feedback Topology. Electronics 2022, 11, 3158. https://doi.org/10.3390/electronics11193158
Drzazga J, Cyganek B. Offset Compensation in Resistive Stretch Sensors Using Low-Frequency Feedback Topology. Electronics. 2022; 11(19):3158. https://doi.org/10.3390/electronics11193158
Chicago/Turabian StyleDrzazga, Jakub, and Bogusław Cyganek. 2022. "Offset Compensation in Resistive Stretch Sensors Using Low-Frequency Feedback Topology" Electronics 11, no. 19: 3158. https://doi.org/10.3390/electronics11193158




