Emergency Mechanical Ventilator Design: Low-Cost and Accessible Components
Abstract
:1. Introduction
2. Methods
3. Materials
3.1. Materials for the Equipment
3.2. Materials for the Components
3.3. Structural Materials
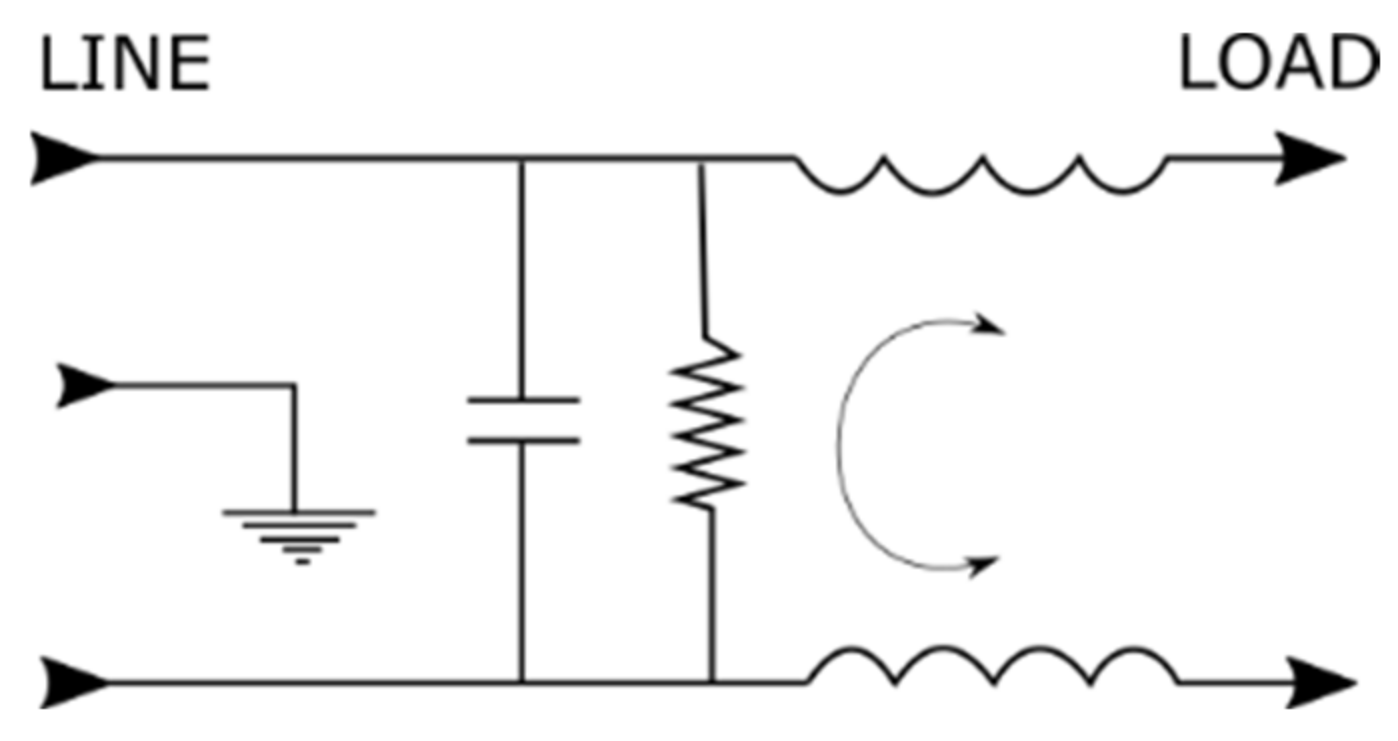
3.4. Insulation of Electrical and Electronic Systems
3.5. Airway Equipment
4. Design
4.1. Mechanical Aspects
4.2. Electrical and Electronic Aspects
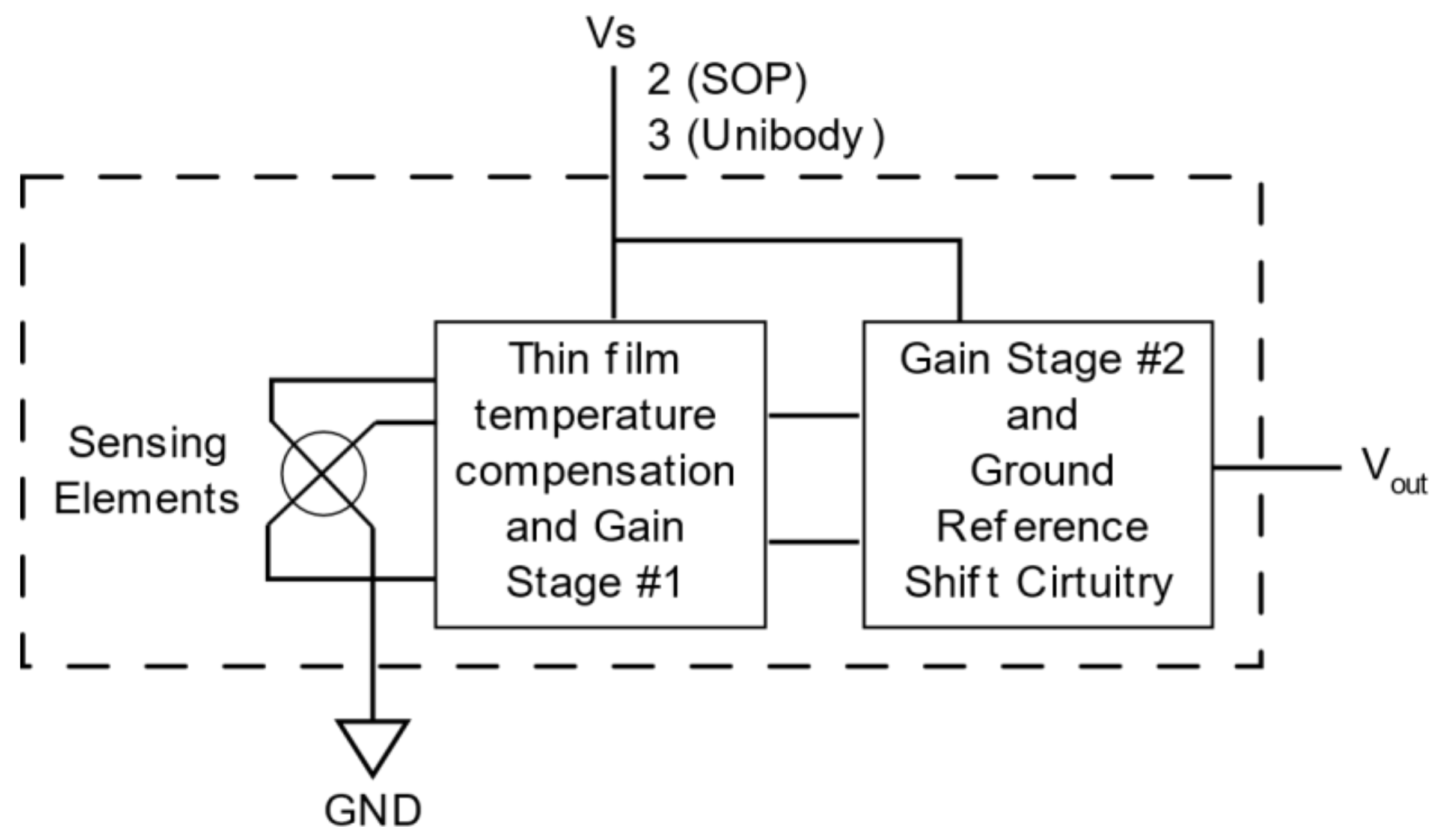
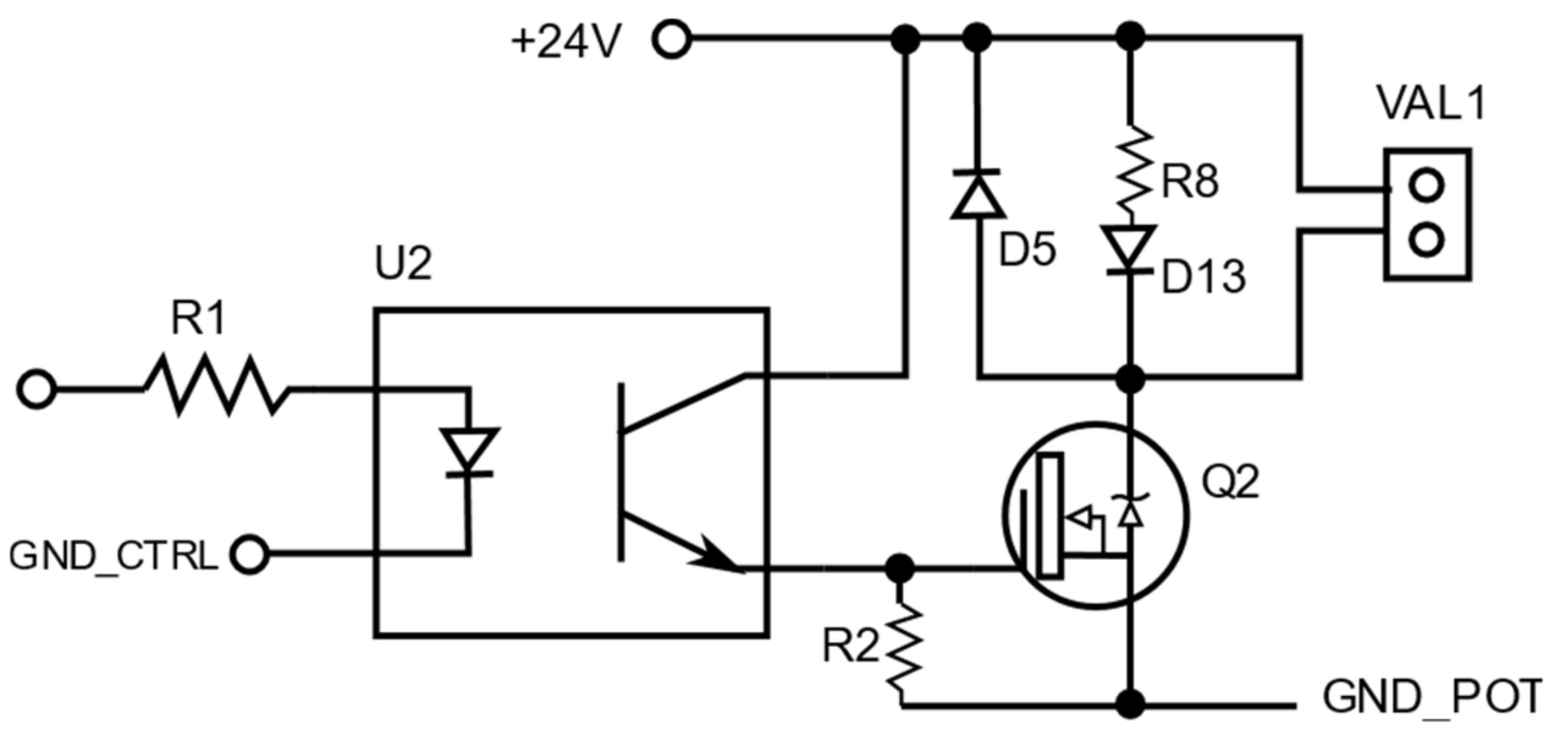
4.2.1. Electrical Design
4.2.2. Electronic Design
4.3. Interface and Control
4.4. Recommended GUI Components
4.5. Ventilatory Variable Control Algorithms
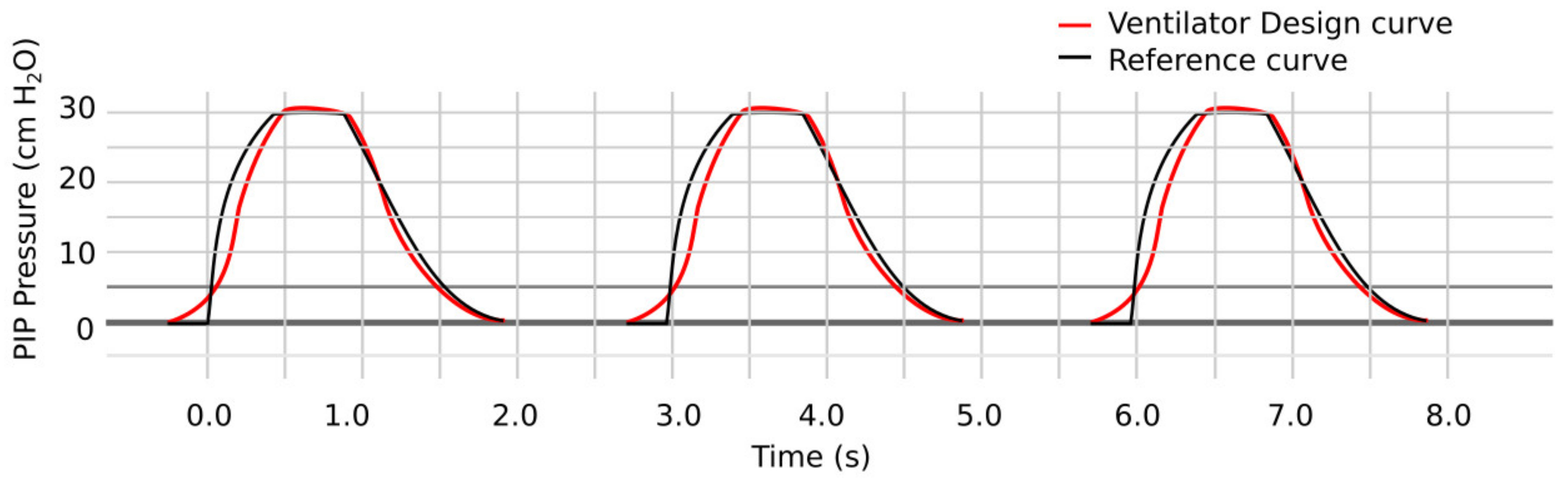
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Universidad Salvadoreña Desarrolla Respirador Mecánico para Apoyar Hospitales. Diario La Tribuna, 26 March 2020. Available online: https://www.latribuna.hn/2020/03/26/universidad-salvadorena-desarrolla-respirador-mecanico-para-apoyar-hospitales/ (accessed on 22 October 2022).
- MPS Created an Open-Source Ventilator to Battle COVID-19. EEWeb, 14 April 2020. Available online: https://www.eeweb.com/mps-created-an-open-source-ventilator-to-battle-covid-19/ (accessed on 22 October 2022).
- Esta Iniciativa Española Diseñará Respiradores Artificiales Baratos Contra el Coronavirus. Available online: https://www.elespanol.com/omicrono/20200314/iniciativa-espanola-disenara-respiradores-artificiales-baratos-coronavirus/474454447_0.html (accessed on 22 October 2022).
- OEDK—Rice University—ApolloBVM. Available online: http://www.oedk.rice.edu/apollobvm (accessed on 22 October 2022).
- Coventor Emergency Ventilator—Adult Manual Resuscitator Compressor Available from Technology Commercialization. Available online: https://license.umn.edu/product/coventor-emergency-ventilator---adult-manual-resuscitator-compressor (accessed on 22 October 2022).
- VU Engineers and VUMC Doctors Team up for Open-Source Ventilator Design. Vanderbilt University, 27 March 2020. Available online: https://news.vanderbilt.edu/2020/03/27/vu-engineers-and-vumc-doctors-team-up-for-open-source-ventilator-design/ (accessed on 22 October 2022).
- Diseño de Placa para Respirador de Código Abierto. Revista Electrónica Convertronic—Noticias y Actualidad Electrónica. Available online: https://convertronic.net/semiconductores/diseno-2/8660-diseno-placaorespiradorocodigo-abierto.html (accessed on 22 October 2022).
- Pcb Prototype Manufacturing Factory Medical Ventilator. FASTPCBA, 11 April 2020. Available online: https://www.medicalpcba.com/product/pcb-prototype-manufacturing-factory/ (accessed on 22 October 2022).
- TheOpenVentilator. Available online: https://www.theopenventilator.com/ (accessed on 22 October 2022).
- Würth Elektronik Circuit Board Technology Produces PCBs for Ventilators | Würth Elektronik: Würth Elektronik Group > News. Available online: https://www.we-online.com/web/en/wuerth_elektronik/news_weg/News_Detail_WE_Gruppe_112665.php (accessed on 22 October 2022).
- Zhu, J.; Ren, Z.; Lee, C. Toward Healthcare Diagnoses by Machine-Learning-Enabled Volatile Organic Compound Identification. ACS Nano 2020, 15, 894–903. [Google Scholar] [CrossRef]
- Björklund, L.J.; Ingimarsson, J.; Curstedt, T.; John, J.; Robertson, B.; Werner, O.; Vilstrup, C.T.; Bj, L.J. Manual Ventilation with a Few Large Breaths at Birth Compromises the Therapeutic Effect of Subsequent Surfactant Replacement in Immature Lambs. Pediatr. Res. 1997, 42, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasha, S.; Babar, E.T.R.; Schneider, J.; Heithaus, J.; Mujeeb-U-Rahman, M. A Low-cost, Automated, Portable Mechanical Ventilator for Developing World. In Proceedings of the 2021 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 19–23 October 2021; pp. 112–118. [Google Scholar] [CrossRef]
- Ochukpue, C.O.; Kingsley, T.U.; Ekwere, I. Abstract PR118: Mechanical Ventilation in the Intensive Care Unit in A Nigerian Hospital. Anesth. Analg. 2016, 123, 160. [Google Scholar] [CrossRef]
- Bayram, B.; Şancı, E. Invasive mechanical ventilation in the emergency department. Turk. J. Emerg. Med. 2019, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vasan, A.; Weekes, R.; Connacher, W.; Sieker, J.; Stambaugh, M.; Suresh, P.; Lee, D.E.; Mazzei, W.; Schlaepfer, E.; Vallejos, T.; et al. MADVent: A low-cost ventilator for patients with COVID-19. Med. Devices Sens. 2020, 3, e10106. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R.; Kacmarek, R.M. Essentials of Mechanical Ventilation, 3rd ed.; McGraw Hill Education, Medical Publishing Division: New York, NY, USA, 2014. [Google Scholar]
- Flor, O.C.; Suarez, F.M.; Toapanta, C.F.; Rosales, L.D.; Alvarez, G.A. Alvarez, Evaluaciones matemáticas de los mecanismos para ventiladores artificiales emergentes. Rev. Espac. 2020, 41, 383–394. Available online: https://www.revistaespacios.com/a20v41n29/20412928.html (accessed on 22 October 2022).
- El Majid, B.; El Hammoumi, A.; Motahhir, S.; Lebbadi, A.; El Ghzizal, A. Preliminary design of an innovative, simple, and easy-to-build portable ventilator for COVID-19 patients. Euro-Mediterr. J. Environ. Integr. 2020, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Chatburn, R.L.; Mireles-Cabodevila, E.; Tobin, M.J. Chapter 3. Basic Principles of Ventilator Design. In Principles and Practice of Mechanical Ventilation, 3rd ed.; Tobin, M.J., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2013; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=520§ionid=41692239 (accessed on 22 October 2022).
- Anda, G.F.V.-D.; Chávez, M.R.-D.; Pérez-Castañeda, A.I.; Vázquez-Moreno, P.; Dávila-Fernández, J.C.; Delaye-Aguilar, M.G. Mechanical ventilator as a shared resource for the COVID-19 pandemic. Gac. Médica México 2020, 156, 4892. [Google Scholar] [CrossRef]
- Garmendia, O.; Rodríguez-Lazaro, M.A.; Otero, J.; Phan, P.; Stoyanova, A.; Dinh-Xuan, A.T.; Gozal, D.; Navajas, D.; Montserrat, J.M.; Farré, R. Low-cost, easy-to-build noninvasive pressure support ventilator for under-resourced regions: Open source hardware description, performance and feasibility testing. Eur. Respir. J. 2020, 55, 2000846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flor, O.; Naranjo, C.; Tapia, J.; Flores, E.; Coba, A.; Chango, E. Diseño de ventilador mecánico emergente en modo asistido/controlado y espontáneo por presión. SciELO Prepr. 2020, 1, 130–137. [Google Scholar] [CrossRef]
- UNE EN ISO 10993-12:2022; Biological Evaluation of Medical devices—Part 12: Sample Preparation and Reference Materials. UNE: Madrid, Spain, 2022. Available online: https://www.en-standard.eu/une-en-iso-10993-12-2022-biological-evaluation-of-medical-devices-part-12-sample-preparation-and-reference-materials-iso-10993-12-2021/ (accessed on 22 October 2022).
- Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. Available online: https://www.legislation.gov.uk/eudr/1993/42/2007-10-11 (accessed on 22 October 2022).
- UNE-EN ISO 10993-15:2009; Evaluación Biológica de Productos Sanitarios. UNE: Madrid, Spain, 2009. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0044133 (accessed on 22 October 2022).
- UNE-EN ISO 10993-13:2000; Evaluación Biológica de Productos San. UNE: Madrid, Spain, 2000. Available online: https://tienda.aenor.com/norma-une-en-iso-10993-13-2000-n0022378 (accessed on 22 October 2022).
- Rubio-Romero, J.C.; del Carmen Pardo-Ferreira, M.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef] [PubMed]
- AAMI—Association for the Advancement of Medical Instrumentation. Dialysis Water Solutions, 2 June 2015. Available online: https://dialysiswatersolution.com/regulations-and-guidelines/ansiaami/ (accessed on 22 October 2022).
- Kalpakjian, S.; Schmid, S.R. Manufactura, Ingeniería y Tecnología. Pearson Educación: London, UK, 2002. [Google Scholar]
- Ashby, M.F.; Shercliff, H.; Cebon, D. Materials: Engineering, Science, Processing and Design, 1st ed.; Butterworth-Heinemann: Amsterdam, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Torres Velásquez, J.A.; Viáfara Avila, R.R.; Chacón Cardona, C.A. Study of Magnetic Shielding in Conductors Materials as a Function of the Frequency. Cienc. Desarro. 2016, 7, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Williams, T. EMC for Product Designers, 5th ed.; Newnes: Oxford, UK, 2017. [Google Scholar]
- Posada, A.O.G.; Téllez, D.A.L.; Rojas, J.R.; Barrado, J.R. Ciencia e Ingeniería Neogranadina, Materiales Compuestos de Matriz Polimérica Usados para el Blindaje de Interferencia Electromagnética. Available online: https://scholar.google.com/citations?view_op=view_citation&hl=es&user=CdPkP1AAAAAJ&citation_for_view=CdPkP1AAAAAJ:9yKSN-GCB0IC (accessed on 23 October 2022).
- Miño, C.; Flor, O.; Quiroga, J.; Cuaycal, A. Remote Variable Monitoring App for Mechanical Ventilators Used in COVID-19. In Artificial Intelligence, Computer and Software Engineering Advances; Botto-Tobar, M., Cruz, H., Díaz Cadena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1326, pp. 303–315. [Google Scholar] [CrossRef]
- Wilson, R.; Kuruvilla, J.; George, G. Materials for Potential Emi Shielding Applications: Processing, Properties and Current Trends; Elsevier: Waltham, MA, USA, 2019. [Google Scholar]
- ISO—ISO/CD 11137-1; Sterilization of Health Care Products—Radiation—Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/81721.html (accessed on 23 October 2022).
- UNE-EN ISO 11135-1:2007; Esterilización de Productos Sanitarios. UNE: Madrid, Spain, 2007. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=norma-une-en-iso-11135-1-2007-n0039866 (accessed on 23 October 2022).
- UNE-EN ISO 17665-1:2007; Esterilización de Productos Sanitarios. UNE: Madrid, Spain, 2007. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?Tipo=N&c=N0039217 (accessed on 23 October 2022).
- Dempsey, D.J.; Thirucote, R.R. Sterilization of Medical Devices: A Review. J. Biomater. Appl. 1988, 3, 454–523. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.R.; Jerina, K.L. (Eds.) Fatigue and Fracture of Medical Metallic Materials and Devices; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar] [CrossRef]
- Jain, A.; Ganesh, N.; Venkatesh, M.P. Quality Standards for Medical Devices. Int. J. Drug Regul. Aff. 2018, 2, 19–24. [Google Scholar] [CrossRef] [Green Version]
- John, K.R.S.; Narayan, R.J.; Gibbons, D.; Drummond, C.K.; Hdeib, A.; Khan, F.R.; Khan, M.; Zhou, Y.; Nag, S.; Banerjee, R.; et al. Biocompatibility of Metallic Materials for Medical Devices—The Effects of Corrosion and Corrosion Products. In Materials for Medical Devices; Narayan, R.J., Ed.; ASM International: Almere, The Netherlands, 2012; pp. 73–78. [Google Scholar] [CrossRef]
- Williams, D.; Flory, S.; King, R.; Thornton, M.; Dingley, J. A low oxygen consumption pneumatic ventilator for emergency construction during a respiratory failure pandemic. Anaesthesia 2010, 65, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Muñoz, F. Ventilación mecánica. Acta Médica Peru. 2011, 28, 87–104. [Google Scholar]
- Hemmati, R.; Wu, F.; El-Refaie, A. Survey of Insulation Systems in Electrical Machines. In Proceedings of the 2019 IEEE International Electric Machines & Drives Conference (IEMDC), San Diego, CA, USA, 12–15 May 2019; pp. 2069–2076. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.H.; Al-Arainy, A.A.; Qureshi, M.I. Electrical Insulation in Power Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Manosalva, O.A.N.; Traslaviña, N.M.P. Reporte de la experiencia en la realización de ensayos de compatibilidad electromagnética sobre ventiladores mecánicos en Colombia. Rev. Ing. 2020, 50, 54–71. [Google Scholar] [CrossRef]
- Restrepo, R.D.; Serrato, D.M.; Adasme, R. Assessing Respiratory System Mechanical Function. Clin. Chest Med. 2016, 37, 615–632. [Google Scholar] [CrossRef] [PubMed]









| Material | Electrical Resistivity (Ohm-cm) | Relative Permeability | Modulus of Elasticity (GPA) | Tensile Strength (MPa) | Specific Weight (kN/m3) | The Frequency Range of Attenuation (Hz) [7] |
|---|---|---|---|---|---|---|
| Copper | 0.00000170 | 0.99999 | 110 | 210 | 76,165 | 32,000 a 100,000 |
| Low-carbon steel | 0.0000143 | 1.075 | 205 | 365 | 76,520 | 60–12,000 |
| Aluminum | 0.00000270 | 1.000023 | 68 | 125 | 26,477 | 60–32,000 |
| Mu metal | 0.0000850 | 8000 | 206 | 715 | 86,328 | 1000–10,000 |
| Permalloy | 0.0000850 | 20,000 | 206 | 715 | 682 | 1000–10,000 |
| Element | Quantity | Unit Power Consumption (W) | Total Power Consumption (W) |
|---|---|---|---|
| Embedded system (with graphics generation capability) | 1 | 12.5 | 12.5 |
| Proportional valve (inhalation and exhalation) | 2 | 8 | 16 |
| Relief valve | 1 | 2 | 2 |
| Cooling Fan | 1 | 10 | 10 |
| Electronic board with sensors | 1 | 10 | 10 |
| Total Power Consumed | 45.5 |
| Component | Application | Characteristics |
|---|---|---|
| Embedded system Raspberry P13 B Embedded system Arduino Nano | Master controller and graphics emitter Proportional valve controller | Broadcom BCNQ 83 7B Corter-A53 64 bit SoC at 1.4 GHz 1 GB LPDDR2 SDRAM 5 V/2.5ADC via micro USB connector 1 × full-size HDMI, Amel AT mega 328 5 V DC via micro USB connector |
| Proportional valve Humphrey 10032300 Relief valve ASCO 411L3112FVO | Inhalation Line Control Valve Valve for rapid pressure relief | Ideal design for precision air or inert gas delivery control (0–100% proportional opening) Operator voltage: 12 V DC, 2.0 W |
| Convertor DC/DC MDS20A-06 | Allows converting the supply voltage (12 V) to the power supply voltage of the embedded system | Ideal input voltage: 12 V (9–18 V) Output: 5 V, 400 W Max, Efficiency: 86% |
| Pressure sensor MXP5010DP | Allows measurement of airway pressure | Measuring range: 0 to 10 kPa, Supply voltage: 5VDC, Sensitivity:4 413 mV/cmH2O |
| POSIFA Flow Sensor PMF4101 V | Allows measurement of airway pressure | Measuring range: 0–10 kPa Supply voltage: 5 V DC Sensitivity: 4,413 mV/cmH2O |
| Measuring range: 0–1001 min Supply voltage: 6–10 V DC Analog output: 1–5 V DC | Allows the oxygen level of the gas entering the patient to be measured with a small amount of conditioning | Measuring range: 21–100%. Analog output: 8–40 mV |
| Parameter Control | Graphics | Sensed Parameter Viewer | Other Relevant Functions |
|---|---|---|---|
| PIP PEEP FR Ti Inspiration pause Trigger | Pressure vs. time Flow vs. time | PIP Tidal Volume Minute Volume FR FiO2 Trigger | Patient identification Alarms Configuration of equipment buttons Ventilation mode identification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flor, O.; Fuentes, M.; Carvajal, H.; Quiroga, J.; Luzuriaga, V.; Tapia, J.; Acosta-Vargas, P. Emergency Mechanical Ventilator Design: Low-Cost and Accessible Components. Electronics 2022, 11, 3910. https://doi.org/10.3390/electronics11233910
Flor O, Fuentes M, Carvajal H, Quiroga J, Luzuriaga V, Tapia J, Acosta-Vargas P. Emergency Mechanical Ventilator Design: Low-Cost and Accessible Components. Electronics. 2022; 11(23):3910. https://doi.org/10.3390/electronics11233910
Chicago/Turabian StyleFlor, Omar, Mauricio Fuentes, Henry Carvajal, Josué Quiroga, Verónica Luzuriaga, Jeysson Tapia, and Patricia Acosta-Vargas. 2022. "Emergency Mechanical Ventilator Design: Low-Cost and Accessible Components" Electronics 11, no. 23: 3910. https://doi.org/10.3390/electronics11233910
APA StyleFlor, O., Fuentes, M., Carvajal, H., Quiroga, J., Luzuriaga, V., Tapia, J., & Acosta-Vargas, P. (2022). Emergency Mechanical Ventilator Design: Low-Cost and Accessible Components. Electronics, 11(23), 3910. https://doi.org/10.3390/electronics11233910











