Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization
Abstract
1. Introduction
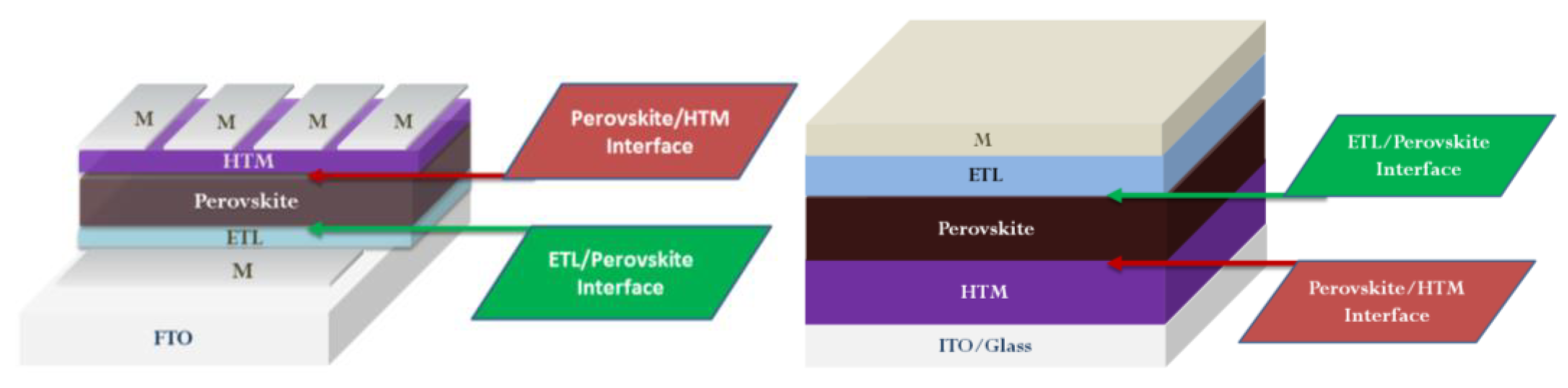
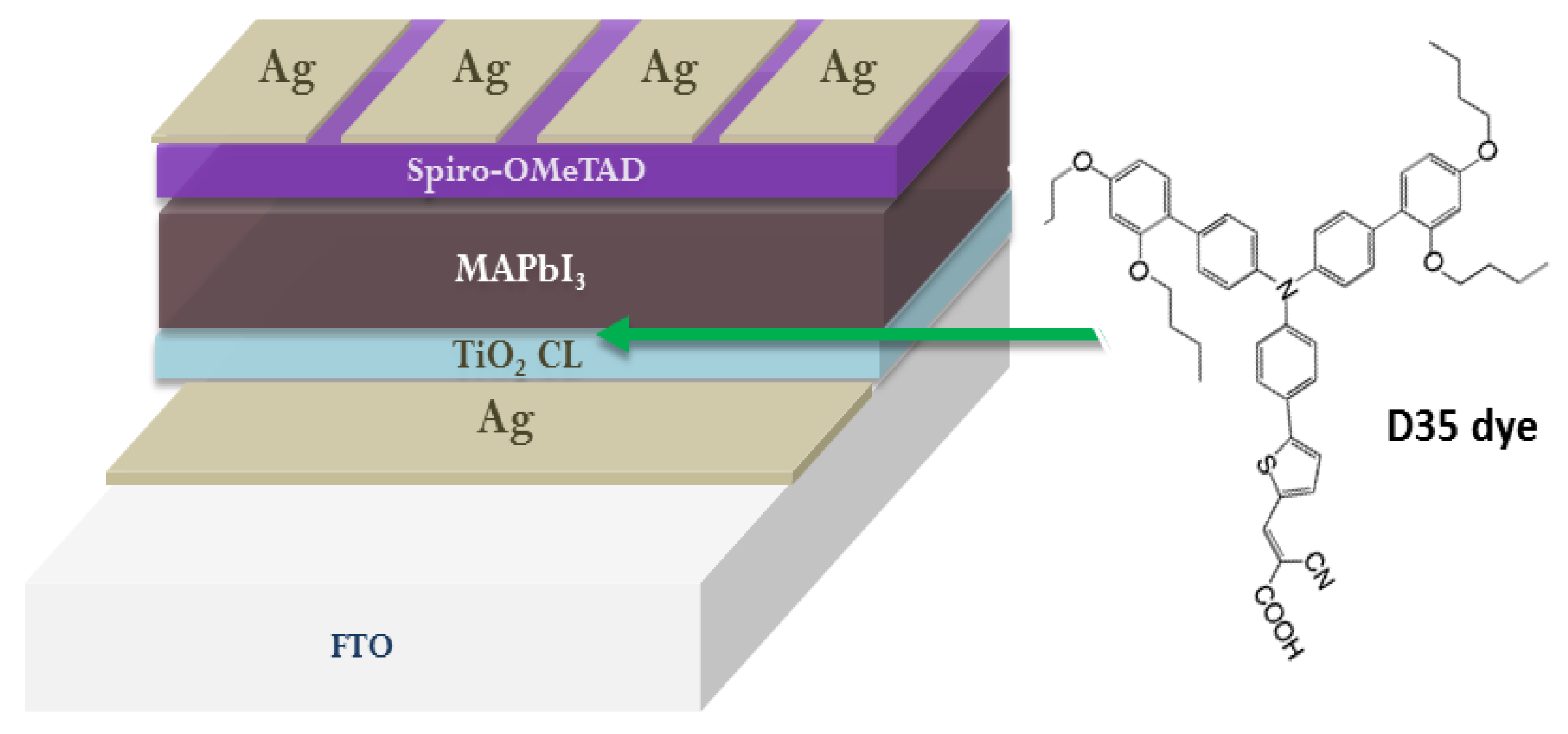
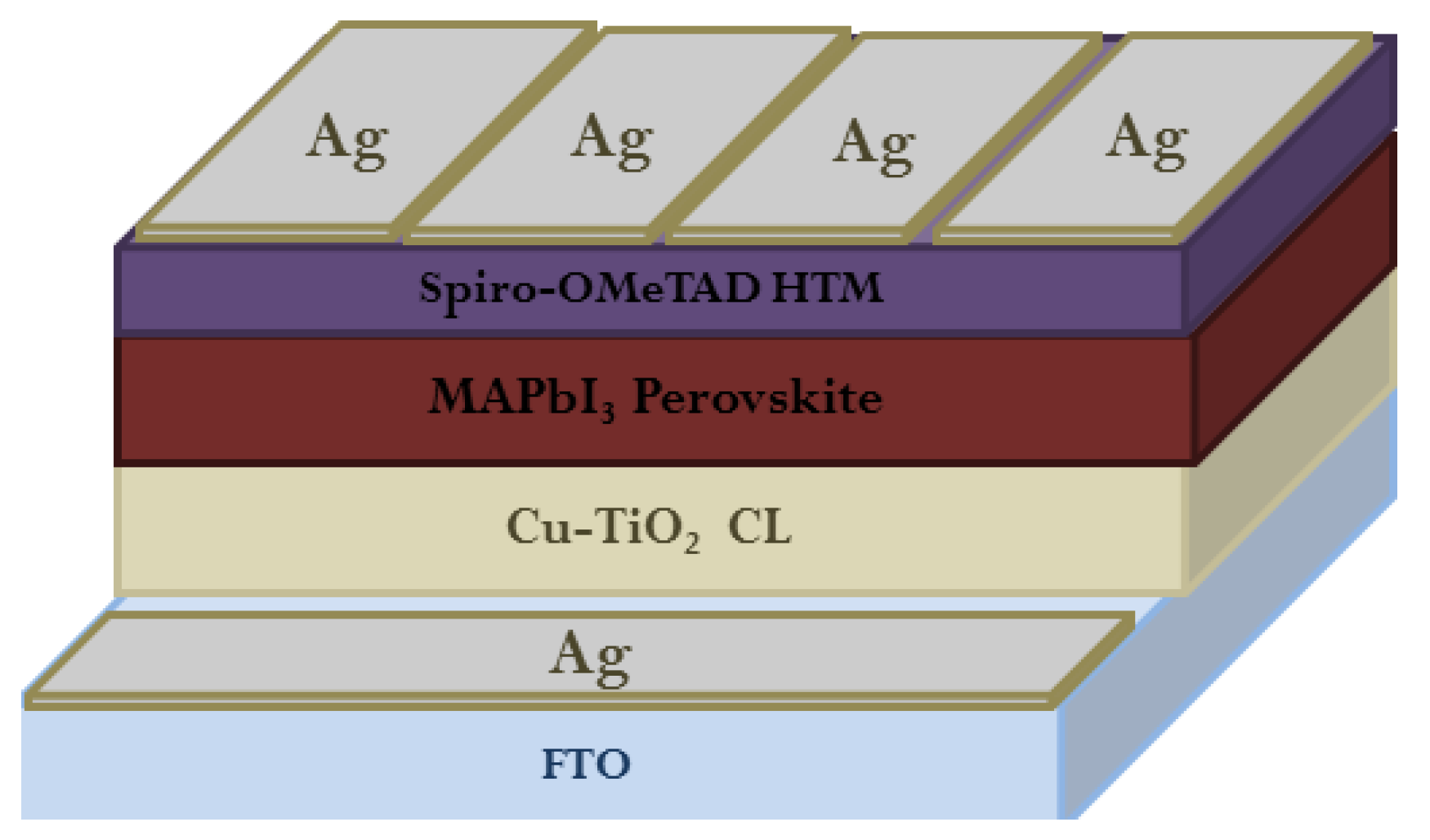
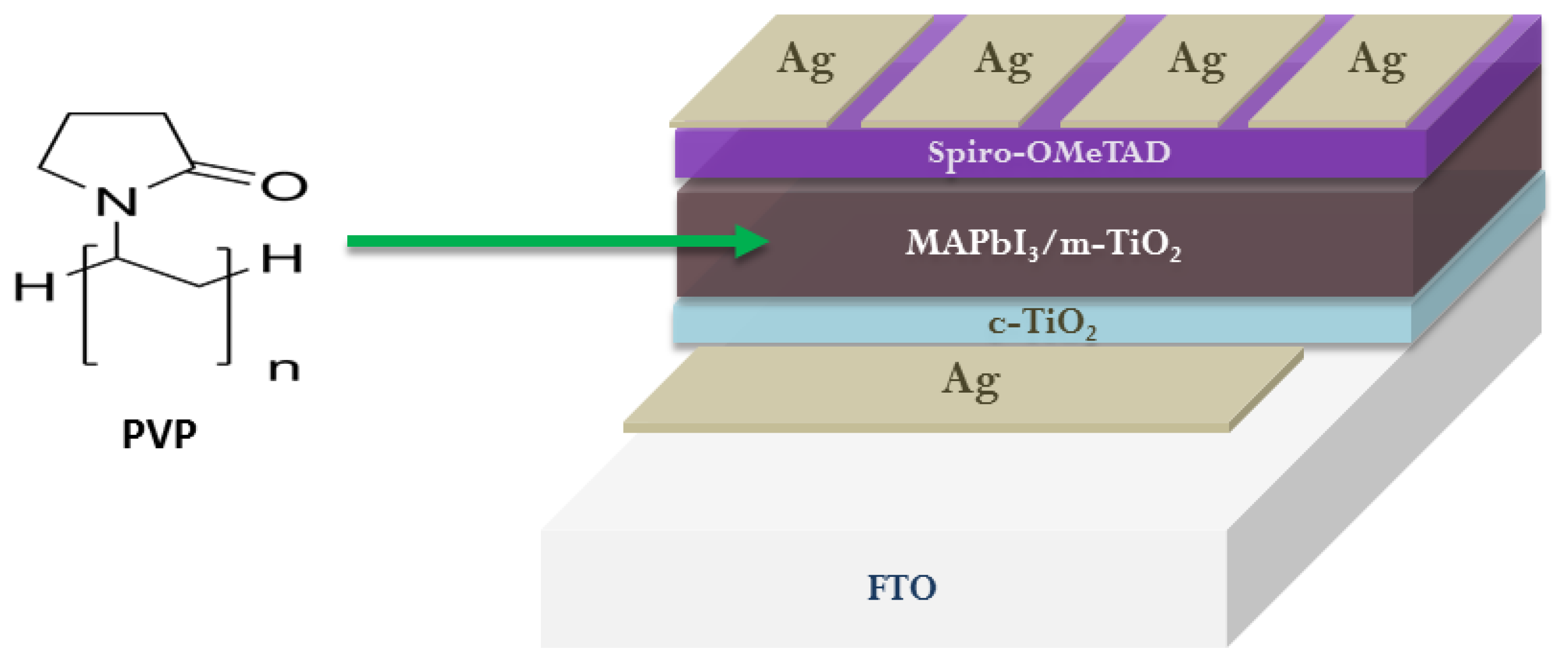
2. Functionalization of ETL/Perovskite Interface
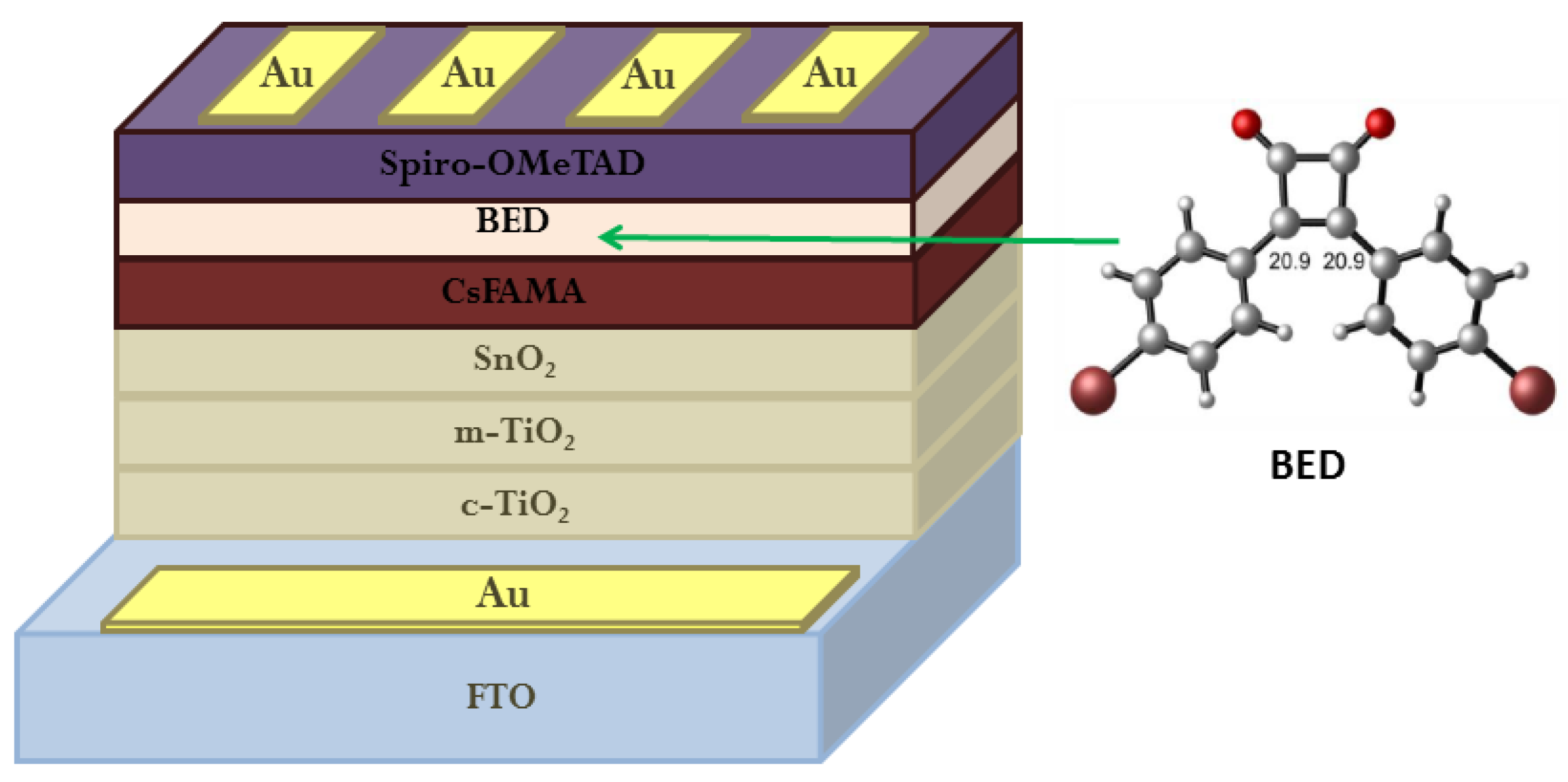
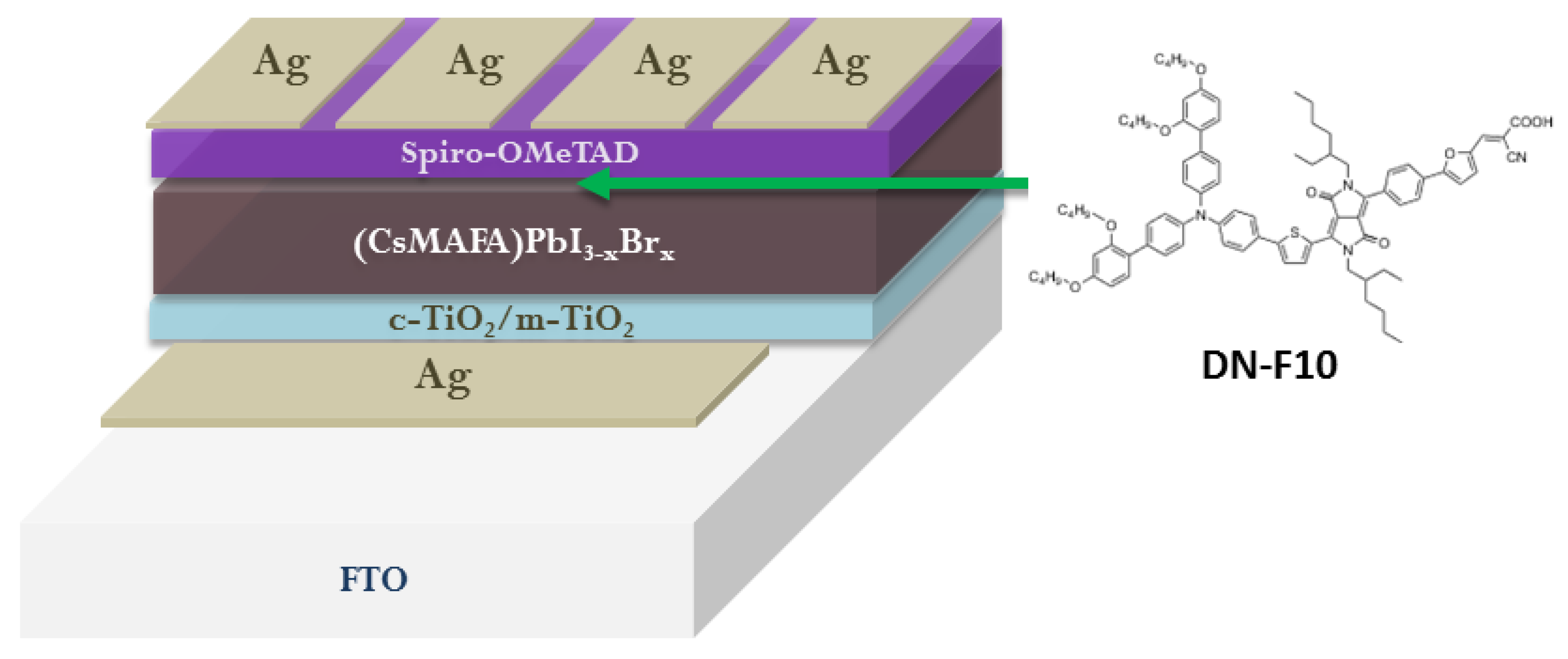
3. Functionalization of Perovskite/HTM Interface
4. Conclusions and Perspectives (and Future Directions)
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-Functionalized Interfaces for Highly Efficient Inverted Perovskite Solar Cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef]
- Chen, C.-I.; Wu, S.; Lu, Y.-A.; Lee, C.-C.; Ho, K.-C.; Zhu, Z.; Chen, W.-C.; Chueh, C.-C. Enhanced Near-Infrared Photoresponse of Inverted Perovskite Solar Cells Through Rational Design of Bulk-Heterojunction Electron-Transporting Layers. Adv. Sci. 2019, 6, 1901714. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for Commercializing Perovskite Solar Cells. Science 2018, 361, 1214. [Google Scholar] [CrossRef] [PubMed]
- Correa-Baena, J.P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Stranks, S.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2017, 2, 17102. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat. Energy 2016, 1, 15001–15006. [Google Scholar] [CrossRef]
- Bu, T.; Li, J.; Zheng, F.; Chen, W.; Wen, X.; Ku, Z.; Peng, Y.; Zhong, J.; Cheng, Y.-B.; Huang, F. Universal passivation strategy to slot-die printed SnO2 for hysteresis-free efficient flexible perovskite solar module. Nat. Commun. 2018, 9, 4609. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S.F. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef] [PubMed]
- Yun, A.J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef]
- Raiford, J.A.; Belisle, R.A.; Bush, K.A.; Prasanna, R.; Palmstrom, A.F.; McGehee, M.D.; Bent, S.F. Atomic layer deposition of vanadium oxide to reduce parasitic absorption and improve stability in n–i–p perovskite solar cells for tandems. Sustain. Energy Fuels 2019, 3, 1517–1525. [Google Scholar] [CrossRef]
- Kamat, P.V.; Kuno, M. Halide Ion Migration in Perovskite Nanocrystals and Nanostructures. Acc. Chem. Res. 2021, 54, 520–531. [Google Scholar] [CrossRef]
- Ricciarelli, D.; Kaiser, W.; Mosconi, E.; Wiktor, J.; Ashraf, M.W.; Malavasi, L.; Ambrosio, F.; De Angelis, F. Reaction Mechanism of Photocatalytic Hydrogen Production at Water/Tin Halide Perovskite Interfaces. ACS Energy Lett. 2022, 7, 1308–1315. [Google Scholar] [CrossRef]
- Shin, S.S.; Lee, S.J.; Seok, S.I. Metal Oxide Charge Transport Layers for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900455. [Google Scholar] [CrossRef]
- Xing, G.; Wu, B.; Chen, S.; Chua, J.; Yantara, N.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Interfacial Electron Transfer Barrier at Compact TiO2/CH3NH3PbI3 Heterojunction. Small 2015, 11, 3606. [Google Scholar] [CrossRef]
- Jung, K.; Lee, J.; Im, C.; Do, J.; Kim, J.; Chae, W.-S.; Lee, M.-J. Highly Efficient Amorphous Zn2SnO4 Electron-Selective Layers Yielding over 20% Efficiency in FAMAPbI3-Based Planar Solar Cells. ACS Energy Lett. 2018, 3, 2410–2417. [Google Scholar] [CrossRef]
- Zhong, Y.; Hufnagel, M.; Thelakkat, M.; Li, C.; Huettner, S. Role of PCBM in the Suppression of Hysteresis in Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1908920. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, H.L.; Vikraman, D.; Jaffery, S.H.A.; Nazir, G.; Shahzad, F.; Batoo, K.M.; Jung, J.W.; Kang, J.W.; Kim, H.-S. Tuning of electron transport layers using MXene/metal-oxide nanocomposites for perovskite solar cells and X-ray detectors. Nanoscale 2023, 15, 7329–7343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, J.; Zai, H.; Ni, D.; Wang, J.; Xue, P.; Li, N.; Jia, B.; Lu, H.; Zhang, Y.; et al. Reducing Energy Disorder in Perovskite Solar Cells by Chelation. J. Am. Chem. Soc. 2022, 144, 5400–5410. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, H.; Xia, X.; Wang, X.; Li, F. Suppressing interfacial defect formation derived from in-situ-generated polyethylenimine-based 2D perovskites to boost the efficiency and stability NiOx-based inverted planar perovskite solar cells. Appl. Surf. Sci. 2021, 548, 149276. [Google Scholar] [CrossRef]
- Ho, I.-H.; Huang, Y.-J.; Cai, C.-E.; Liu, B.-T.; Wu, T.-M.; Lee, R.-H. Enhanced Photovoltaic Performance of Inverted Perovskite Solar Cells through Surface Modification of aNiOx-Based Hole-Transporting Layer with Quaternary Ammonium Halide–Containing Cellulose Derivatives. Polymers 2023, 15, 437. [Google Scholar] [CrossRef]
- Balis, N.; Zaky, A.A.; Perganti, D.; Kaltzoglou, A.; Sygellou, L.; Katsaros, F.; Stergiopoulos, T.; Kontos, A.G.; Falaras, P. Dye Sensitization of Titania Compact Layer for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6161–6171. [Google Scholar] [CrossRef]
- Zaky, A.A.; Balis, N.; Gkini, K.; Athanasekou, C.; Kaltzoglou, A.; Stergiopoulos, T.; Falaras, P. Dye Engineered Perovskite Solar Cells under Accelerated Thermal Stress and Prolonged Light Exposure. ChemistrySelect 2020, 5, 4454–4462. [Google Scholar] [CrossRef]
- Chouk, R.; Haouanoh, D.; Aguir, C.; Bergaoui, M.; Toubane, M.; Bensouici, F.; Tala-Ighil, R.; Erto, A.; Khalfaoui, M. Dye Sensitized TiO2 and ZnO Charge Transport Layers for Efficient Planar Perovskite Solar Cells: Experimental and DFT Insights. J. Electron. Mater. 2020, 49, 1396–1403. [Google Scholar] [CrossRef]
- Noh, Y.W.; Lee, J.H.; Jin, I.S.; Park, S.H.; Jung, J.W. Enhanced efficiency and ambient stability of planar heterojunction perovskite solar cells by using organic-inorganic double layer electron transporting material. Electrochim. Acta 2019, 294, 337–344. [Google Scholar] [CrossRef]
- Qi, Y.; Ndaleh, D.; Meador, W.E.; Delcamp, J.H.; Hill, G.; Pradhan, N.R.; Dai, Q. Interface Passivation of Inverted Perovskite Solar Cells by Dye Molecules. ACS Appl. Energy Mater. 2021, 4, 9525–9533. [Google Scholar] [CrossRef]
- He, Q.; Worku, M.; Liu, H.; Lochner, E.; Robb, A.J.; Lteif, S.; Raaj, J.S.; Winfred, V.; Hanson, K.; Schlenoff, J.B.; et al. Highly Efficient and Stable Perovskite Solar Cells Enabled by Low Cost Industrial Organic Pigment. Angew. Chem. Int. Ed. 2021, 60, 2485. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Ales, V.G. Cesium compounds as interface modifiers for stable and efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 172–186. [Google Scholar] [CrossRef]
- Ruankham, P.; Wongratanaphisan, D.; Gardchareon, A.; Phadungdhitidhada, S.; Choopun, S.; Sagawa, T. Full coverage of perovskite layer onto ZnO nanorods via a modified sequential two-step deposition method for efficiency enhancement in perovskite solar cells. Appl. Surf. Sci. 2017, 410, 393–400. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite solar cells with ZnO electron-transporting materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Lei, L.; Zhu, J.; Yu, Y.; Liu, Y.; Yang, S.; Bao, S.; Jin, P. Controllable deposition of TiO2 nanopillars at room temperature for high performance perovskite solar cells with suppressed hysteresis. Sol. Energy Mater. Sol. Cells 2017, 168, 172–182. [Google Scholar] [CrossRef]
- Huang, X.; Hu, Z.; Xu, J.; Wang, P.; Wang, L.; Zhang, J.; Zhu, Y. Low-temperature processed SnO2 compact layer by incorporating TiO2 layer toward efficient planar heterojunction perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 164, 87–92. [Google Scholar] [CrossRef]
- Ren, X.; Yang, D.; Yang, Z.; Feng, J.; Zhu, X. Solution-processed Nb: SnO2 electron transport layer for efficient planar perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 2421–2429. [Google Scholar] [CrossRef]
- Song, J.; Zheng, E.; Bian, J.; Wang, X.; Tian, W.; Sanehira, Y.; Miyasaka, T. Low temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A. 2015, 3, 10837–10844. [Google Scholar] [CrossRef]
- Eze, V.O.; Seike, Y.; Mori, T. Efficient planar perovskite solar cells using solution processed amorphous WOx/fullerene C60 as electron extraction layers. Org. Electron. 2017, 46, 253–262. [Google Scholar] [CrossRef]
- Pintilie, I.; Stancu, V.; Tomulescu, A.; Radu, R.; Besleaga Stan, C.; Trinca, L.; Pintilie, L. Properties of perovskite ferroelectrics deposited on F doped SnO2 electrodes and the prospect of their integration into perovskite solar cells. Mater. Des. 2017, 135, 112–121. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Sun, S.Y.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.C.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef]
- Wu, M.C.; Chan, S.H.; Jao, M.H.; Su, W.F. Enhanced short-circuit current density of perovskite solar cells using Zn-doped TiO2 as electron transport layer. Sol. Energy Mater. Sol. Cells 2016, 157, 447–453. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Fang, S.D.; Abate, A.; Sadoughi, G.; Sadhanala, A.; Kopidakis, N.; Rumbles, G.; Li, C.Z.; Friend, R.H.; Jen, A.K.Y.; et al. Heterojunction Modification for Highly Efficient Organic-Inorganic Perovskite Solar Cells. ACS Nano 2014, 8, 12701–12709. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Murugadoss, G.; Tanaka, S.; Mizuta, G.; Kanaya, S.; Nishino, H.; Umeyama, T.; Imahori, H.; Ito, S. Light stability tests of methylammonium and formamidinium Pb-halide perovskite for solar cell applications. Jpn. J. Appl. Phys. 2015, 54, 08KF08. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Sidhik, S.; Cerdan Pasarán, A.; Esparza, D.; López Luke, T.; Carriles, R.; De la Rosa, E. Improving the optoelectronic properties of mesoporous TiO2 by cobalt doping for high-performance hysteresis-free perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Abate, A.; Correa Baena, J.P.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, A.; Thambidurai, M.; Shini, F.; Muthukumarasamy, N.; Velauthapillai, D. Effect of doped TiO2 film as electron transport layer for inverted organic solar cell. Mater. Sci. Energy Technol. 2019, 2, 385–388. [Google Scholar] [CrossRef]
- Su, T.-S.; Wei, T.-C. Co-Electrodeposition of Sn-Doped TiO2 Electron-Transporting Layer for Perovskite Solar Cells. Phys. Status Solidi A 2020, 217, 1900491. [Google Scholar] [CrossRef]
- Wu, T.; Zhen, C.; Zhu, H.; Wu, J.; Jia, C.; Wang, L.; Liu, G.; Park, N.-G.; Cheng, H.-M. Gradient Sn-Doped Heteroepitaxial Film of Faceted Rutile TiO2 as an Electron Selective Layer for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 19638–19646. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, Y.; Zhang, T.; Liu, D.; Zhang, R.; Zhang, P.; Wu, J.; Chen, Z.D.; Li, S. Enhanced electronic transport in Fe3+-doped TiO2 for high efficiency perovskite solar cells. J. Mater. Chem. C 2017, 5, 10754–10760. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, X.; Guo, Y.; Pu, Y.; He, M. Ru-Doping in TiO2 electron transport layers of planar heterojunction perovskite solar cells for enhanced performance. J. Mater. Chem. C 2018, 6, 4746–4752. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhu, Y.; Ma, Z.; Liu, B.; Miao, X.; Ma, R.; Wang, C. Enhanced performance of TiO2-based perovskite solar cells with Ru-doped TiO2 electron transport layer. Sol. Energy 2018, 169, 335–342. [Google Scholar] [CrossRef]
- Chen, B.-X.; Rao, H.-S.; Li, W.-G.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Achieving high-performance planar perovskite solar cell with Nb-doped TiO2 compact layer by enhanced electron injection and efficient charge extraction. J. Mater. Chem. A 2016, 4, 5647–5653. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chan, S.-H.; Lee, K.-M.; Chen, S.-H.; Jao, M.-H.; Chen, Y.-F.; Su, W.-F. Enhancing the efficiency of perovskite solar cells using mesoscopic zinc-doped TiO2 as the electron extraction layer through band alignment. J. Mater. Chem. A 2018, 6, 16920–16931. [Google Scholar] [CrossRef]
- Lv, M.; Lv, W.; Fang, X.; Sun, P.; Lin, B.; Zhang, S.; Xu, X.; Ding, J.; Yuan, N. Performance enhancement of perovskite solar cells with a modified TiO2 electron transport layer using Zn-based additives. RSC Adv. 2016, 6, 35044–35050. [Google Scholar] [CrossRef]
- Ranjan, R.; Prakash, A.; Singh, A.; Singh, A.; Garg, A.; Gupta, R.K. Effect of tantalum doping in a TiO2 compact layer on the performance of planar spiro-OMeTAD free perovskite solar cells. J. Mater. Chem. A 2018, 6, 1037–1047. [Google Scholar] [CrossRef]
- Wang, J.; Qin, M.; Tao, H.; Ke, W.; Chen, Z.; Wan, J.; Qin, P.; Xiong, L.; Lei, H.; Yu, H.; et al. Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 2015, 106, 121104. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Xu, X.; Zhu, L.; Luo, Y.; Li, D.; Meng, Q. Mg-doped TiO2 boosts the efficiency of planar perovskite solar cells to exceed 19%. J. Mater. Chem. A 2016, 4, 15383–15389. [Google Scholar] [CrossRef]
- Acchutharaman, K.R.; Santhosh, N.; Senthil Pandian, M.; Ramasamy, P. Improved optoelectronic properties of rutile TiO2 nanorods through strontium doping for the economical and efficient perovskite solar cells. Mater. Res. Bull. 2023, 160, 112141. [Google Scholar] [CrossRef]
- Wu, M.-C.; Liao, Y.-H.; Chan, S.-H.; Lu, C.-F.; Su, W.-F. Enhancing organolead halide perovskite solar cells performance through interfacial engineering using Ag-doped TiO2 hole blocking layer. Sol. RRL 2018, 2, 1800072. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wang, J.; Qin, P.; Tao, H.; Lei, H.; Liu, Q.; Dai, X.; Zhao, X. Perovskite solar cell with an efficient TiO2 compact film. ACS Appl. Mater. Interfaces 2014, 6, 15959–15965. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, Z.; Yang, G.; Wang, H.; Ye, F.; Tao, C.; Fang, G. Achieving High Open-Circuit Voltage on Planar Perovskite Solar Cells via Chlorine-Doped Tin Oxide Electron Transport Layers. ACS Appl. Mater. Interfaces 2019, 11, 23152–23159. [Google Scholar] [CrossRef] [PubMed]
- Arman, S.Y.; Omidvar, H.; Tabaian, S.H.; Sajjadnejad, M.; Fouladvand, S.; Afshar, S. Evaluation of nanostructured S-doped TiO2 thin films and their photoelectrochemical application as photoanode for corrosion protection of 304 stainless steel. Surf. Coat. Technol. 2014, 251, 162–169. [Google Scholar] [CrossRef]
- Abd Mutalib, M.; Ahmad Ludin, N.; Su’ait, M.S.; Davies, M.; Sepeai, S.; Mat Teridi, M.A.; Mohamad Noh, M.F.; Ibrahim, M.A. Performance-Enhancing Sulfur-Doped TiO2 Photoanodes for Perovskite Solar Cells. Appl. Sci. 2022, 12, 429. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chan, S.-H.; Lin, Y.-T.; Wu, M.-C. Enhanced power conversion efficiency of perovskite solar cells based on mesoscopic Ag-doped TiO2 electron transport layer. Appl. Surface Sci. 2019, 469, 18–26. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Xie, L.; Wang, S.; Yang, C.; Fang, C.; Hao, F. Recent Advances and Perspectives of Photostability for Halide Perovskite Solar Cells. Adv. Opt. Mater. 2022, 10, 2101822. [Google Scholar] [CrossRef]
- Zaky, A.A.; Christopoulos, E.; Gkini, K.; Arfanis, M.K.; Sygellou, L.; Kaltzoglou, A.; Stergiou, A.; Tagmatarchis, N.; Balis, N.; Falaras, P. Enhancing efficiency and decreasing photocatalytic degradation of perovskite solar cells using a hydrophobic copper-modified titania electron transport layer. Appl. Catal. B Environ. 2021, 284, 119714. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, S.; Yang, X.; Zhou, Y.; Jin, J.; Sun, J.; Zhao, L.; Wang, S. The dual interfacial modification of 2D g-C3N4 for high-efficiency and stable planar perovskite solar cells. Nanoscale Adv. 2020, 2, 5396–5402. [Google Scholar] [CrossRef]
- Gkini, K.; Martinaiou, I.; Botzakaki, M.; Tsipas, P.; Theofylaktos, L.; Dimoulas, A.; Katsaros, F.; Stergiopoulos, T.; Krontiras, C.; Georga, S.; et al. Energy band tuning induced by g-C3N4 interface engineering for efficient and stable perovskite solar cells. Mater. Today Commun. 2022, 32, 103899. [Google Scholar] [CrossRef]
- Haque, M.A.; Sheikh, A.D.; Guan, X.; Wu, T. Metal Oxides as Efficient Charge Transporters in Perovskite Solar Cells. Adv. Ener. Mat. 2017, 7, 1602803. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Tremolet de Villers, B.J.; Schulz, P.; Reese, M.O.; Ferrere, S.; Zhu, K.; Lin, L.Y.; Berry, J.J.; Luther, J.M. Influence of Electrode Interfaces on the Stability of Perovskite Solar Cells: Reduced Degradation Using MoOx/Al for Hole Collection. ACS Energy Lett. 2016, 1, 38–45. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wang, Y.; Shao, J.Y.; Lan, Y.; Lan, Z.R.; Zhong, Y.W.; Song, Y. Defect Passivation by a D–A–D Type Hole-Transporting Interfacial Layer for Efficient and Stable Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 2030–2037. [Google Scholar] [CrossRef]
- Wang, C.; Gao, P. Radicalize the Performance of Perovskite Solar Cells with Radical Compounds. Chem. Res. Chin. Univ. 2023, 39, 176–186. [Google Scholar] [CrossRef]
- Jiang, Q.; Ni, Z.; Xu, G.; Lin, Y.; Rudd, P.N.; Xue, R.; Li, Y.; Li, Y.; Gao, Y.; Huang, J. Interfacial Molecular Doping of Metal Halide Perovskite for Highly Efficient Solar Cells. Adv. Mater. 2020, 32, 2001581. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Peng, Y.; Zhang, X.; Ren, Y.; Ghadari, R.; Zhu, J.; Tulloch, G.; Zhang, H.; Falaras, P.; Hu, L. Resonant molecular treatment for efficient and stable perovskite solar cells. ACS Energy Lett. 2022, 7, 3104–3111. [Google Scholar] [CrossRef]
- Niazi, Z.; Hagfeldt, A.; Goharshadi, E.K. Recent progress on the use of graphene-based nanomaterials in perovskite solar cells. J. Mater. Chem. A 2023, 11, 6659–6687. [Google Scholar] [CrossRef]
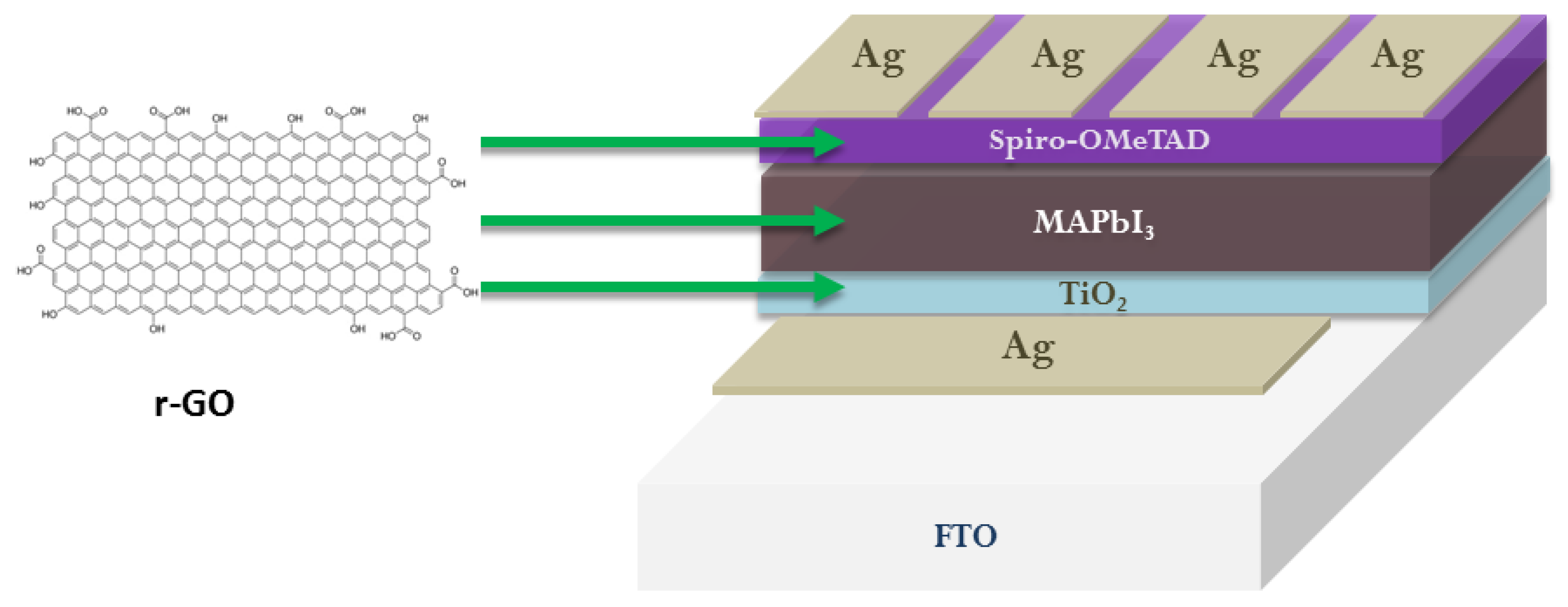
- Cho, T.; Grancini, G.; Lee, Y.; Konios, D.; Paek, S.; Kymakis, E.; Nazeeruddin, M.K. Beneficial Role of Reduced Graphene Oxide for Electron Extraction in Highly Efficient Perovskite Solar Cells. ChemSusChem 2016, 9, 3040–3044. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Cinà, L.; Konios, D.; Kakavelakis, G.; Kymakis, E.; Di Carlo, A. Efficiency and Stability Enhancement in Perovskite Solar Cells by Inserting Lithium-Neutralized Graphene Oxide as Electron Transporting Layer. Adv. Funct. Mater. 2016, 6, 2686–2694. [Google Scholar] [CrossRef]
- Yang, Q.-D.; Li, J.; Cheng, Y.; Li, H.-W.; Guan, Z.; Yu, B.; Tsang, S.-W. Graphene oxide as an efficient hole-transporting material for high-performance perovskite solar cells with enhanced stability. J. Mater. Chem. A 2017, 5, 9852–9858. [Google Scholar] [CrossRef]
- Nouri, E.; Wang, Y.-L.; Chen, Q.; Xu, J.-J.; Paterakis, G.; Dracopoulos, V.; Xu, Z.-X.; Tasis, D.; Mohammadi, M.R.; Lianos, P. Introduction of Graphene Oxide as Buffer Layer in Perovskite Solar Cells and the Promotion of Soluble n-Butyl-substituted Copper Phthalocyanine as Efficient Hole Transporting Material. Electrochim. Acta 2017, 233, 36–43. [Google Scholar] [CrossRef]
- Palma, A.L.; Cinà, L.; Pescetelli, S.; Agresti, A.; Raggio, M.; Paolesse, R.; Bonaccorso, F.; Di Carlo, A. Reduced graphene oxide as efficient and stable hole transporting material in mesoscopic perovskite solar cells. Nano Energy 2016, 22, 349–360. [Google Scholar] [CrossRef]
- Balis, N.; Zaky, A.A.; Athanasekou, C.; Silva, A.M.T.; Sakellis, E.; Vasilopoulou, M.; Stergiopoulos, T.; Kontos, A.G.; Falaras, P. Investigating the Role of Reduced Graphene Oxide as a Universal Additive in Planar Perovskite Solar Cells. J. Photochem. Photobiol. A Chem. 2020, 386, 112141. [Google Scholar] [CrossRef]
- Lin, X.; Su, H.; He, S.; Song, Y.; Wang, Y.; Qin, Z.; Wu, Y.; Yang, X.; Han, Q.; Fang, J.; et al. In situ growth of graphene on both sides of a Cu–Ni alloy electrode for perovskite solar cells with improved stability. Nat. Energy 2022, 7, 520–527. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Barbaud, J.; Kong, W.; Cui, D.; Chen, H.; Yang, X.; Han, L. Stabilizing heterostructures of soft perovskite semiconductors. Science 2019, 365, 687–691. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, C.; Zhang, H.; Dong, H.; Chen, D.; Zhu, W.; Xi, H.; Chang, J.; Lin, Z.; Zhang, J.; et al. Boosting performance of perovskite solar cells with Graphene quantum dots decorated SnO2 electron transport layers. Appl. Surf. Sci. 2020, 507, 145099. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K.; et al. Luminescent Zero-Dimensional Organic Metal Halide Hybrids with near-Unity Quantum Efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef]
- Fan, J.; Ma, Y.; Zhang, C.; Liu, C.; Li, W.; Schropp, R.E.I.; Mai, Y. Thermodynamically Self-Healing 1D–3D Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703421. [Google Scholar] [CrossRef]
- Ma, C.; Shen, D.; Huang, B.; Li, X.; Chen, W.-C.; Lo, M.-F.; Wang, P.; Hon-Wah Lam, M.; Lu, Y.; Ma, B.; et al. High Performance Low-Dimensional Perovskite Solar Cells Based on a One Dimensional Lead Iodide Perovskite. J. Mater. Chem. A 2019, 7, 8811–8817. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, S.; Zheng, H.; Wu, M.; Zhang, S.; Lan, J.; Li, W.; Fan, J. Structurally Dimensional Engineering in Perovskite Photovoltaics. Adv. Energy Mater. 2023, 356, 2300188. [Google Scholar] [CrossRef]
- Elsenety, M.M.; Kaltzoglou, A.; Antoniadou, M.; Koutselas, I.; Kontos, A.G.; Falaras, P. Synthesis, Characterization and Use of Highly Stable Trimethyl Sulfonium Tin(IV) Halide Defect Perovskites in Dye Sensitized Solar Cells. Polyhedron 2018, 150, 83–91. [Google Scholar] [CrossRef]
- Elsenety, M.M.; Antoniadou, M.; Kaltzoglou, A.; Kontos, A.G.; Philippopoulos, A.I.; Mitsopoulou, C.A.; Falaras, P. Synthesis, Characterization of ((CH3)3S)2SnI6-nCln and ((CH3)3S)2SnI6-nBrn (n = 1, 2) Perovskites and Use in Dye-Sensitized Solar Cells. Mater. Chem. Phys. 2020, 239, 122310. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Stoumpos, C.C.; Kontos, A.G.; Manolis, G.K.; Papadopoulos, K.; Papadokostaki, K.G.; Psycharis, V.; Tang, C.C.; Jung, Y.K.; Walsh, A.; et al. Trimethylsulfonium Lead Triiodide: An Air-Stable Hybrid Halide Perovskite. Inorg. Chem. 2017, 56, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Kim, G.; Paik, M.J.; Lee, S.; Yang, W.S.; Jung, M.; Seok, S.I. Stabilization of Precursor Solution and Perovskite Layer by Addition of Sulfur. Adv. Energy Mater. 2019, 9, 1803476. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Elsenety, M.M.; Koutselas, I.; Kontos, A.G.; Papadopoulos, K.; Psycharis, V.; Raptopoulou, C.P.; Perganti, D.; Stergiopoulos, T.; Falaras, P. Synthesis, Characterization and Optoelectronic Properties of Chemically Stable (CH3)3SPbI3−xBrx and (CH3)3SPbI3−xClx (X = 0, 1, 2, 3) Perovskites. Polyhedron 2018, 140, 67–73. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Manolis, G.K.; Elsenety, M.M.; Koutselas, I.; Psycharis, V.; Kontos, A.G.; Falaras, P. Synthesis and Characterization of Lead-Free (CH3)3SSnI3 1-D Perovskite. J. Electron. Mater. 2019, 48, 7533–7538. [Google Scholar] [CrossRef]
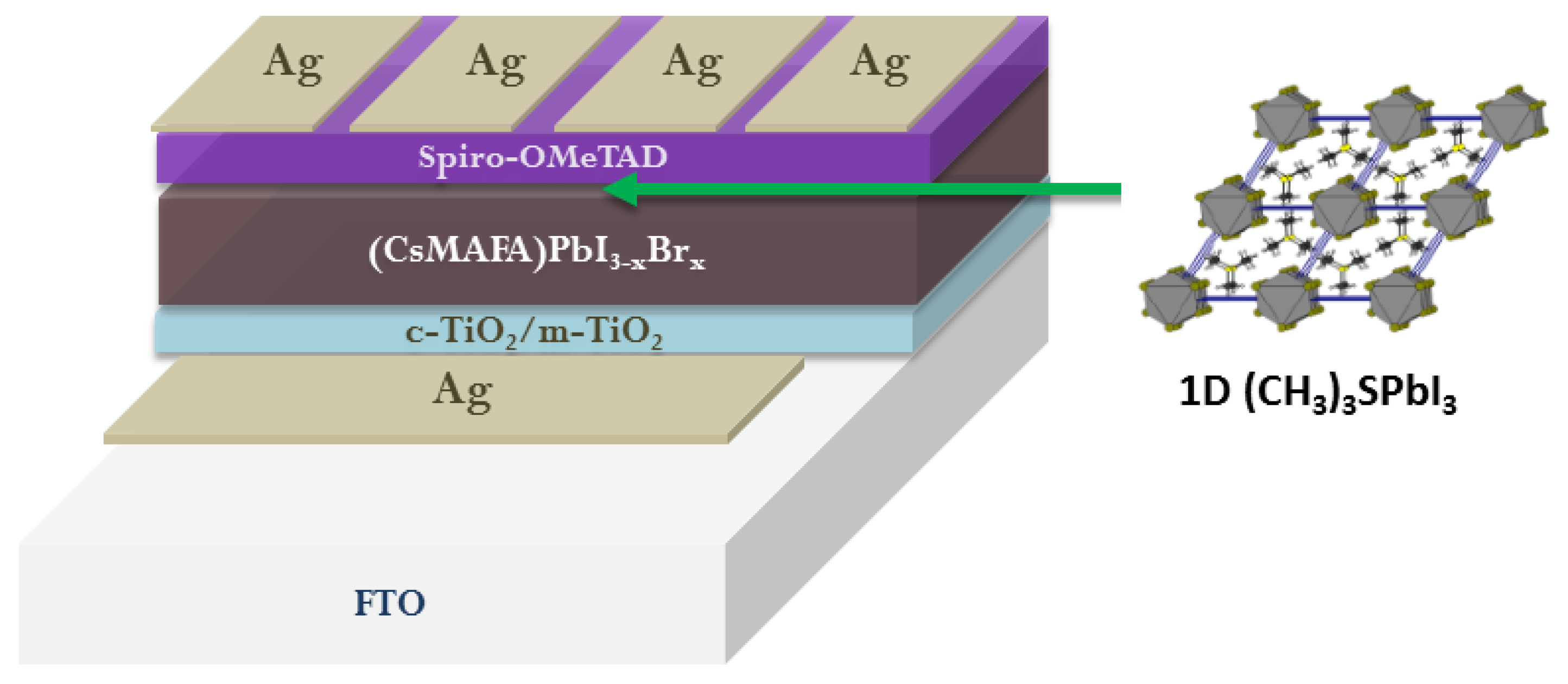
- Elsenety, M.; Antoniadou, M.; Balis, N.; Kaltzoglou, A.; Sygellou, L.; Stergiou, A.; Tagmatarchis, N.; Falaras, P. Stability Improvement and Performance Reproducibility Enhancement of Perovskite Solar Cells Following (FA/MA/Cs) PbI3−xBrx/(CH3)3SPbI3 Dimensionality Engineering. ACS Appl. Energy Mater. 2020, 3, 2465–2477. [Google Scholar] [CrossRef]
- Parashar, M.; Singh, R.; Yoo, K.; Lee, J.-J. Formation of 1-D/3-D Fused Perovskite for Efficient and Moisture Stable Solar Cells. ACS Appl. Energy Mater. 2021, 4, 2751–2760. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, L.; Dai, J.; Deng, Y.; Wu, Y.; Bai, Y.; Zheng, X.; Wang, J.; Fang, Y.; Wei, H.; et al. π-Conjugated Lewis Base: Efficient Trap-Passivation and Charge-Extraction for Hybrid Perovskite Solar Cells. Adv. Mater. 2017, 29, 1604545. [Google Scholar] [CrossRef]
- Wu, Z.; Raga, S.R.; Juarez-Perez, E.J.; Yao, X.; Jiang, Y.; Ono, L.K.; Ning, Z.; Tian, H.; Qi, Y. Improved Efficiency and Stability of Perovskite Solar Cells Induced by C=O Functionalized Hydrophobic Ammonium-Based Additives. Adv. Mater. 2018, 3, 1703670. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Bae Park, J.; Kim, H.; Kim, K.; Park, J.; Cho, S.; Lee, H.; Pak, Y.; Jung, G.Y. Enhanced long-term stability of perovskite solar cells by passivating grain boundary with polydimethylsiloxane (PDMS). J. Mater. Chem. A 2019, 7, 20832–20839. [Google Scholar] [CrossRef]
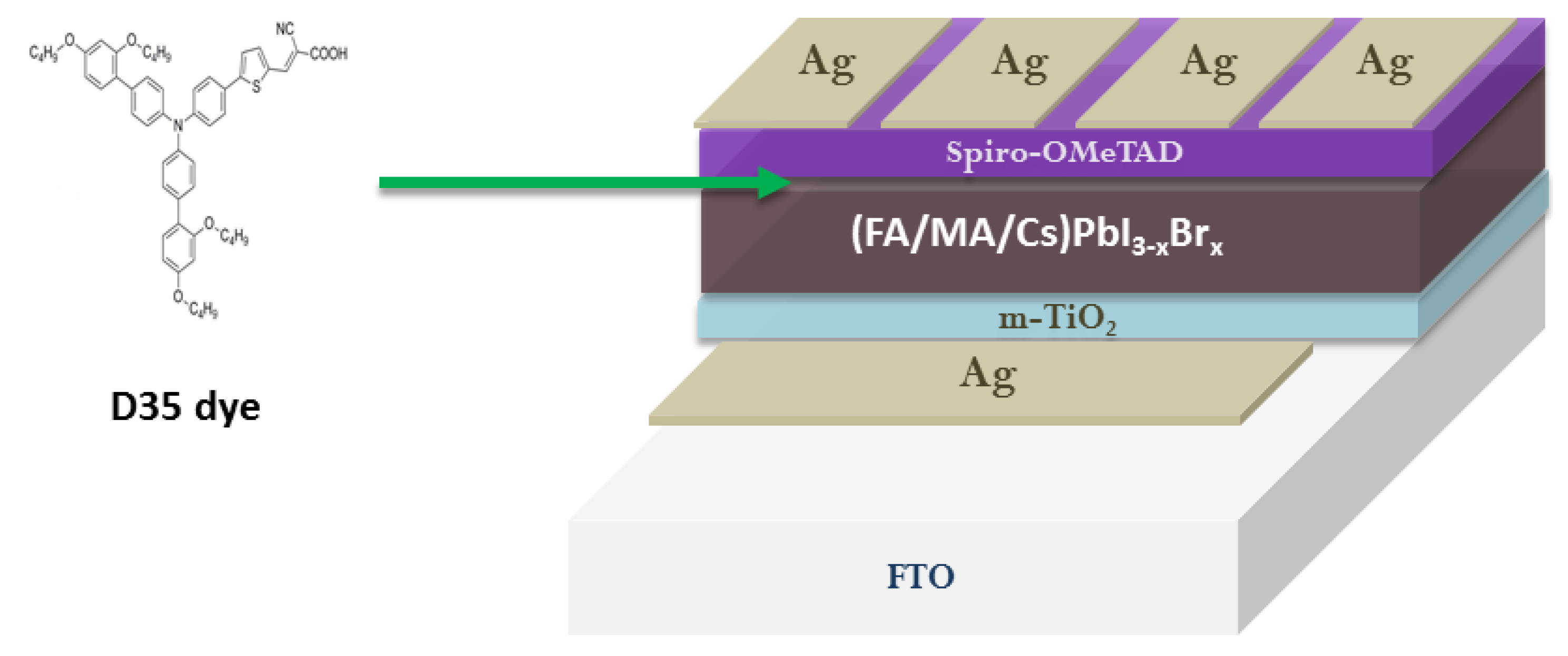
- Li, X.; Chen, C.-C.; Cai, M.; Hua, X.; Xie, F.; Liu, X.; Hua, J.; Long, Y.-T.; Tian, H.; Han, L. Efficient Passivation of Hybrid Perovskite Solar Cells Using Organic Dyes with -COOH Functional Group. Adv. Energy Mater. 2018, 8, 1800715. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Y.; Li, X.; Wu, Y.; Meng, X.; Cui, D.; Yang, X.; Han, L. Efficient Defect Passivation for Perovskite Solar Cells by Controlling the Electron Density Distribution of Donor-π-Acceptor Molecules. Adv. Energy Mater. 2019, 9, 1803766. [Google Scholar] [CrossRef]
- Fu, S.; Li, X.; Wan, L.; Wu, Y.; Zhang, W.; Wang, Y.; Bao, Q.; Fang, J. Efficient Passivation with Lead Pyridine-2-Carboxylic for High-Performance and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1901852. [Google Scholar] [CrossRef]
- Cao, J.; Li, C.; Lv, X.; Feng, X.; Meng, R.; Wu, Y.; Tang, Y. Efficient Grain Boundary Suture by Low-Cost Tetra-ammonium Zinc Phthalocyanine for Stable Perovskite Solar Cells with Expanded Photoresponse. J. Am. Chem. Soc. 2018, 140, 11577. [Google Scholar] [CrossRef] [PubMed]
- Elsenety, M.; Stergiou, A.; Sygellou, L.; Tagmatarchis, N.; Balis, N.; Falaras, P. Boosting Perovskite Nanomorphology and Charge Transport Properties via a Functional D-π-A Organic Layer at the Absorber/Hole Transporter Interface. Nanoscale 2020, 12, 15137–15149. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Zhang, L.; Deng, Z.; Yang, K.; Cui, H.; Hou, Q.; Ji, W. A D–π–A Organic Dye as a Passivator to Effectively Regulate the Performance of Perovskite Solar Cells. Energy Technol. 2022, 10, 220026. [Google Scholar] [CrossRef]
- Koh, T.M.; Shanmugam, V.; Guo, X.; Lim, S.S.; Filonik, O.; Herzig, E.M.; Muller-Buschbaum, P.; Swamy, V.; Chien, S.T.; Mhaisalkar, S.G.; et al. Enhancing moisture tolerance in efficient hybrid 3D/2D perovskite photovoltaics. J. Mater. Chem. A 2018, 6, 2122–2128. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, J.; Tian, C.; Wu, J.; Li, H.; Li, Y.; Yu, B.; Luo, Y.; Wu, H.; Xie, Z.; et al. CdS Induced Passivation toward High Efficiency and Stable Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 9771–9780. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, M.; Liu, Z.; Jamshaid, A.; Ono, L.K.; Qi, Y. Highly Efficient Perovskite Solar Cells Enabled by Multiple Ligand Passivation. Adv. Energy Mater. 2020, 10, 1903696. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Wang, G.; Wang, J.; Li, Y.; Qian, W.; Zheng, S.; Xiao, S.; Yang, S. A prenucleation strategy for ambient fabrication of perovskite solar cells with high device performance uniformity. Nat. Commun. 2020, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
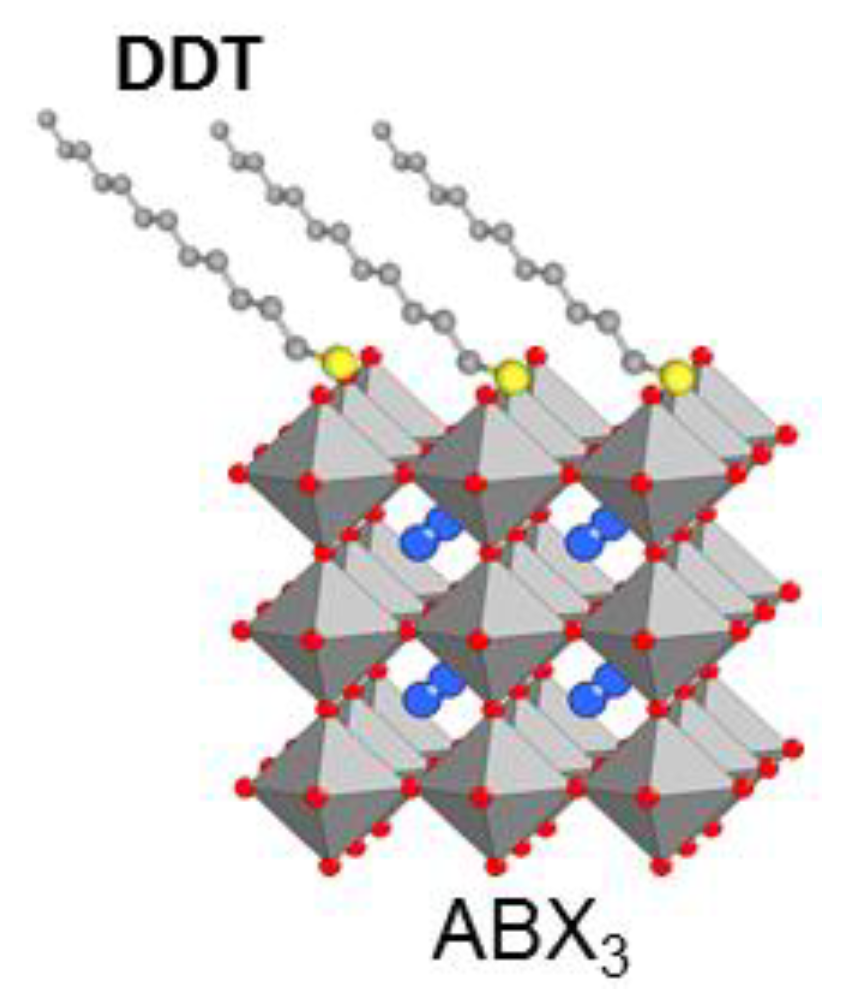
- He, D.; Xu, X.; Liang, Z.; Niu, Y.; Sun, Y.; Tulloch, G.; Falaras, P.; Hu, L. Defect passivation and humidity protection for perovskite solar cells enabled by 1-dodecanethiol. J. Mater. Chem. C 2021, 9, 9584–9591. [Google Scholar] [CrossRef]
- Kara, D.A.; Cirak, D.; Gultekin, B. Decreased surface defects and non-radiative recombination via the passivation of the halide perovskite film by 2-thiophenecarboxylic acid in triple-cation perovskite solar cells. Phys. Chem. Chem. Phys. 2022, 24, 10384. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, H.; Lee, G.; Jeong, Y.R.; Hong, S.Y.; Keum, K.; Yoon, J.; Kim, M.S.; Ha, J.S. Paper-Like, Thin, Foldable, and Self-Healable Electronics Based on PVA/CNC Nanocomposite Film. Adv. Funct. Mater. 2019, 29, 1905968. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, L.; Juarez-Perez, E.J.; Ono, L.K.; Hu, Z.; Liu, Z.; Wu, Z.; Meng, L.; Wang, Q.; Qi, Y. Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation. Nat. Energy 2019, 4, 585–593. [Google Scholar] [CrossRef]
- Gong, Z.; He, B.; Zhu, J.; Yao, X.; Wang, S.; Chen, H.Y.; Duan, Y.Y.; Tang, Q. Tri-Brominated Perovskite Film Management and Multiple-Ionic Defect Passivation for Highly Efficient and Stable Solar Cells. Sol. RRL 2021, 5, 2000819. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Cao, F.; Tian, W.; Li, L. Moisture-Triggered Self-Healing Flexible Perovskite Photodetectors with Excellent Mechanical Stability. Adv. Mater. 2021, 33, 2100625. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, M.; He, B.; Shen, K.; Zhang, W.; Sun, X.; Sun, M.; Chen, H.; Duan, Y.; Tang, Q. Ultraviolet filtration and defect passivation for efficient and photostable CsPbBr3 perovskite solar cells by interface engineering with ultraviolet absorber. Chem. Eng. J. 2021, 404, 126548. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, H.; Nie, J.; Shao, W.; Wang, X.; Zhang, B.; Ouyang, X. Effect of methylammonium lead tribromide perovskite based-photoconductor under gamma photons radiation. Radiat. Phys. Chem. 2021, 181, 109337. [Google Scholar] [CrossRef]
- Niu, Y.; He, D.; Zhang, Z.; Zhu, J.; Tulloch, G.; Falaras, P.; Hua, L. Improved crystallinity and self-healing effects in perovskite solar cells via functional incorporation of polyvinylpyrrolidone. J. Energy Chem. 2022, 68, 12–18. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, C.; Chen, Y.; Liu, L.; Yin, L.; Cui, F.; Zhang, N.; Wen, S.; Zhu, G. Crystallinity Regulation and Defects Passivation for Efficient and Stable Perovskite Solar Cells Using Fully Conjugated Porous Aromatic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202301234. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Tseng, C.; Chien, W.-C.; Hsu, H.-L.; Chen, C.-P. Photovoltaic Performance Enhancement of Perovskite Solar Cells Using Polyimide and Polyamic Acid as Additives. J. Phys. Chem. C 2019, 123, 23826–23833. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; bin Zafar, M.A.; Islam, M.S.-U.; Shahinuzzaman, M.M.; Islam, M.A.; Khandaker, M.U. Stability of perovskite solar cells: Issues and prospects. RSC Adv. 2023, 13, 1787. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bai, S.; Tress, W.F.; Hagfeldt, A.; Gao, F. Defects engineering for high-performance perovskite solar cells. npj Flex Electron. 2018, 2, 22. [Google Scholar] [CrossRef]
- Sherkar, T.S.; Momblona, C.; Gil-Escrig, L.; Ávila, J.; Sessolo, M.; Bolink, H.J.; Koster, L.J.A. Recombination in Perovskite Solar Cells: Significance of Grain Boundaries, Interface Traps, and Defect Ions. ACS Energy Lett 2017, 2, 1214. [Google Scholar] [CrossRef]
- Yin, W.J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Gong, X.; Guan, L.; Pan, H.; Sun, Q.; Zhao, X.; Li, H.; Pan, H.; Shen, Y.; Shao, Y.; Sun, L.; et al. Highly Efficient Perovskite Solar Cells via Nickel Passivation. Adv. Funct. Mater. 2018, 28, 1804286. [Google Scholar] [CrossRef]
- Feng, X.; Chen, R.; Nan, Z.-A.; Lv, X.; Meng, R.; Cao, J.; Tang, Y. Perfection of Perovskite Grain Boundary Passivation by Eu-Porphyrin Complex for Overall-Stable Perovskite Solar Cells. Adv. Sci. 2019, 6, 1802040. [Google Scholar] [CrossRef]
- Tao, Q.; Xu, P.; Li, M.; Lu, W. Machine learning for perovskite materials design and discovery. NPJ Comput. Mater. 2021, 7, 23. [Google Scholar] [CrossRef]
- Yılmaz, B.; Yıldırım, R. Critical review of machine learning applications in perovskite solar research. Nano Energy 2021, 80, 105546. [Google Scholar] [CrossRef]
- Raccuglia, P.; Elbert, K.C.; Adler, P.D.F.; Falk, C.; Wenny, M.B.; Mollo, A.; Zeller, M.; Friedler, S.A.; Schrier, J.; Norquist, A.J. Machine-learning-assisted materials discovery using failed experiments. Nature 2016, 533, 73. [Google Scholar] [CrossRef]
- Balachandran, P.V.; Kowalski, B.; Sehirlioglu, A.; Lookman, T. Experimental search for high-temperature ferroelectric perovskites guided by two-step machine learning. Nat. Commun. 2018, 9, 1668. [Google Scholar] [CrossRef]
- She, C.; Huang, Q.; Chen, C.; Jiang, Y.; Fan, Z.; Gao, J. Machine learning-guided search for high-efficiency perovskite solar cells with doped electron transport layers. J. Mater. Chem. A 2021, 9, 25168–25177. [Google Scholar] [CrossRef]
- Elsenety, M.M.; Christopoulos, E.; Falaras, P. Passivation Engineering Using Ultra-Hydrophobic Organic D-π-A dye with Machine Learning insights for Efficient and Stable Perovskite Solar Cells. Solar RRL 2023, 7, 2201016. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, W. Molecular design for perovskite solar cells. Int. J. Energy Res. 2022, 46, 14740–14765. [Google Scholar] [CrossRef]
- Zhang, L.; Wenguang Hu, W.; Li, S. Optimizing Photoelectrochemical Photovoltage and Stability of Molecular Interlayer-Modified Halide Perovskite in Water: Insights from Interpretable Machine Learning and Symbolic Regression. ACS Appl. Energy Mater. 2023, 6, 5177–5187. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaras, C.; Stathatos, E. Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization. Electronics 2023, 12, 3319. https://doi.org/10.3390/electronics12153319
Falaras C, Stathatos E. Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization. Electronics. 2023; 12(15):3319. https://doi.org/10.3390/electronics12153319
Chicago/Turabian StyleFalaras, Christos, and Elias Stathatos. 2023. "Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization" Electronics 12, no. 15: 3319. https://doi.org/10.3390/electronics12153319
APA StyleFalaras, C., & Stathatos, E. (2023). Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization. Electronics, 12(15), 3319. https://doi.org/10.3390/electronics12153319






