Decoding Electroencephalography Underlying Natural Grasp Tasks across Multiple Dimensions
Abstract
:1. Introduction
2. Materials and Methods
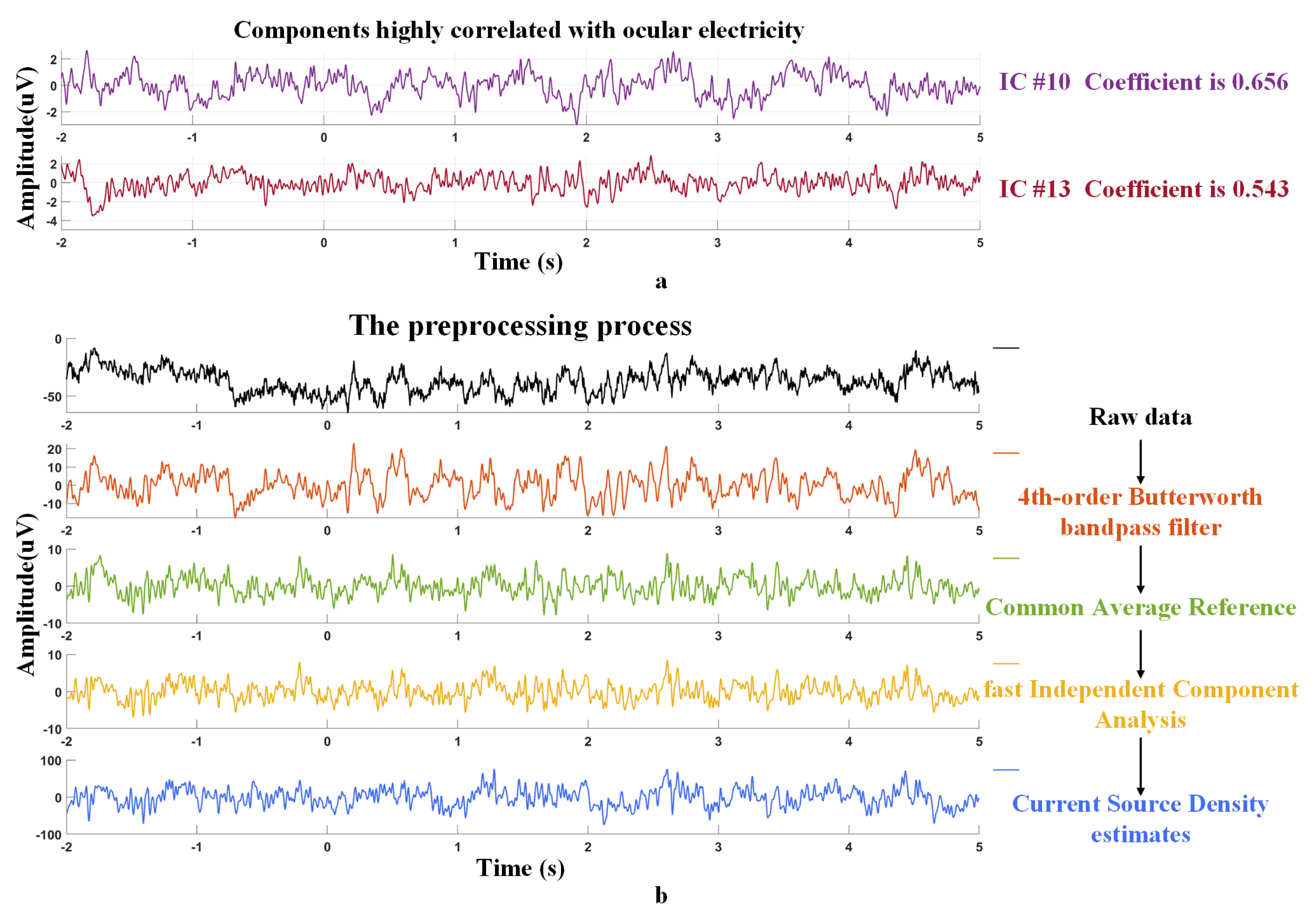
2.1. Data and Preprocessing
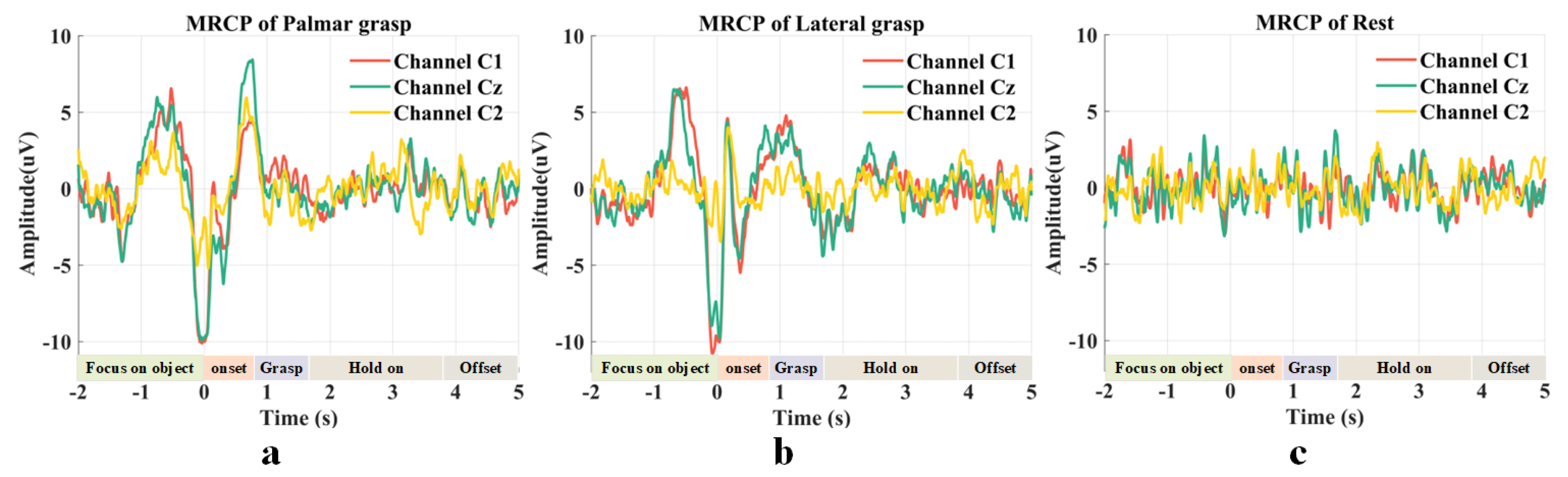
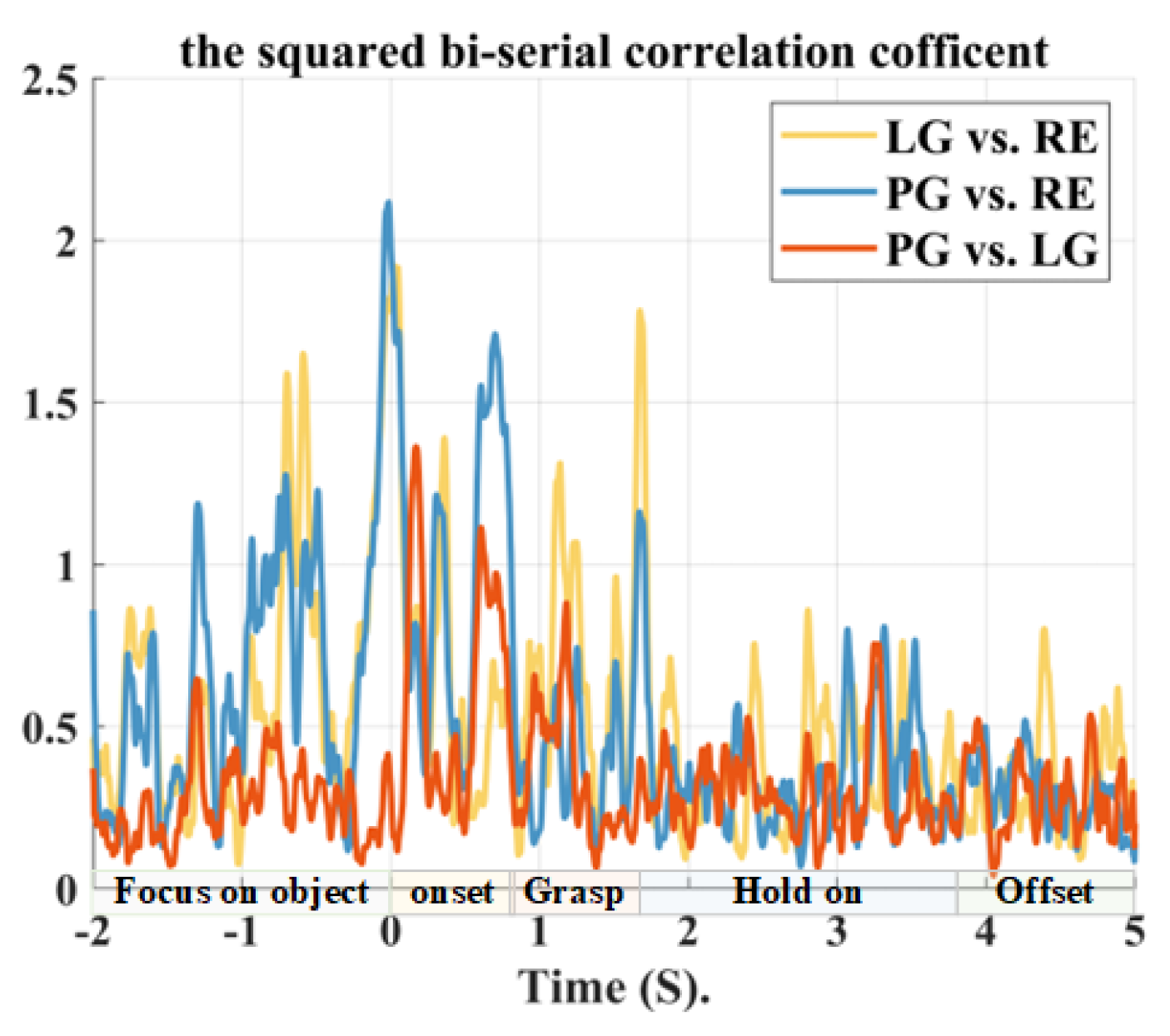
2.2. Movement-Related Cortical Potentials
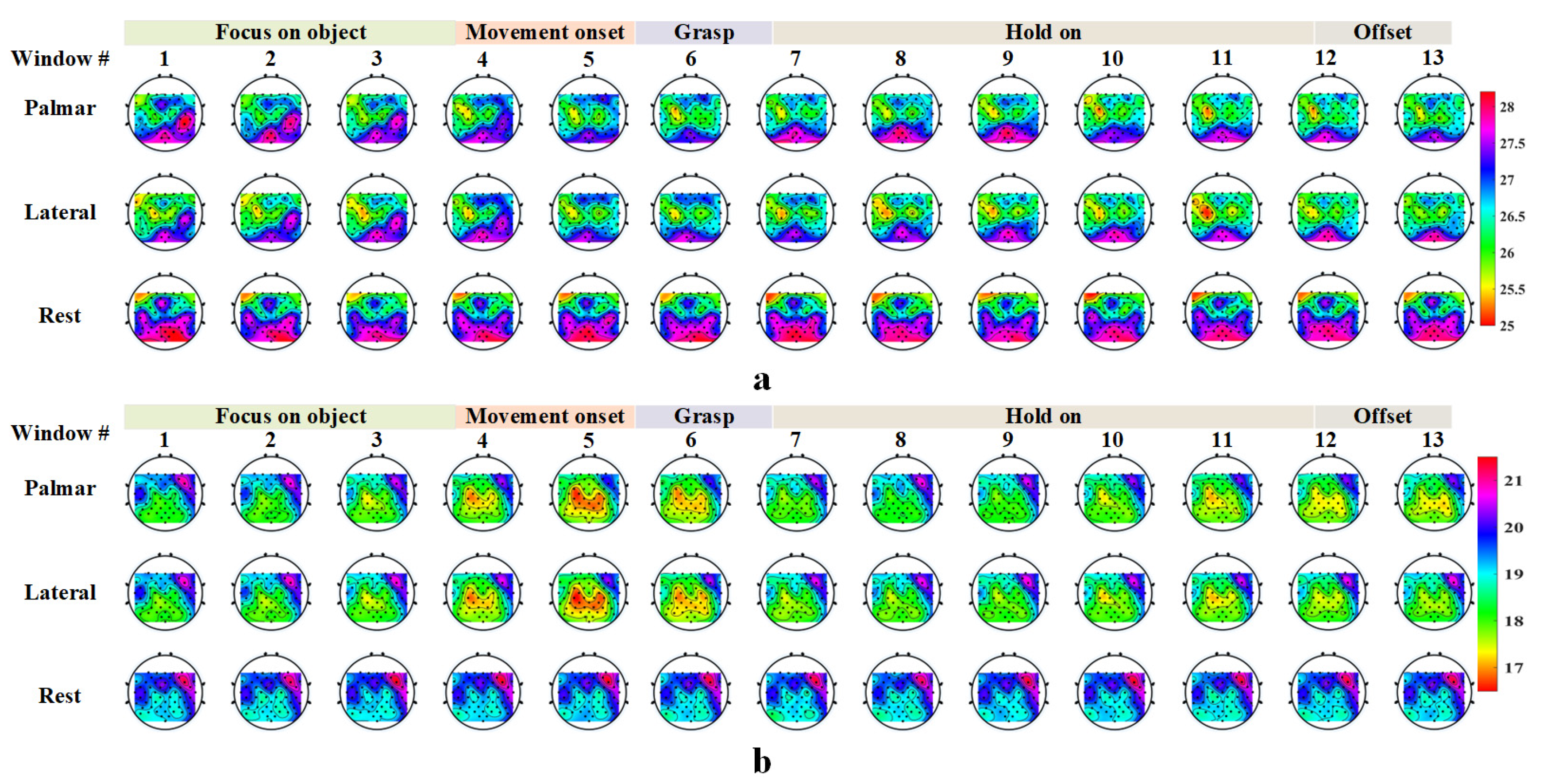
2.3. Event-Related Desynchronization and Synchronization
2.4. Brain Functional Connectivity
2.5. Classification
2.5.1. Wavelet Packet Decomposition
2.5.2. Random Forest
2.6. Difference Evaluation
3. Results
3.1. Motor Cortex-Related Potentials
3.2. Event-Related Desynchronization and Synchronization
3.3. Brain Functional Connectivity
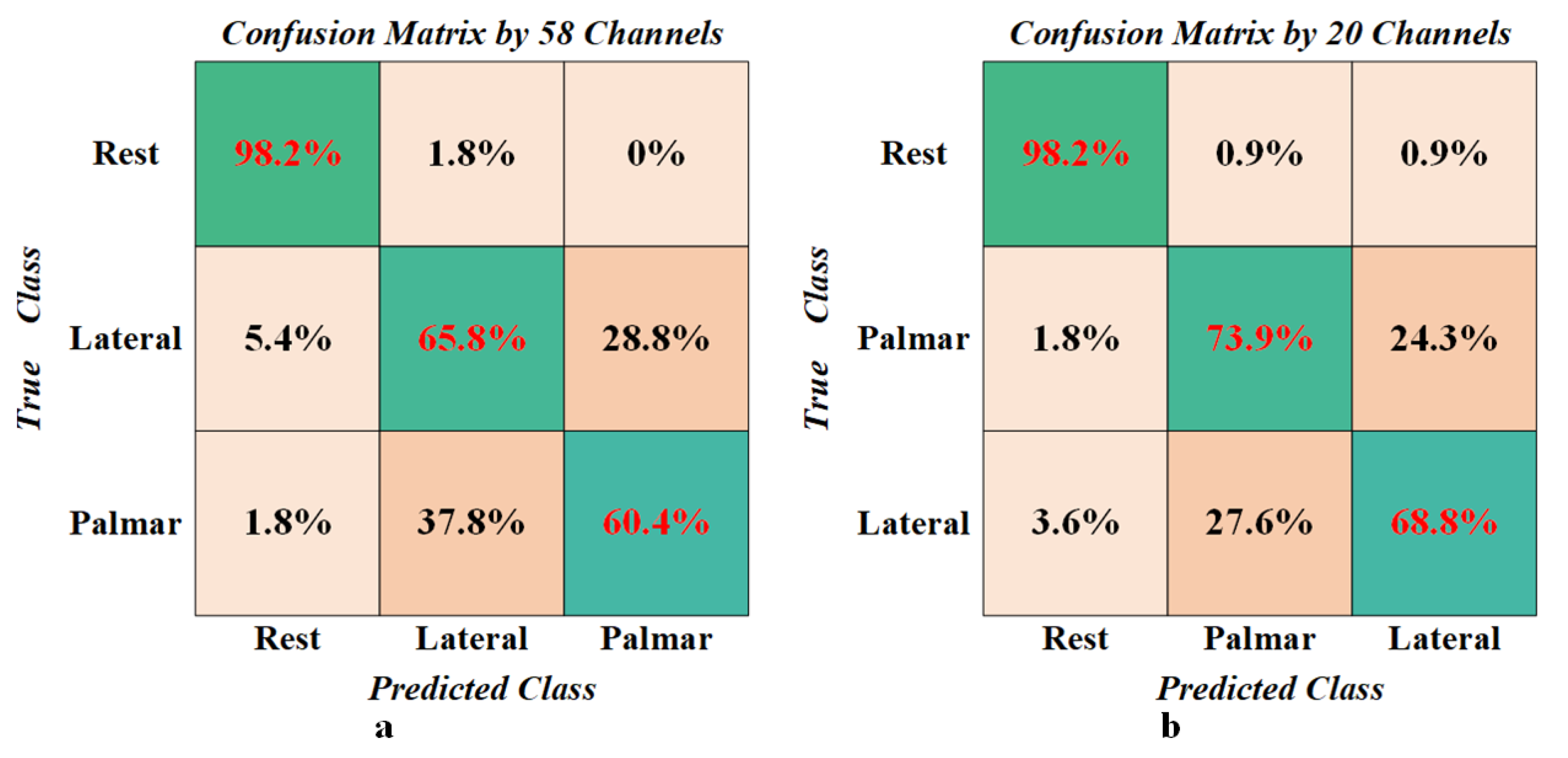
3.4. Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biasiucci, A.; Leeb, R.; Iturrate, I.; Perdikis, S.; Al-Khodairy, A.; Corbet, T.; Schnider, A.; Schmidlin, T.; Zhang, H.; Bassolino, M.; et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat. Commun. 2018, 9, 2421. [Google Scholar] [CrossRef]
- Snoek, G.J.; MJ, I.J.; Hermens, H.J.; Maxwell, D.; Biering-Sorensen, F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004, 42, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Edelman, B.J.; Meng, J.; Suma, D.; Zurn, C.; Nagarajan, E.; Baxter, B.S.; Cline, C.C.; He, B. Noninvasive neuroimaging enhances continuous neural tracking for robotic device control. Sci. Robot. 2019, 4, eaaw6844. [Google Scholar] [CrossRef] [PubMed]
- Muller-Putz, G.R.; Scherer, R.; Pfurtscheller, G.; Rupp, R. Brain-computer interfaces for control of neuroprostheses: From synchronous to asynchronous mode of operation. Biomed. Tech. 2006, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Muller-Putz, G.R.; Scherer, R.; Pfurtscheller, G.; Rupp, R. EEG-based neuroprosthesis control: A step towards clinical practice. Neurosci. Lett. 2005, 382, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.A.; Lynch, C.J.; Cheng, K.M.; Phillips, J.; Supekar, K.; Ryali, S.; Uddin, L.Q.; Menon, V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc. Natl. Acad. Sci. USA 2013, 110, 12060–12065. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Ofner, P.; Pereira, J.; Sburlea, A.I.; Muller-Putz, G.R. Decoding natural reach-and-grasp actions from human EEG. J. Neural Eng. 2018, 15, 016005. [Google Scholar] [CrossRef]
- Vuckovic, A.; Sepulveda, F. Delta band contribution in cue based single trial classification of real and imaginary wrist movements. Med. Biol. Eng. Comput. 2008, 46, 529–539. [Google Scholar] [CrossRef]
- Jochumsen, M.; Niazi, I.K.; Dremstrup, K.; Kamavuako, E.N. Detecting and classifying three different hand movement types through electroencephalography recordings for neurorehabilitation. Med. Biol. Eng. Comput. 2016, 54, 1491–1501. [Google Scholar] [CrossRef]
- Jochumsen, M.; Niazi, I.K.; Mrachacz-Kersting, N.; Farina, D.; Dremstrup, K. Detection and classification of movement-related cortical potentials associated with task force and speed. J. Neural Eng. 2013, 10, 056015. [Google Scholar] [CrossRef] [PubMed]
- Ofner, P.; Schwarz, A.; Pereira, J.; Müller-Putz, G.R. Movements of the same upper limb can be classified from low-frequency time-domain EEG signals. In Proceedings of the Sixth International Brain-Computer Interface Meeting: BCI Past, Present, and Future, Pacific Grove, CA, USA, 30 May–3 June 2016. [Google Scholar]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef]
- de Melo, G.C.; Martes Sternlicht, V.; Forner-Cordero, A. EEG Analysis in Coincident Timing Task towards Motor Rehabilitation. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3027–3030. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Rimbert, S.; Fleck, S. Long-term kinesthetic motor imagery practice with a BCI: Impacts on user experience, motor cortex oscillations and BCI performances. Comput. Hum. Behav. 2023, 146, 107789. [Google Scholar] [CrossRef]
- Lopes da Silva, F.H.; van Rotterdam, A.; Barts, P.; van Heusden, E.; Burr, W. Models of neuronal populations: The basic mechanisms of rhythmicity. Prog. Brain Res. 1976, 45, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Berghold, A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 250–258. [Google Scholar] [CrossRef]
- Paek, A.Y.; Prashad, S. Repetitive execution of a reach-and-lift task causes longitudinal attenuation in movement-related EEG features. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lu, C.F.; Teng, S.; Hung, C.I.; Tseng, P.J.; Lin, L.T.; Lee, P.L.; Wu, Y.T. Reorganization of functional connectivity during the motor task using EEG time-frequency cross mutual information analysis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 1569–1579. [Google Scholar] [CrossRef]
- Gong, A.; Liu, J.; Chen, S.; Fu, Y. Time-Frequency Cross Mutual Information Analysis of the Brain Functional Networks Underlying Multiclass Motor Imagery. J. Mot. Behav. 2018, 50, 254–267. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsieh, J.C.; Wu, Y.Z.; Lee, P.L.; Chen, S.S.; Niddam, D.M.; Yeh, T.C.; Wu, Y.T. Mutual-information-based approach for neural connectivity during self-paced finger lifting task. Hum. Brain Mapp. 2008, 29, 265–280. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Han, Y. Decoding of Brain Functional Connections Underlying Natural Grasp Task Using Time-Frequency Cross Mutual Information. IEEE Access 2023, 11, 84912–84921. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Muller, G.R.; Pfurtscheller, J.; Gerner, H.J.; Rupp, R. ‘Thought’—control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci. Lett. 2003, 351, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, D.; Wang, Y.; Deng, L.; Wang, X.; Wu, C.; Song, A. Decoding Different Reach-and-Grasp Movements Using Noninvasive Electroencephalogram. Front. Neurosci. 2021, 15, 684547. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Escolano, C.; Montesano, L.; Muller-Putz, G.R. Analyzing and Decoding Natural Reach-and-Grasp Actions Using Gel, Water and Dry EEG Systems. Front. Neurosci. 2020, 14, 849. [Google Scholar] [CrossRef]
- Schwarz, A.; Pereira, J.; Lindner, L.; Muller-Putz, G.R. Combining frequency and time-domain EEG features for classification of self-paced reach-and-grasp actions. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3036–3041. [Google Scholar] [CrossRef]
- Schwarz, A.; Holler, M.K.; Pereira, J.; Ofner, P.; Muller-Putz, G.R. Decoding hand movements from human EEG to control a robotic arm in a simulation environment. J. Neural Eng. 2020, 17, 036010. [Google Scholar] [CrossRef]
- Pereira, J.; Kobler, R.; Ofner, P.; Schwarz, A.; Muller-Putz, G.R. Online detection of movement during natural and self-initiated reach-and-grasp actions from EEG signals. J. Neural Eng. 2021, 18, 046095. [Google Scholar] [CrossRef]
- Schwarz, A.; Pereira, J.; Kobler, R.; Muller-Putz, G.R. Unimanual and Bimanual Reach-and-Grasp Actions Can Be Decoded From Human EEG. IEEE Trans. Biomed. Eng. 2020, 67, 1684–1695. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Hjorth, B. Principles for transformation of scalp EEG from potential field into source distribution. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1991, 8, 391–396. [Google Scholar] [CrossRef]
- do Nascimento, O.F.; Nielsen, K.D.; Voigt, M. Movement-related parameters modulate cortical activity during imaginary isometric plantar-flexions. Exp. Brain Res. 2006, 171, 78–90. [Google Scholar] [CrossRef]
- Cunnington, R.; Iansek, R.; Bradshaw, J.L.; Phillips, J.G. Movement-related potentials associated with movement preparation and motor imagery. Exp. Brain Res. 1996, 111, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, H.H.; Deecke, L. Brain potential changes in voluntary and passive movements in humans: Readiness potential and reafferent potentials. Pflug. Arch. 2016, 468, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.M.; Ray, W.J. Movement-related potentials with reference to isometric force output in discrete and repetitive tasks. Exp. Brain Res. 1998, 123, 461–473. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, O.F.; Nielsen, K.D.; Voigt, M. Relationship between plantar-flexor torque generation and the magnitude of the movement-related potentials. Exp. Brain Res. 2005, 160, 154–165. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Neuper, C.; Wortz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Aranibar, A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 138–146. [Google Scholar] [CrossRef]
- Duncan, T.E. On the Calculation of Mutual Information. SIAM J. Appl. Math. 1970, 19, 215–220. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Bostanov, V. BCI Competition 2003—Data sets Ib and IIb: Feature extraction from event-related brain potentials with the continuous wavelet transform and the t-value scalogram. IEEE Trans. Biomed. Eng. 2004, 51, 1057–1061. [Google Scholar] [CrossRef]
- Fraser, A.M.; Swinney, H.L. Independent coordinates for strange attractors from mutual information. Phys. Rev. A Gen. Phys. 1986, 33, 1134–1140. [Google Scholar] [CrossRef]
- Zandi, A.S.; Javidan, M.; Dumont, G.A.; Tafreshi, R. Automated Real-Time Epileptic Seizure Detection in Scalp EEG Recordings Using an Algorithm Based on Wavelet Packet Transform. IEEE Trans. Biomed. Eng. 2010, 57, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Stat. Sci. 1986, 1, 54–75. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Müller, K.R.; Krauledat, M.; Dornhege, G.; Curio, G.; Blankertz, B. Machine learning techniques for brain-computer interfaces. Biomed. Tech. 2004, 49, 11–22. [Google Scholar]
- Deecke, L. Planning, preparation, execution, and imagery of volitional action. Cogn. Brain Res. 1996, 3, 59–64. [Google Scholar] [CrossRef]
- Lang, W.; Beisteiner, R.; Lindinger, G.; Deecke, L. Changes of cortical activity when executing learned motor sequences. Exp. Brain Res. 1992, 89, 435–440. [Google Scholar] [CrossRef]
- Zhang, W.; Han, X.; Qiu, S.; Li, T.; Chu, C.; Wang, L.; Wang, J.; Zhang, Z.; Wang, R.; Yang, M.; et al. Analysis of Brain Functional Network Based on EEG Signals for Early-Stage Parkinson’s Disease Detection. IEEE Access 2022, 10, 21347–21358. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A., Jr.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network—Anatomy, function, and relevance to disease. In Year in Cognitive Neuroscience 2008; Kingstone, A., Miller, M.B., Eds.; Annals of the New York Academy of Sciences: New York, NY, USA, 2008; Volume 1124, pp. 1–38. [Google Scholar]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020–11025. [Google Scholar] [CrossRef]
- Sehatpour, P.; Molholm, S.; Schwartz, T.H.; Mahoney, J.R.; Mehta, A.D.; Javitt, D.C.; Stanton, P.K.; Foxe, J.J. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc. Natl. Acad. Sci. USA 2008, 105, 4399–4404. [Google Scholar] [CrossRef]
- Binkofski, F.; Buccino, G.; Posse, S.; Seitz, R.J.; Rizzolatti, G.; Freund, H. A fronto-parietal circuit for object manipulation in man: Evidence from an fMRI-study. Eur. J. Neurosci. 1999, 11, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Ferraina, S.; Bianchi, L.; Caminiti, R. Cortical networks for visual reaching: Physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb. Cortex 1996, 6, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Culham, J.C.; Valyear, K.F. Human parietal cortex in action. Curr. Opin. Neurobiol. 2006, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.S.A.; Taylor, C.S.R.; Moore, T. Complex Movements Evoked by Microstimulation of Precentral Cortex. Neuron 2002, 34, 841–851. [Google Scholar] [CrossRef] [PubMed]




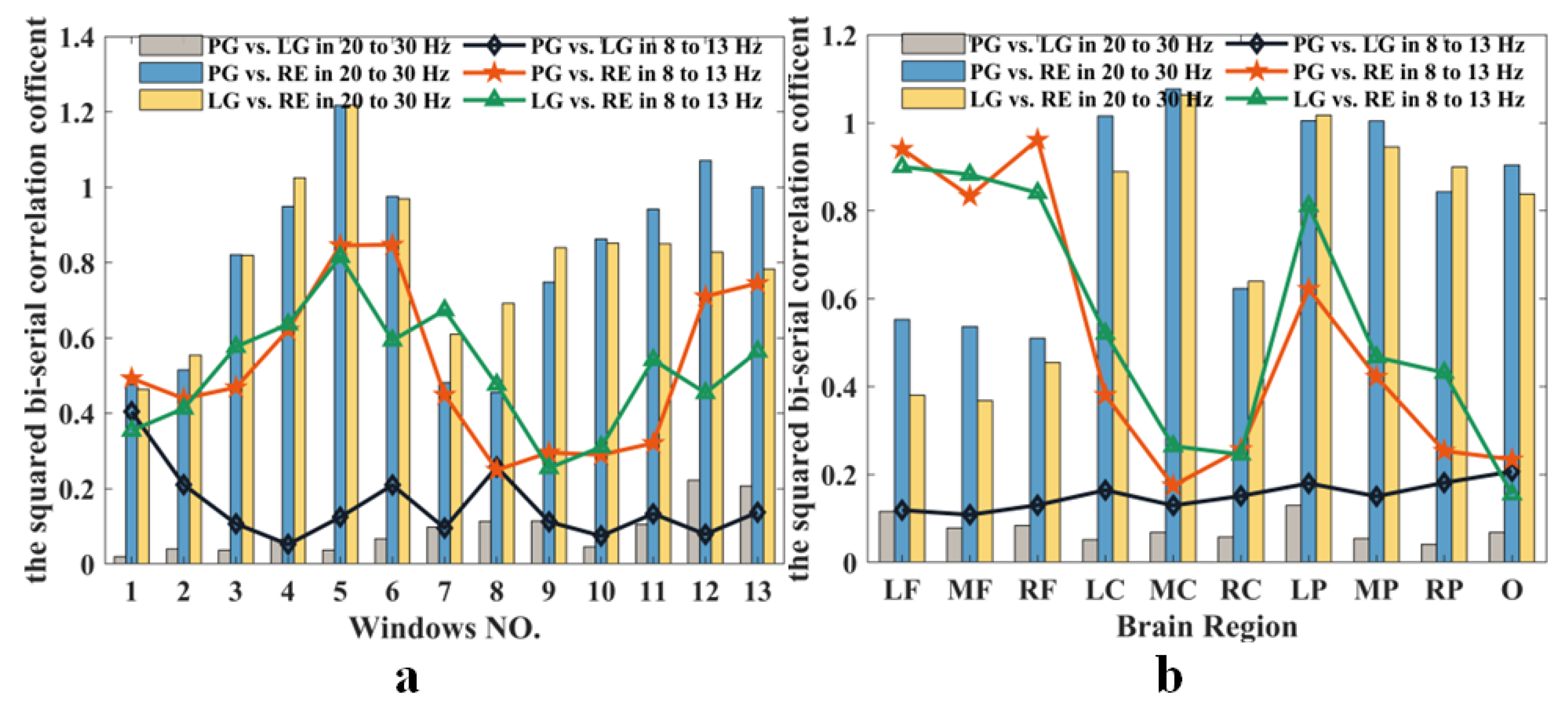
| Cortical location | Channels |
|---|---|
| Left frontal area (LF) | F3, FFC3h |
| Middle frontal area (MF) | F1, FZ, F2, FFC1h, FFC2h |
| Right frontal area (RF) | F4, FFC4h |
| Left central area (LC) | FC5, FC3, FCC5h, FCC3h, C5, C3, CCP5h, CCP3h, CP5, CP3 |
| Middle central area (MC) | FC1, FCz, FC2, FCC1h, FCC2h, C1, Cz, C2, CCP1h, CCP2h, CP1, CPz, CP2 |
| Right central area (RC) | FC4, FC6, FCC4h, FCC6h, C4, C6, CCP4h, CCP6h, CP4, CP6 |
| Left parietal area (LP) | CPP5h, CPP3h, P5, P3 |
| Middle parietal area (MP) | CPP1h, CPP2h, Pz, P1, P2 |
| Right parietal area (RP) | CPP4h, CPP6h, P4, P6 |
| Occipital area (O) | PPO1h, PPO2h, POz |
| Subject | Palmar (%) | Lateral (%) | Rest (%) | AVG (%) |
|---|---|---|---|---|
| G01 | 78.57 | 60.00 | 100.00 | 79.52 |
| G02 | 85.71 | 78.57 | 92.86 | 85.71 |
| G03 | 78.57 | 78.57 | 92.86 | 83.33 |
| G04 | 85.71 | 66.67 | 92.86 | 81.75 |
| G05 | 85.71 | 78.57 | 100.00 | 88.09 |
| G06 | 71.43 | 73.33 | 100.00 | 81.59 |
| G07 | 60.00 | 71.43 | 100.00 | 77.14 |
| G08 | 63.64 | 63.64 | 91.67 | 72.98 |
| STD | 9.52 | 6.81 | 3.74 | 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Wang, J.; Jiao, F.; Han, Y.; Xu, W.; Zhao, X. Decoding Electroencephalography Underlying Natural Grasp Tasks across Multiple Dimensions. Electronics 2023, 12, 3894. https://doi.org/10.3390/electronics12183894
Gu H, Wang J, Jiao F, Han Y, Xu W, Zhao X. Decoding Electroencephalography Underlying Natural Grasp Tasks across Multiple Dimensions. Electronics. 2023; 12(18):3894. https://doi.org/10.3390/electronics12183894
Chicago/Turabian StyleGu, Hao, Jian Wang, Fengyuan Jiao, Yan Han, Wang Xu, and Xin Zhao. 2023. "Decoding Electroencephalography Underlying Natural Grasp Tasks across Multiple Dimensions" Electronics 12, no. 18: 3894. https://doi.org/10.3390/electronics12183894
APA StyleGu, H., Wang, J., Jiao, F., Han, Y., Xu, W., & Zhao, X. (2023). Decoding Electroencephalography Underlying Natural Grasp Tasks across Multiple Dimensions. Electronics, 12(18), 3894. https://doi.org/10.3390/electronics12183894





