Abstract
Along with the continuous aging of the population, various diseases have brought a great threat to human health and a large economic burden. The development of advanced medical devices has gained global attention for disease treatment. Electrical stimulation refers to stimulation and treatment of cells by high output voltage, which is an important rehabilitation and therapeutic strategy in medical treatment. Triboelectric nanogenerators (TENGs), which are lightweight and feature high-voltage output and flexible structure, have drawn great attention in the field of disease treatment for health care. The conversion of the body’s mechanical energy into electrical pulses to stimulate cells for health treatment through TENG has promising applications. Using uniquely designed TENGs to convert human mechanical energy into electrical impulses to stimulate cells is considered a promising health treatment. Here, we review the recent progress of TENG-based electrical stimulation for disease treatments, focusing on the structure, materials, and performances of the TENGs used in diverse facets of healthcare. More importantly, we systematically discuss the application of TENG-based electrical stimulation in wound healing, osteoblast proliferation and differentiation, muscle stimulation, nerve stimulation, and pacemakers. Finally, several developmental challenges of and prospective solutions for TENG-based electrical stimulation are discussed and summarized in light of recent advances.
1. Introduction
The aging of the population has become a global phenomenon, and healthcare expenditure has put a heavy burden on social and economic development. Effective and low-cost treatment equipment contributes to the diagnosis and therapy of diseases over time, which can not only minimize the illness caused by diverse diseases but also reduce medical expenditures and alleviate the family’s economic situation. Electrical stimulation therapy [1,2] which stimulates the tissues and organs or innervates their nerves to control tissues and organs by controlling the parameters of a pulse current to achieve therapeutic effects, has been applied to clinical practice in the 20th century. Since electrical stimulation often requires a stable high-voltage output, and traditional therapeutic equipment requires regular replacement of the power supplies, this process not only incurs additional medical expenses but also increases the complexity of the treatment process. With the development of science and technology, the emergence of triboelectric nanogenerators has allowed researchers to explore the possibility of electrical stimulation therapy devices by utilizing them [3,4]. Compared with the piezoelectric nanogenerator (PENG), the TENG shows much higher output voltage with smaller size, biocompatibility with selection of diverse materials, and flexible structural design, and it is considered a potential choice for electrical stimulation.
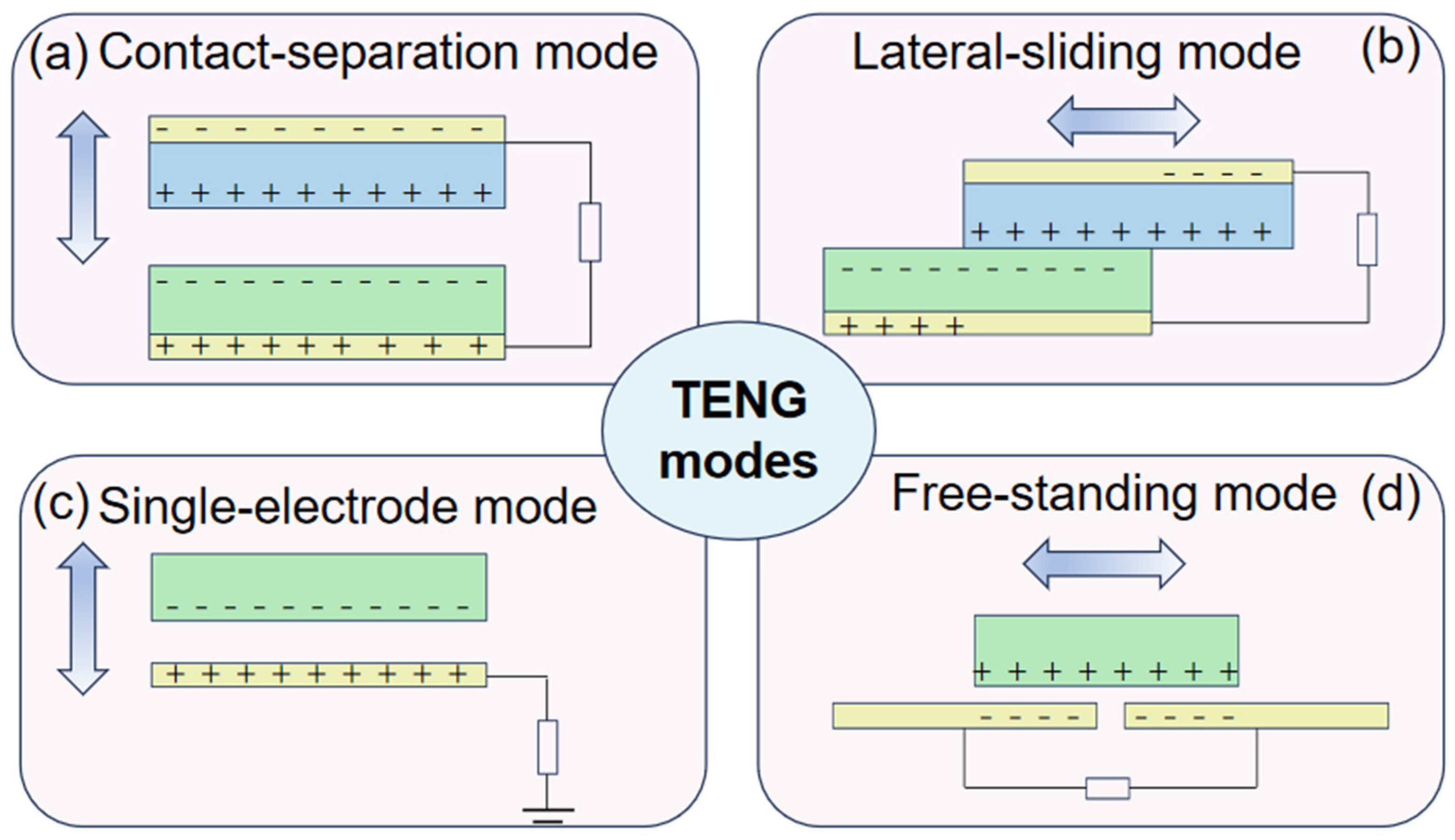
Triboelectric nanogenerators have attracted a lot of attention since their invention, and they are based on the coupling effect of triboelectrification and electrostatic induction. It is well known that when two different materials come into contact with each other, a frictional charge is generated. However, if the materials are dielectric, the frictional charge generated will be preserved for a long time [5,6]. Driven by external forces, the frictionally charged interface causes relative movement between each other, leading the periodical change of the potential difference. The periodical potential difference will drive the electron flow through the external load, generating an alternating-current output. As demonstrated in Figure 1, the TENG can work in four different modes, including contact-separation mode, lateral-sliding mode, single-electrode mode, and free-standing mode, which contribute to harvesting mechanical energy efficiently in the environments around us, such as human motions, wind energy [7,8], and wave energy in the ocean [9,10], converting it into electrical energy to power the electronics. Given this unique principle, the TENG has the advantages of high-voltage output, flexible structure, light weight, and a wide range of material choices, which make it possible for researchers to invent high-voltage-source, biocompatible, and sustainable self-powered electrical stimulation devices based on the TENG [11,12].
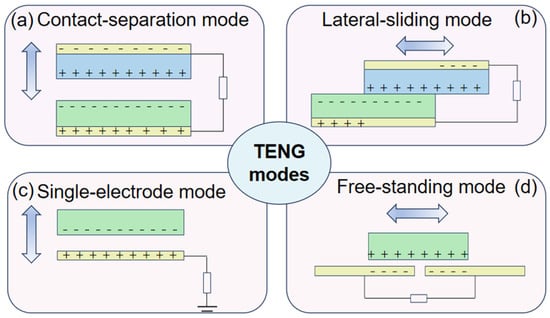
Figure 1.
Four TENG modes. (a) Contact-separation mode. (b) Lateral-sliding mode. (c) Single-electrode mode. (d) Free-standing mode.
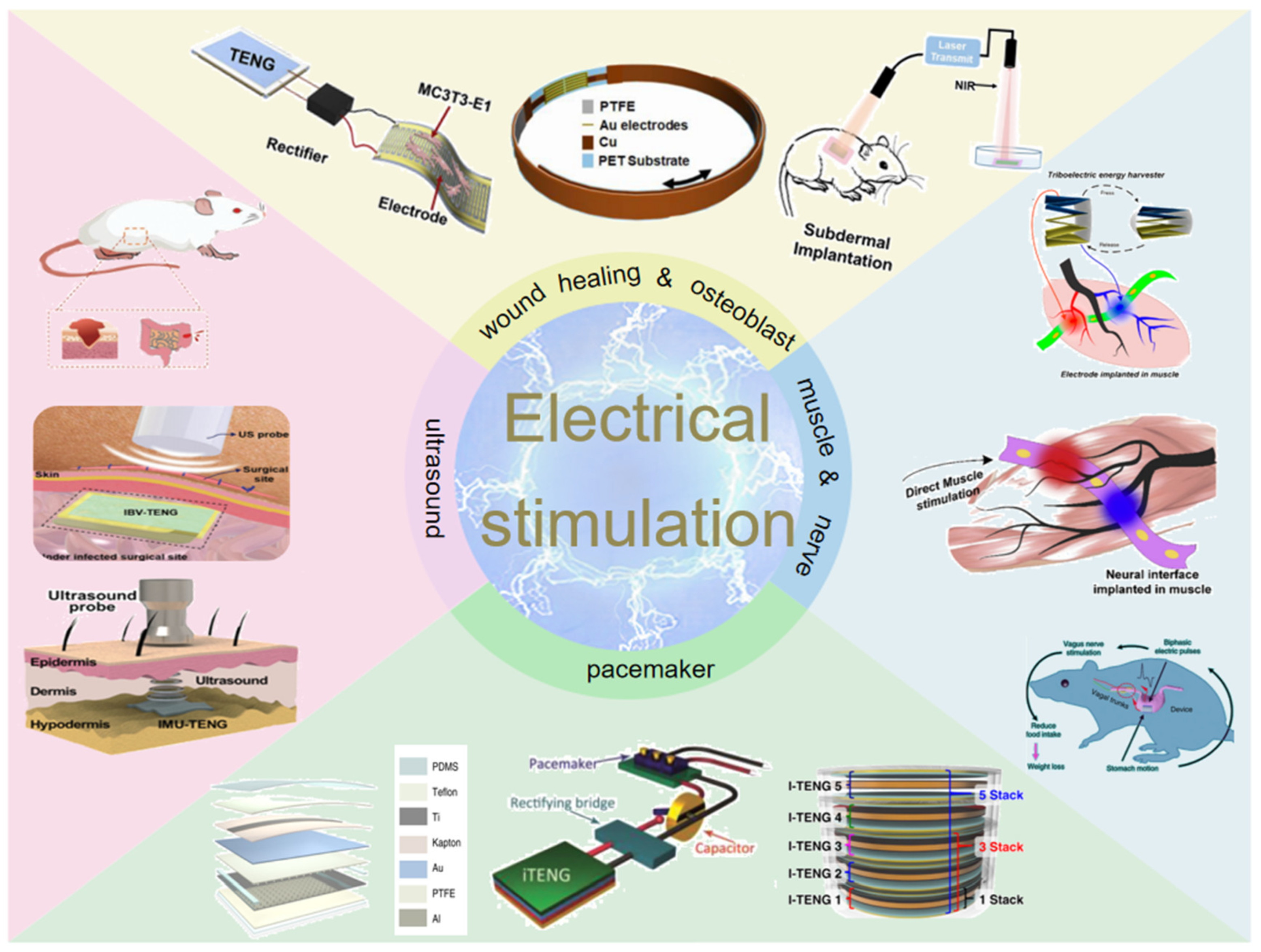
In this review, recent advances of TENG-based electrical stimulation devices will be presented comprehensively. As shown in Figure 2, the progress of wound healing and osteoblast proliferation and differentiation will be described, and then the application of muscle stimulation and nerve stimulation is systematically discussed. In addition, the pacemaker module for cardiovascular disease is presented from the aspects of the materials, structures, and properties of the TENG components. We also discuss the applications of ultrasound-driven TENG for antibacterial and wound healing and finally summarize the challenges and prospects of TENG-based medical devices in the future.
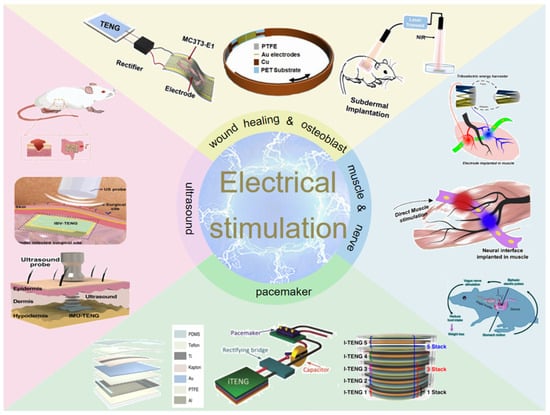
Figure 2.
Diagram of the four application modules of electrical stimulation discussed.
2. Wound Healing, Osteoblast Proliferation and Differentiation
Bruises, cuts, and other phenomena often occur in life, and for the general population, slow wound healing only affects the quality of life of patients. However, for some special groups, such as patients with diabetes and rheumatoid arthritis, a little carelessness will lead to serious health problems [13,14]. The slow healing or nonhealing of the wound increases the risk of wound infection, and the infection can even spread to the surrounding tissues or other parts of the body, resulting in chronic diseases, even fatal in some cases. Many years of research have shown that electrical stimulation can promote the recovery of skin wounds [15,16,17]. However, due to the limitation of bulky electrical equipment, electrical stimulation has not been put into large-scale treatment. Since 2012, the TENG’s invention [18] has provided new ideas for wound healing. Because of its lightweight, flexible structure and low-cost self-generating characteristics, the TENG has become an important candidate for preparing wound-healing devices [19,20,21].
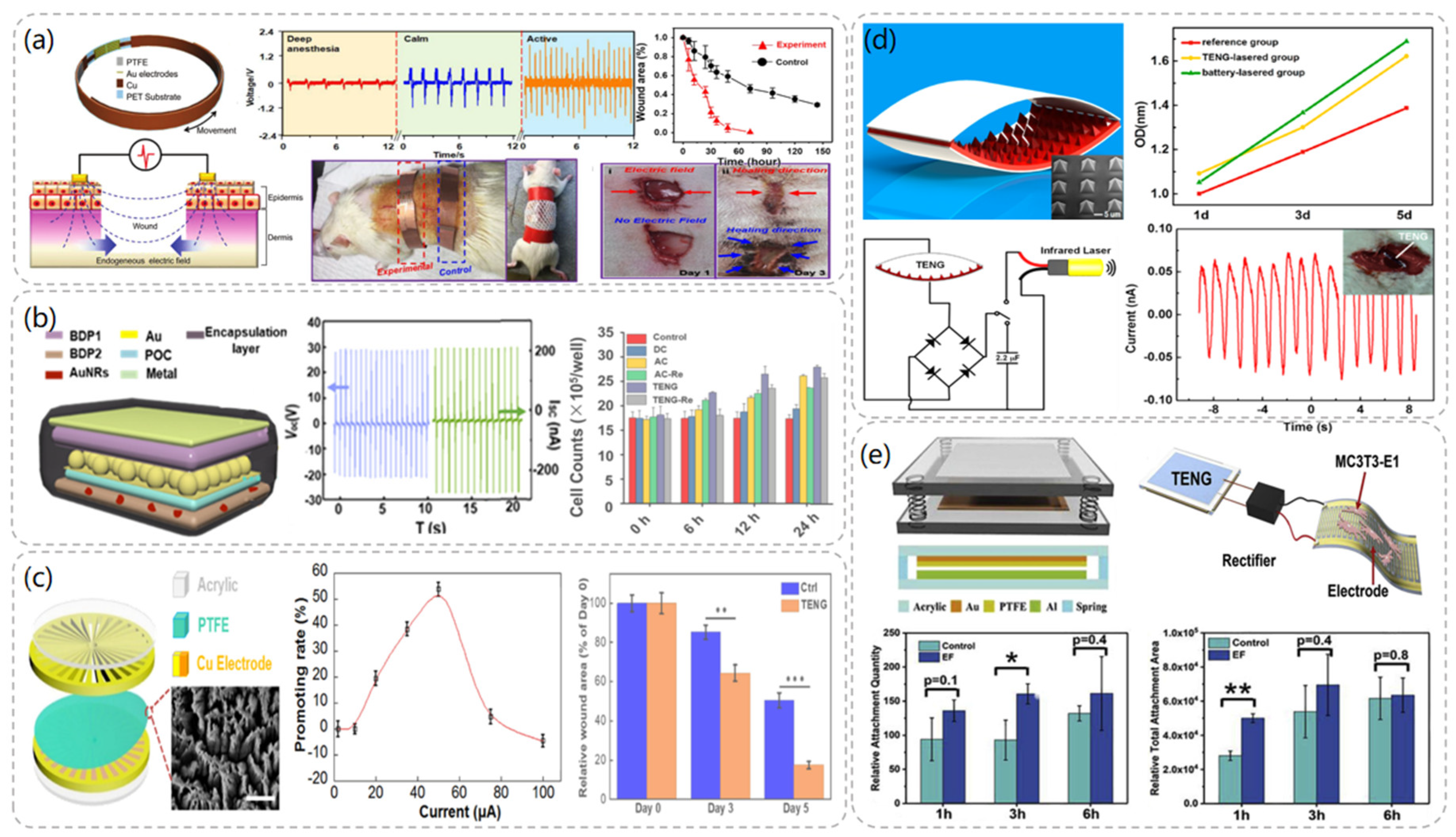
In 2018, Wang et al. demonstrated that a TENG-based wearable electronic bandage can achieve effective wound recovery [22]. The device can locally convert the mechanical energy generated by rat respiration into discrete AC voltage signals and directly apply the electric field to the wound to promote fibroblast migration, proliferation, and transdifferentiation to enhance skin regeneration. The electronic bandage consists of two parts: the TENG part and the dressing electrode. As demonstrated in Figure 3a, the TENG is designed as a multilayer structure consisting of a polyethylene terephthalate (PET) layer, a Cu foil, and a PTFE layer with Au electrodes, respectively. The multilayer structure allows the device to have better flexibility and to fit better on the skin’s surface. The amplitude of the peak-to-peak voltage generated by the normal activity of the rat’s respiration was able to reach a maximum level of 102.2 V at a rate of 110/min when the electronic bandage was implanted in the rat. By stimulating the dorsal wounds of the rats with the electronic bandage, the recovery period of the wounds in the experimental group was shortened from the normal 12 days to 3 days, and the present study was proven to be an effective therapeutic strategy in the treatment of wound healing.
In 2018, Wang et al. fabricated a tunable biodegradable (BD) implantable TENG (BD-iTENG) responding to near-infrared (NIR) photothermal manipulation [23]. The peak output voltage of the BD-iTENG is about 2V in the implanted rat, and the output of the BD-iTENG can accelerate the process of narrowing the wound by carving the L929 cells to accelerate the wound-healing process. As demonstrated in Figure 3b, BD-iTENG uses a hemispherical array structure deposited with Au as the friction layer, which increases the contact area between the two friction layers and increases the output effect. This is one of the highlights of this TENG. In addition, BD-iTENG demonstrated a controlled biodegradation process by using Au nanorods in response to NIR treatment, where the output voltage of BD-iTENG was reduced to 0 V within 24 h, and the device was able to degrade substantially within 14 days. For short-term therapeutic devices, the controlled biodegradation of the device avoids the need for another surgical removal step after the device has completed its work cycle, which not only reduces medical expenses but also alleviates patient pain.
In 2019, Wen et al. [24] designed a TENG-driven electrical stimulation system to achieve proliferative behavior on fibroblasts and thus promote wound healing. The TENG was designed as a rotatory disc-shaped TENG (RD-TENG) and consists of a rotor and a stator mounted coaxially. As depicted in Figure 3c, the rotor consists of a disc-shaped printed circuit board (PCB) deposited with radial copper, while the stator consists of the same PCB coated with cross-finger copper electrodes with a piece of PTFE film adhered. Due to the different output currents at different speeds, a rheostat was connected externally in the circuit to obtain different currents at the same frequency for experimental studies. The experimental data showed that when the peak current of RD-TENG was greater than 10 μA, it had an obvious promotion effect on cell proliferation, and its relative promotion rate could reach more than 50% when the current was 50 μA, which had the best promotion effect. Moreover, the expression of Fgf 2 (fibroblast growth factor 2) and Dlk 1 (delta-like non-canonical Notch ligand 1), which play an important role in promoting wound healing, was significantly upregulated after RD-TENG stimulation of L929 cells. This also demonstrated the promising application of the device in wound healing.
Osteoporosis has now become a major health problem for people over 50 years of age, especially for middle-aged and elderly women [25]. It is a chronic disease that seriously endangers bone health, and most people may lack sufficient understanding and attention to it. Once it develops seriously, not only will it make the patients suffer, but its follow-up medical and nursing care requires a large amount of human, material, and economic resources, which will result in a heavy burden on the family and society [26,27]. As one of the most common orthopedic diseases, osteoporosis is mainly caused by bone resorption by osteoclasts being greater than bone formation by osteoblasts, resulting in a state of low bone mass, impaired bone tissue microstructure, increased bone fragility, and susceptibility to fracture. Numerous studies have shown that physical electrical stimulation, such as direct current, pulsed current, electric field, and electromagnetic field, can promote bone formation of osteoblasts [28,29], thus providing a certain alleviation and therapeutic effect on osteoporosis, and treatment through electrical stimulation has become a popular research topic.
After Wang et al. invented the TENG in 2012, they combined the TENG with an infrared laser excitation unit to develop a self-powered low-level laser therapy (SPLC) [30] system for osteogenesis in 2015. In the TENG, polydimethylsiloxane (PDMS) and indium tin oxide (ITO) were used as friction materials, and the PDMS layer was patterned with pyramidal arrays to increase its current output. Tests revealed that this TENG outputs a short-circuit current of about 30 μA and an open-circuit voltage of 115 V. As shown in Figure 3d, a series of comparative experiments demonstrated that the TENG-driven laser therapy group showed a significant increase in the number of MC3T3-E1 cells after two days of irradiation and a 15% increase in their cell proliferation rate compared to the reference group without laser therapy. Moreover, the system can be driven by human walking or mouse breathing, which is convenient compared with traditional battery laser devices, and thus has great potential for development in the field of portable or implantable therapeutic devices.
In 2019, Li et al. [31] proposed a self-powered flexible implantable electrical stimulator, which consists of a TENG, a rectifier, and flexible forked-finger electrodes. The TENG converts mechanical energy from the daily movements of rats into electrical energy and connects the energy to the electrodes through the rectifier to stimulate the MC3T3-E1 cells to up-regulate intracellular calcium ions and promote adhesion, proliferation, and differentiation of osteoblasts. The rectified TENG has an output open-circuit voltage of 100 V and a short-circuit current of 1.6 µ A. As illustrated in Figure 3e, the TENG uses Al and PTFE films as friction layers, where the PTFE films are treated with inductively coupled plasma reaction ion etching (ICP) to form nanostructure arrays to increase the friction area, and springs are used between the friction layers to ensure contact and separation of the friction layers. The results showed that the number of MC3T3-E1 cells attached to the cell wall increased by 72.76% after 3 h of electrical stimulation compared with the group without stimulation, and the cell proliferation rate increased by 23.82% after 3 days of stimulation. The flexible TENG was prepared by adjusting the TENG material and was implanted on the femoral surface of rats; it successfully converted the mechanical energy of the rats’ daily movement into electrical energy.
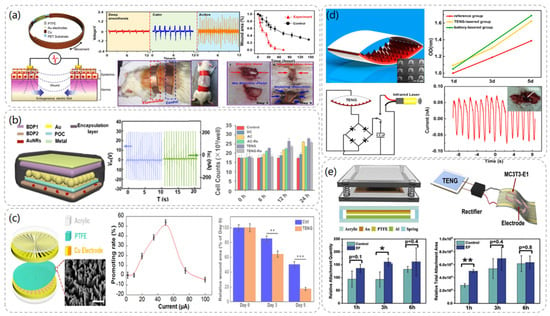
Figure 3.
Wound healing and osteoblast module after TENG chart and related data. (a) Structural diagram of TENG−based wearable electronic bandage and data comparison with other wound healing devices in comparative experiments. Reproduced with permission from ref. [22]. (b) The structural diagram of BD-iTENG and the changes of cell proliferation degree after electrical stimulation of fibroblasts L929 by DC, AC, and BD-iTENG. Reproduced with permission from ref. [23]. (c) RD-TENG schematic diagram and output performance: The effect of the peak current value of RD-TENG on the promotion rate of L929 cells is the best at 50 μA. Reproduced with permission from ref. [24]. (d) Structural design and characterization of TENG. Reproduced with permission from ref. [30]. (e) Schematic diagram of self-powered electrical stimulation device and structure of TENG. Reproduced with permission from ref. [31].
Figure 3.
Wound healing and osteoblast module after TENG chart and related data. (a) Structural diagram of TENG−based wearable electronic bandage and data comparison with other wound healing devices in comparative experiments. Reproduced with permission from ref. [22]. (b) The structural diagram of BD-iTENG and the changes of cell proliferation degree after electrical stimulation of fibroblasts L929 by DC, AC, and BD-iTENG. Reproduced with permission from ref. [23]. (c) RD-TENG schematic diagram and output performance: The effect of the peak current value of RD-TENG on the promotion rate of L929 cells is the best at 50 μA. Reproduced with permission from ref. [24]. (d) Structural design and characterization of TENG. Reproduced with permission from ref. [30]. (e) Schematic diagram of self-powered electrical stimulation device and structure of TENG. Reproduced with permission from ref. [31].
3. Muscle Stimulation and Nerve Stimulation
Muscle atrophy is very harmful to the body. It will lead to muscle weakness or loss of motor function. At present, the main treatment for this symptom is neuromuscular electrical stimulation [32,33,34,35]. Its basic principle is to use the electrical excitability of nerve cells to stimulate the nerves that dominate the muscle to make the muscle contract through pulse current. In recent years, commercial neuromuscular electrical stimulators have been developed, but for the implantable electrical muscle stimulation system, the power supply problem has been a huge challenge that has not been solved. The continuous development of TENG technology makes it possible to maintain the self-sustainable power supply of implanted equipment through mechanical energy collection equipment.
Chengkuo Lee at the National University of Singapore presented a treatment for muscle stimulation via stacked TENG with multichannel intramuscular electrodes in 2019 [36]. The ability of the multichannel intramuscular electrodes to localize motor neurons sparsely distributed in muscle tissue allows the device to achieve efficient TENG muscle electrical stimulation at a short-circuit current of 35 μA. As depicted in Figure 4a, the use of a PET sheet folded into a serrated structure as a support for the TENG ensures that the TENG can be restored to its original position after being pressed. The PTFE film and Al layer are then attached to the PET sheet separately as a friction layer. Intramuscular electrodes were implanted into the anterior tibial (TA) muscle belly of the rat, and the electrodes were connected to the TENG via FPC connectors. It was found that the use of site-specific electrodes activated muscle contraction. The possibility of electrical stimulation of the muscle using a direct power supply from the TENG was demonstrated.
Li et al. proposed the use of diode-amplified TENG (D-TENG) [37] for muscle stimulation in 2017. Not only does D-TENG amplify the current generated by the TENG, the exponential current pulses it generates are the optimal waveform for muscle stimulation, and thus the stimulation efficiency can be greatly improved. As demonstrated in Figure 4b, D-TENG is also designed as a sawtooth-shaped layer, using an Al layer and a PTFE layer as the friction layer, and requires an additional mechanical switch to control the diode in parallel. It was found that the electrical pulses generated by the D-TENG were more effective for muscle stimulation than commercial stimulators, overcoming the high threshold barrier for direct muscle stimulation and making the TENG an effective muscle stimulator. In addition, the device is a versatile configuration that can be applied to TENGs of other materials and structures to further improve output.
In fact, there are nerves distributed in every part of the human body, and all kinds of physiological behaviors are controlled by nerves. Neuromodulation, as a non-destructive and reversible treatment method, has attracted extensive attention [38,39,40]. Unlike drug therapy, it may have side effects. Only the corresponding nerve cells need to be electrically stimulated to control or treat the human body. The combination of TENG device and stimulator or neural interface can achieve more convenient, safe, and effective treatment in the future.
In 2017, Lee et al. proposed a stacked TENG as a neural interface for neural stimulation [41]. The TENG has an output voltage of 160 V and a short-circuit current of 6.7 µ A. The TENG was designed as a zigzag-shaped structure, and PET material was chosen as the mechanical support for this structure, using Cu and PDMS layers as friction electric layers. As depicted in Figure 4c, it is driven by human muscle movement to generate electrical energy, and the common peroneal nerve (CP) is then stimulated through the nerve interface to activate the tibialis anterior (TA) muscle. The experiments have demonstrated that the TENG can generate enough electrical charge to directly stimulate the CP to control the TA muscle. The TENG with an integrated flexible neural interface has important developmental value for future battery-free neuromodulation.
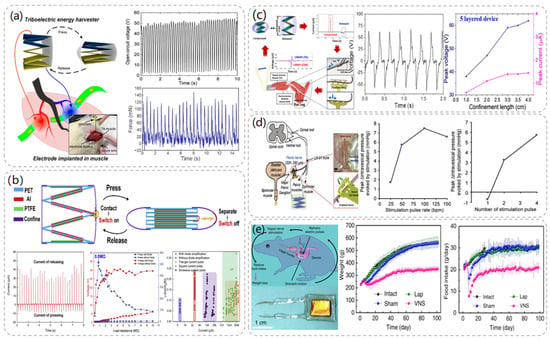
Figure 4.
Muscle stimulation and nerve stimulation module TENG structure and related data. (a) Structural diagram of the TA muscle stimulated by TENG with electrodes. Reproduced with permission from ref. [36]. (b) Structure, constituent materials, and operation process of D-TENG. Reproduced with permission from ref. [37]. (c) Schematic diagram of the TENG in the compressed and released state and the output data diagram of the 5–layer structure of the TENG. Reproduced with permission from ref. [41]. (d) A rendering of bladder nerve stimulation using a stacked TENG combined with a flexible nerve clip (FNC) interface. Reproduced with permission from ref. [42]. (e) Schematic diagram of the operating principle of the VNS system and comparison of body weight changes in mice stimulated by the device. Reproduced with permission from ref. [43].
Figure 4.
Muscle stimulation and nerve stimulation module TENG structure and related data. (a) Structural diagram of the TA muscle stimulated by TENG with electrodes. Reproduced with permission from ref. [36]. (b) Structure, constituent materials, and operation process of D-TENG. Reproduced with permission from ref. [37]. (c) Schematic diagram of the TENG in the compressed and released state and the output data diagram of the 5–layer structure of the TENG. Reproduced with permission from ref. [41]. (d) A rendering of bladder nerve stimulation using a stacked TENG combined with a flexible nerve clip (FNC) interface. Reproduced with permission from ref. [42]. (e) Schematic diagram of the operating principle of the VNS system and comparison of body weight changes in mice stimulated by the device. Reproduced with permission from ref. [43].
Similarly, the regulation of bladder function can also rely on neural stimulation, and in 2019, Lee et al. proposed a way to control bladder function using a stacked TENG interfaced with a flexible nerve clip (FNC) [42]. As illustrated in Figure 4d, a four-layer zigzag PET sheet was used as the body of the TENG, and PTFE and Al layers were used as frictional and electrical layers, respectively. The TENG was connected to the FNC, which was implanted in the pelvic nerve next to the bladder in rats, and the effects of different factors on the experimental results were investigated by monitoring bladder pressure changes and urination. The research team investigated not only the frequency stimulation parameters but also the effects of different numbers of stimulation pulses on bladder regulation. The results of the experiments showed that when pulses of 50 BPM and above were applied to the pelvic nerves of rats, or when two or more numbers of pulses were applied, significant changes in bladder pressure and micturition occurred. This work demonstrates the possibility of controlling bladder contraction and voiding through this device.
In 2018, Wang et al. successfully developed an implantable vagus nerve stimulation (VNS) device for weight control [43]. As shown in Figure 4e, the flexible TENG is attached to the surface of the stomach to reduce food intake by stimulating the vagus nerve with electrical pulses generated by the peristaltic movement of the stomach, ultimately achieving weight control. A series of controlled experiments using rats with an initial body weight of 250 g found that under the same feeding conditions, rats implanted with VNS consumed only about 2/3 of the daily food consumption of control rats, and weighed about 350 g after 100 days, which was 63% of the final body weight of the control group. When the implanted VNS device was removed, the rats were also able to return to their normal weight status immediately. This work demonstrated that an implantable VNS device has a significant effect on neurostimulation for weight control and that the weight control effect is reversible.
4. Pacemaker
A pacemaker is a special medical electronic device that stimulates the heart by sending out some form of electrical pulse to make it excited and contract. That is, it simulates the impulse formation and conduction of the normal heart in order to treat heart dysfunction due to certain heart rate disorders. With the rapid development of pacemaker manufacturing technology and techniques, and after the first pacemaker was implanted in the human body in 1958, the functions of pacemakers became increasingly sophisticated and began to be applied to diseases such as slow arrhythmias and tachyarrhythmias [44,45]. The battery life of conventional pacemakers is only about 5 years, which means that patients need to undergo several major surgeries to replace the battery regularly, which not only increases the patient’s treatment expenses but also aggravates the risks of the surgery and causes more pain to the patient. Self-powered pacemakers that combine TENG with pacemakers have become a popular direction for researchers.
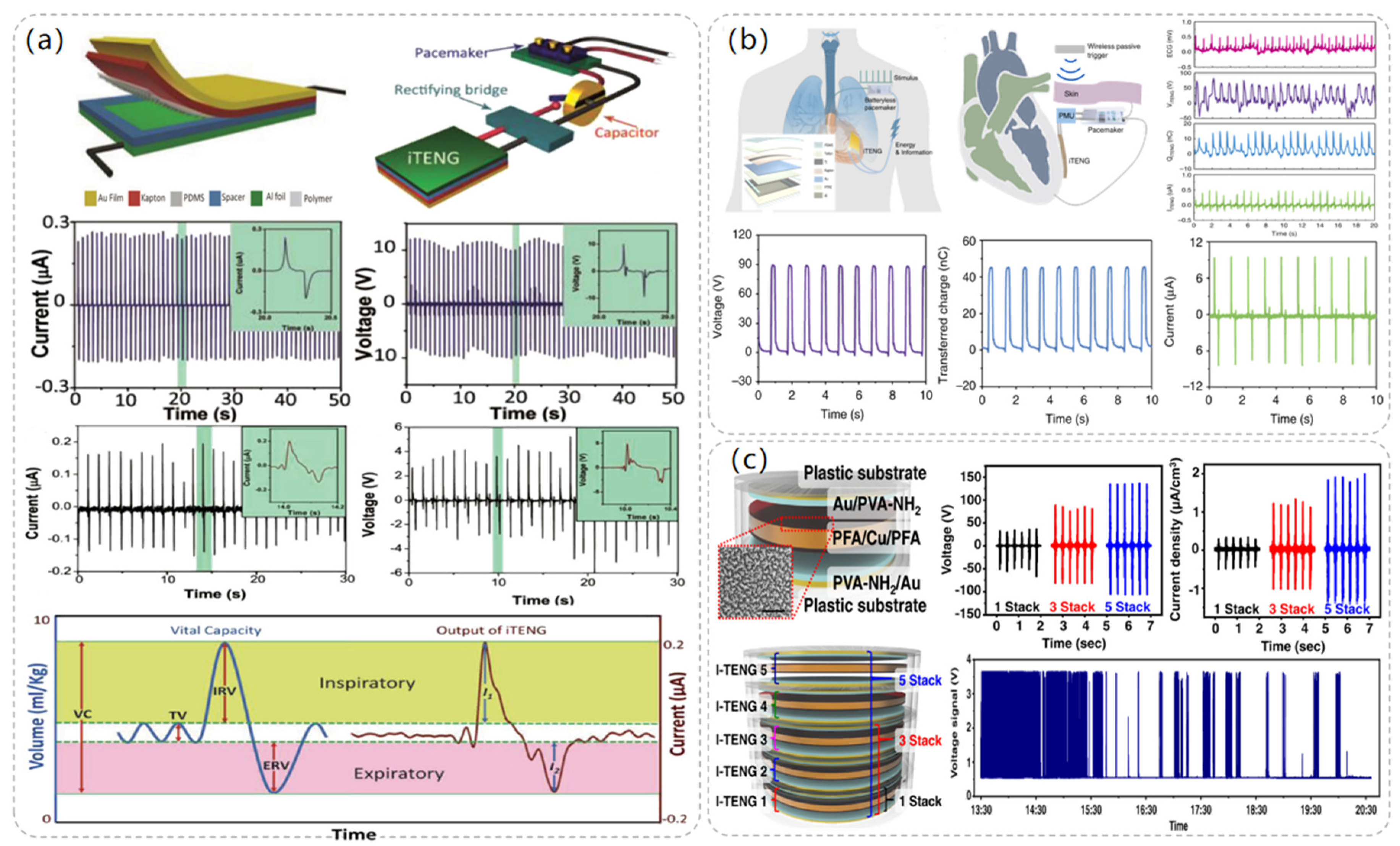
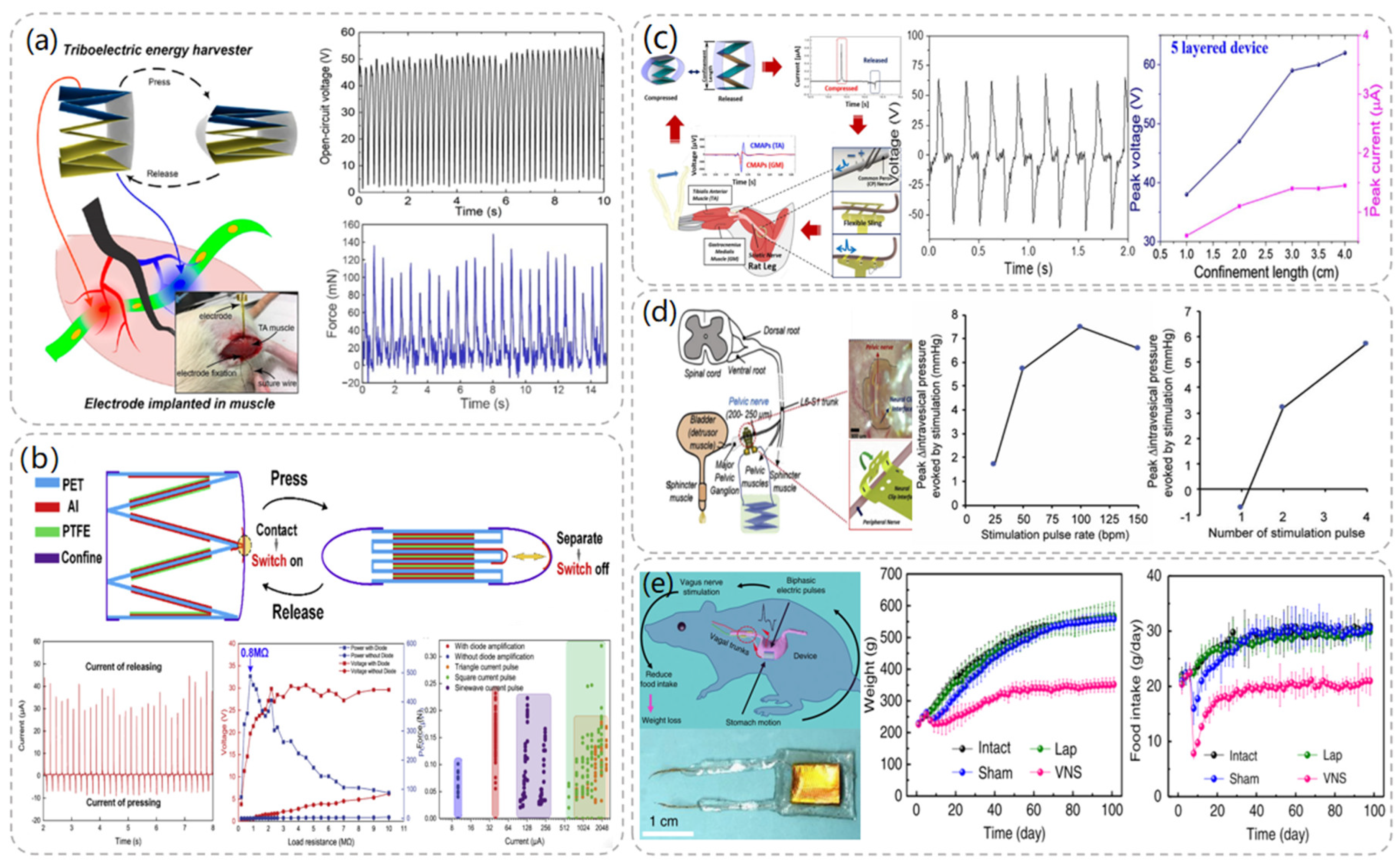
In 2014, Wang et al. proposed an implantable triboelectric nanogenerator (iTENG) powered by collected respiratory energy to power a pacemaker that successfully regulated the heart rate of a rat [46]. Specially treated PDMS and aluminum foil were used as friction layers in the preparation of iTENG, and a flexible PET material of 400 μm thickness was used as a spacer between the two friction layers. Since the environment in which the iTENG was used was in vivo, the entire device was covered with flexible polymer to protect its internal structure from the environment. Since PDMS is flexible, leak-proof, and biocompatible, the entire device was encapsulated with a 50 μm layer of PDMS to ensure that the device could work properly in the in vivo environment. Meanwhile, the overall size of the iTENG was also controlled to be 1.2 cm × 1.2 cm to fit in the in vivo environment. In the experiment, the iTENG was implanted under the skin of the left chest of a rat, and the rat’s breath was generated through the respirator at a frequency of about 50 times per minute. As shown in Figure 5a, the measured voltage and current values of the iTENG were 3.73 V and 0.14 μA. The iTENG-generated current was stored in a capacitor that successfully drove the pacemaker prototype to regulate the heart rate of rats. Since the experimental subjects were rats, the size of the iTENG was limited, which affected the output performance. When used in humans, the structure and size of the iTENG can be redesigned to achieve greater power output.
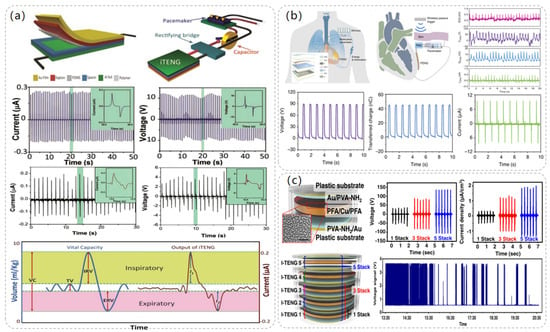
Figure 5.
Pacemaker module TENG structure and related data. (a) Respiration-driven TENG structure and the composition diagram of the self-powered pacemaker. Reproduced with permission from ref. [46]. (b) Radio-triggered symbiotic pacemaker system illustration with I-TENG performance test data in vitro including open circuit voltage, short circuit current, transferred charge, etc. Reproduced with permission from ref. [47]. (c) Diagram of the structure of the five-layer TENG and the amount of energy that the TENG can harvest in a day in the experiment. Reproduced with permission from ref. [48].
In 2019, Wang et al. successfully demonstrated another symbiotic pacemaker (SPM) based on an implantable TENG [47]. As depicted in Figure 5b, the SPM consists of three components: an energy harvesting unit (iTENG), a power management unit (PMU), and a pacemaker unit. Energy is harvested from cardiac motion through the iTENG module, which stores the collected energy in the capacitor of the PMU and drives the pacemaker to generate electrical pulses to control cardiac contraction. The iTENG was designed as a core-shell structure consisting of two triboelectric layers, a support structure, and encapsulated shells. PTFE and Al were chosen as the triboelectric materials, and the entire device was encapsulated using flexible PTFE and PDMS materials to ensure its structural stability. Meanwhile, in order to ensure the safety of the experiment, the encapsulation materials were attached to mouse fibroblasts to test their cytotoxicity, and the experimental data showed that PTFE and PDMS had good cytocompatibility and did not adversely affect the cells. When iTENG was implanted between the heart and pericardium of adult Yorkshire porcine, the open circuit voltage of iTENG was measured to reach 65.2 V. At the same time, the iTENG collected energy of 0.495 μJ in one cardiac exercise cycle, which was much larger than the pacing threshold energy of the pig (0.262 μJ), and successfully achieved cardiac pacing.
In 2021, Sang-woo Kim et al. [48] proposed a high-performance inertia-driven in vivo triboelectric nanogenerator (I-TENG) based on human motion and gravity. I-TENG was designed as a cylindrical shape with a radius of 1.5 cm and a height of 2.4 mm, using PVA-NH2 and PFA as triboelectric materials, and was stacked in five layers to increase its output effect. As illustrated in Figure 5c, compared with a single-layer I-TENG, the output voltage of a five-layer stacked I-TENG increases from 36 V to 136 V, and the current packing density increases from 0.4 μA/cm3 to 2 μA/cm3. The biocompatibility of the device was tested, and it was implanted into the back of the animal in contact with the muscle layer. During the observation period, there were no signs of infection, and all the subjects’ behavior was normal. By integrating the I-TENG with the pacemaker and the battery management device into a system, an adult mongrel was selected as the experimental object and implanted in the body. The system was able to receive and analyze the ECG data of the subjects, and the energy collected by the I-TENG successfully drove the 150 bpm VVI mode and 90 bpm VOO mode of the pacemaker, realizing the therapeutic effect of bradycardia.
5. Ultrasound-Driven TENG for Electrical Stimulation
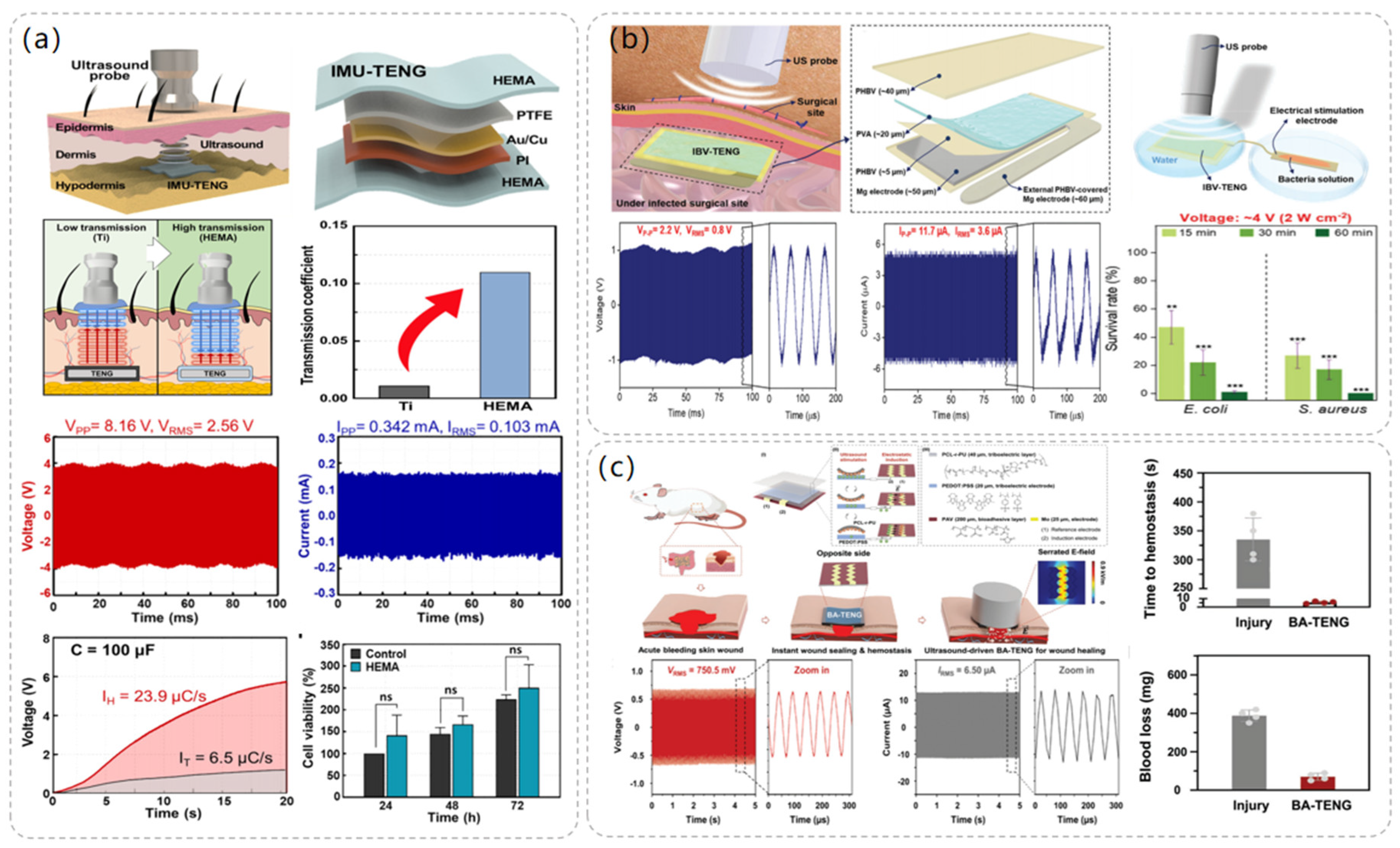
The use of ultrasound in medical imaging and treatment has a history of more than 50 years, is used in the treatment of various diseases, and shows high security. The ultrasound-powered TENG is also an important energy solution for the battery life of implantable medical devices. After the combination of ultrasound-driven TENG and implantable medical devices, the most critical problem is how to transmit ultrasonic energy to the implantable power generation device with the minimum energy loss while ensuring its biocompatibility in vivo. As shown in Figure 6a, Sang-Woo Kim et al. [49] proposed the use of biocompatible poly(2-hydroxyethyl methacrylate) (HEMA) as the friction layer and packaging layer of TENG, and compared with the traditional Ti layer, the ultrasonic transmission coefficient of HEMA is ten times greater. Use of HEMA materials made of a novel implantable, modulus-tunable, ultrasound-driven TENG (IMU-TENG) implanted in the rat dorsal skin tested the output performance, and it was found, under 40 MΩ impedance, to produce 8.16 V peak voltage. It can make 100 μF capacitor charging 3.7 times faster than a Ti plate; because it transmits more ultrasonic waves, it produces higher energy.
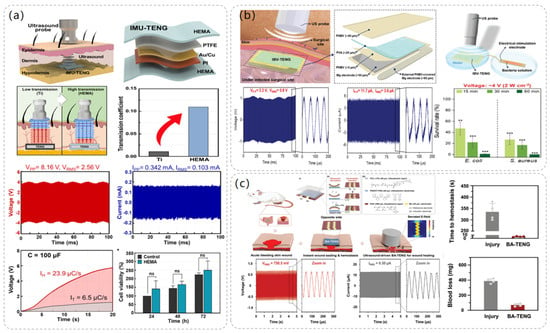
Figure 6.
The structure and performance data of the electrostimulation module of the ultrasonic-driven TENG. (a) The operating process diagram of IMU-TENG, its structure, and its material composition. Reproduced with permission from ref. [49]. (b) Composition and structure diagram of IBV-TENG, experimental diagram, and data diagram of bacteriostatic effect using IBV-TENG. Reproduced with permission from ref. [50]. (c) The structure and composition of BA-TENG, the treatment of acute skin injury by BA-TENG, and the voltage output of BA-TENG in a water experiment. Reproduced with permission from ref. [51].
In 2023, Sang-Woo Kim et al. [50] proposed an implantable, biodegradable, and vibrant triboelectric nanogenerator (IBV-TENG) using electrical stimulation for the elimination of microorganisms in deep tissues, and the IBV-TENG was able to successfully inactivate bacteria such as Staphylococcus aureus and Escherichia coli, which promises to be an effective solution for combating surgical-site infections. As illustrated in Figure 6b, the dimensions of the IBV-TENG are approximately 1 cm × 2 cm, and its thickness is roughly 170 μm. It was designed as a single-electrode model using poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV) and poly(vinyl alcohol) (PVA) as the two friction layer materials, respectively, and Mg foil, which has a fast hydrolysis rate and is biocompatible, was used as the electrode. The IBV-TENG was implanted into porcine tissues, and the voltage output of the device was measured and found to produce a voltage of ≈2.2 V at 40 megohm impedance and a current of ≈11.7 μA at 1 ohm impedance. To test its bacterial inhibitory ability in vitro, the IBV-TENG was attached to a petri dish and placed under an ultrasonic probe, and the bacterial solution was placed in the electrical stimulation portion of the device to be processed, to study the relationship between the ultrasonic strength at different ultrasonic intensities and electrical stimulation voltages and bacterial solutions at different electrical stimulation voltages. The survival rates of Staphylococcus aureus and Escherichia coli under different ultrasonic intensities and stimulation voltages were investigated. It was found that the IBV-TENG was able to kill 99% of E. coli and 100% of S. aureus after 1 h of IBV-TENG induction at an electrical stimulation intensity of 4 V. This proves that the use of the IBV-TENG device is a new strategy for microbial inhibition.
In addition, electrical stimulation of the TENG driven by ultrasound also shows its own advantages in wound healing. In 2023, Sang-Woo Kim et al. [51] proposed a novel bioadhesive TENG (BA-TENG), which can seal a wound and stop bleeding through the adhesion of the bioadhesive layer. Driven by ultrasound, BA-TENG can generate a stable electric field to accelerate the migration and proliferation of cells, thus accelerating the healing effect of the wound. As depicted in Figure 6c, the single electrode mode of BA-TENG selects polycaprolactone-based polyurethane (PCL-r-PU) material as the triboelectric layer and poly (3,4-ethylene dioxythiophene) polystyrene sulfonate (PEDOT:PSS) as the triboelectrode material, uses poly (vinyl alcohol) copolymer (PAV) to prepare the biological adhesive, and the Mo electrode to provide an electric field to the wound. BA-TENG uses biocompatible and biodegradable materials and does not require a second surgical resection after completing the corresponding work. The electrical output performance of BA-TENG was tested experimentally, and it was found that the BA-TENG with a size of 1.5 cm × 1.5 cm could stably produce a voltage output of 1.5 V and a current output of 24.2 µ A under ultrasound at 20 kHz, which was sufficient to accelerate cell migration and proliferation. A Sprague-Dawley rat liver incision model was established to evaluate the hemostatic performance of BA-TENG in vivo. It was found that BA-TENG could induce rapid wound healing after 5 s and reduce blood loss by 82% compared with the control group, which proved its rapid and effective wound sealing and hemostatic effect.
6. Summary and Challenges
TENG is widely investigated in the field of medical therapy, especially in implantable medical devices, due to its advantage of harvesting other mechanical energy and converting it into electrical energy. It can be used not only as an energy harvesting device but also as an alternative therapeutic tool through its electrical stimulation directly acting on the cells in the body to achieve therapeutic effects. In this review, recent advances in different medical devices with electrical stimulation based on TENG are reviewed, and the performance and applications of the TENG-based electrical stimulation devices introduced in this paper are listed in Table 1.

Table 1.
The performance and applications of electrical stimulation devices based on TENGs are listed.
Although the TENG-based electrical stimulation application showed satisfactory therapeutic effect in the experiment, there is a certain distance between the experimental data and the actual applications, and some biological mechanisms are still unknown. Its existing challenges are briefly described below.
(a) For implantable TENG, because the amplitude of motion in vivo is relatively small, how to respond to small movements and use them to produce large electrical output is the focus. In addition, the size of electrical stimulation medical devices is limited in the body, so reducing the size while ensuring their therapeutic effect is another challenge. This requires not only developing more suitable TENG structures but also exploring more advanced materials and improving their production process.
(b) Electrical stimulation used for medical devices must have the reliability and long-term stability of the output power to ensure that it can output the corresponding electrical signals in various environments. However, the performance and structure of TENG devices may be modified by the environment, especially for implantable devices, where the influence factors in the body will be greater. How to select the appropriate packaging material to protect the equipment is also a serious issue.
(c) When using electrical stimulation, improving the precise positioning of the target cells and grasping the degree of electrical stimulation are the keys to realize effective treatments. Firstly, the mislocalization of the target cells or the insufficient sensitivity of the device will be damaged by the electrical stimulation of other cells. Secondly, for the cells in the body, the electrical output of the device has a small controllable range: too much output will cause damage to the cells, and too little output will not achieve the therapeutic effect.
(d) Inappropriate equipment implanted into the body will not only cause mechanical stimulation or wear but also may lead to human allergic reactions, tissue inflammation, infection, and other complications, which will seriously harm the health and safety of patients. Therefore, the device needs to undergo strict biocompatibility tests and safety assessment to ensure that it can be placed in the body for a long time without causing negative effects and that it can safely play a therapeutic role.
(e) Since the performance of TENG is different in different modes, materials, and sizes, in medical applications integrated with TENG and other devices, appropriate TENG should be selected according to different research purposes and application scenarios. It is a hot topic for research to combine TENG with more medical devices and components to form self-powered medical products. In addition, the ultimate goal of researching successful medical devices is to commercialize them, and the research process should also consider the cost and whether the materials or fabrications can be put into large-scale production. The preparation process of some TENG materials is complicated and dependent on advanced instruments, which will bring certain difficulties to large-scale production. At the same time, since most medical devices work in biological bodies, the biocompatibility and water resistance of materials need to be paid more attention. The emergence of materials with better biocompatibility effect and the continuous progress of waterproof packaging materials will promote the final commercialization of TENG-based medical devices.
Author Contributions
Writing—original draft, X.W., Y.W., S.N. and Z.L.; writing—review and editing, X.W. and Z.L. All the authors discussed the results and made suggestions on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Young Elite Scientists Sponsorship Program by CAST (2022QNRC001), Fundamental Research Funds for the Central Universities (SWU120045), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant no. KJQN202200209), the General Program of Chongqing Natural Science Foundation (2022NSCQ-MSX0806), and the Visiting Scholar Foundation of Key Laboratory of Optoelectronic Technology & Systems (Chongqing University), Ministry of Education.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emilia, A.; Simona, F.; Alessandra, P.; Giancarlo, F.; Franco, M. Cycling induced by electrical stimulation improves motor recovery in postacute hemiparetic patients: A randomized controlled trial. Stroke 2011, 42, 1068–1073. [Google Scholar]
- Naaz, K.; Bastien, M.; Popovic, M.R. Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping after Spinal Cord Injury and Stroke. Front. Neurosci. 2020, 14, 718. [Google Scholar]
- Sun, J.; Yang, A.; Zhao, C.; Liu, F.; Li, Z. Recent progress of nanogenerators acting as biomedical sensors in vivo. Sci. Bull. 2019, 64, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Tong, T.; Bu, T.; Cao, Y.; Xu, S.; Qi, Y.; Zhang, C. Overview of Power Management for Triboelectric Nanogenerators. Adv. Intell. Syst. 2020, 2, 1900129. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Li, H.; Lv, S.; Zhang, B.; Liu, B.; Yang, J.; Guo, H.; Xie, Y.; Lin, Z. High power and low crest factor of direct-current triboelectric nanogenerator for self-powered optical computing system. Energy Environ. Sci. 2023, 16, 4641–4649. [Google Scholar] [CrossRef]
- Seol, M.L.; Woo, J.H.; Jeon, S.B.; Kim, D.; Park, S.J.; Hur, J.; Choi, Y.K. Vertically stacked thin triboelectric nanogenerator for wind energy harvesting. Nano Energy 2015, 14, 201–208. [Google Scholar] [CrossRef]
- Lv, S.; Li, H.; Xie, Y.; Zhang, B.; Liu, B.; Yang, J.; Guo, H.; Yang, Z.; Lin, Z. High-Performance and Durable Rotational Triboelectric Nanogenerator Leveraging Soft-Contact Coplanar Charge Pumping Strategy. Adv. Energy Mater. 2023, 2301832. [Google Scholar] [CrossRef]
- Wen, X.; Yang, W.; Jing, Q.; Wang, Z.L. Harvesting broadband kinetic impact energy from mechanical triggering/vibration and water waves. ACS Nano 2014, 8, 7405–7412. [Google Scholar] [CrossRef]
- Lin, Z.; Nie, S.; He, Q.; Wang, X.; Wang, Y.; Zhang, B.; Liu, B.; Yang, J. Wearable and Flexible Helical Pressure Sensor for Noninvasive Respiratory Monitoring. ACS Appl. Eng. Mater. 2023, 1, 2452–2830. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Wang, Z.; Ji, L.; Wang, Z.L. Triboelectric nanogenerators for human-health care. Sci. Bull. 2021, 66, 490–511. [Google Scholar] [CrossRef]
- Khandelwal, G.; Maria Joseph Raj, N.P.; Kim, S.-J. Triboelectric nanogenerator for healthcare and biomedical applications. Nano Today 2020, 33, 100882. [Google Scholar] [CrossRef]
- Leila, Y.; Morteza, N.; Sara, A. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar]
- Glenn, D.J.; Whartenby, A.K. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef]
- Sari, Y.; Isworo, A.; Upoyo, A.S.; Anam, A.; Sutrisna, E. A low-frequency of electrical stimulation improves wound healing. IOP Conf. Ser. Earth Environ. Sci. 2019, 255, 012014. [Google Scholar] [CrossRef]
- Ruizeng, L.; Jieyu, D.; Jiaping, Z.; Zhou, L. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, e2100557. [Google Scholar]
- Jun, C.Y.; Ramdzan, B.M.; Heikal, M.Y.M. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13, 3790. [Google Scholar]
- Feng-Ru, F.; Long, L.; Guang, Z.; Wenzhuo, W.; Rui, Z.; Lin, W.Z. Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2022, 14, 7–52. [Google Scholar] [CrossRef]
- Ha, J.S.; Younghoon, L.; Gyu, L.M.; Jun, S.W.; Ung, P.J.; Yun, S.J. Accelerated Wound Healing with an Ionic Patch assisted by a Triboelectric Nanogenerator. Nano Energy 2020, 79, 105463. [Google Scholar]
- Yin, L.; Hao, W.; Jun, L.; Guang, Y.; Bo, Y.; Dalong, N.; Lf, G.A.; Xiaoli, L.; Yadong, J.; Weibo, C.; et al. Effective Wound Healing Enabled by Discrete Alternative Electric Fields from Wearable Nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar]
- Li, Z.; Feng, H.; Zheng, Q.; Li, H.; Zhao, C.; Ouyang, H.; Noreen, S.; Yu, M.; Su, F.; Liu, R.; et al. Photothermally Tunable Biodegradation of Implantable Triboelectric Nanogenerators for Tissue Repairing. Nano Energy 2018, 54, 390–399. [Google Scholar] [CrossRef]
- Hu, W.; Wei, X.; Zhu, L.; Yin, D.; Wei, A.; Bi, X.; Liu, T.; Zhou, G.; Qiang, Y.; Sun, X.; et al. Enhancing proliferation and migration of fibroblast cells by electric stimulation based on triboelectric nanogenerator. Nano Energy 2019, 57, 600–607. [Google Scholar] [CrossRef]
- Tümay, S.; Lale, Ö.; Çalık, B.N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar]
- Nicholas, H.; Elaine, D.; Cyrus, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar]
- Cotts, K.G.; Cifu, A.S. Treatment of Osteoporosis. JAMA 2018, 319, 1040–1041. [Google Scholar] [CrossRef]
- Weifei, Z.; Yuanrui, L.; Jixuan, X.; Chuan, G.; Jing, S.; Lu, L.; Xiao, S.; Qingquan, K. The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review. Medicina 2023, 59, 121. [Google Scholar]
- Zhang, Y.; Xu, L.; Liu, Z.; Cui, X.; Xiang, Z.; Bai, J.; Jiang, D.; Xue, J.; Wang, C.; Lin, Y.; et al. Self-powered pulsed direct current stimulation system for enhancing osteogenesis in MC3T3-E1. Nano Energy 2021, 85, 106009. [Google Scholar] [CrossRef]
- Tang, W.; Tian, J.; Zheng, Q.; Yan, L.; Wang, J.; Li, Z.; Wang, Z.L. Implantable Self-Powered Low-Level Laser Cure System for Mouse Embryonic Osteoblasts’ Proliferation and Differentiation. ACS Nano 2015, 9, 7867–7873. [Google Scholar] [CrossRef]
- Tian, J.; Shi, R.; Liu, Z.; Ouyang, H.; Yu, M.; Zhao, C.; Zou, Y.; Jiang, D.; Zhang, J.; Li, Z. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy 2019, 59, 705–714. [Google Scholar] [CrossRef]
- Brock Symons, T.; Park, J.; Kim, J.H.; Kwon, E.H.; Delacruz, J.; Lee, J.; Park, Y.; Chung, E.; Lee, S. Attenuation of skeletal muscle atrophy via acupuncture, electro-acupuncture, and electrical stimulation. Integr. Med. Res. 2023, 12, 100949. [Google Scholar] [CrossRef]
- Bao, W.; Yang, J.; Li, M.; Chen, K.; Ma, Z.; Bai, Y.; Xu, Y. Prevention of muscle atrophy in ICU patients without nerve injury by neuromuscular electrical stimulation: A randomized controlled study. BMC Musculoskelet. Disord. 2022, 23, 780. [Google Scholar] [CrossRef]
- Nonoyama, T.; Shigemi, H.; Kubota, M.; Matsumine, A.; Shigemi, K.; Ishizuka, T. Neuromuscular electrical stimulation in the intensive care unit prevents muscle atrophy in critically ill older patients: A retrospective cohort study. Medicine 2022, 101, e29451. [Google Scholar] [CrossRef]
- Sepúlveda-Jofré, P.; Guerra-Vega, P.; Fu, C.; Marzuca-Nassr, G.N. Skeletal muscle atrophy in critical ill patients and the use of electrical stimulation as a treatment strategy: Recommendations for clinical practice. Trends Anaesth. Crit. Care 2021, 40, 14–22. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Thakor, N.V.; Lee, C. Self-Powered Direct Muscle Stimulation Using a Triboelectric Nanogenerator (TENG) Integrated with a Flexible Multiple-Channel Intramuscular Electrode. ACS Nano 2019, 13, 3589–3599. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; He, T.; Li, Z.; Lee, C. Direct muscle stimulation using diode-amplified triboelectric nanogenerators (TENGs). Nano Energy 2019, 63, 103844. [Google Scholar] [CrossRef]
- Choi, H.J.; Oh, I.H.; Choi, S.K.; Lim, Y.J. Clinical Outcomes of Pulsed Radiofrequency Neuromodulation for the Treatment of Occipital Neuralgia. J. Korean Neurosurg. Soc. 2012, 51, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Szymański, J.K.; Słabuszewska-Jóźwiak, A.; Zaręba, K.; Jakiel, G. Neuromodulation—A therapeutic option for refractory overactive bladder. A recent literature review. Videosurg. Other Miniinvasive Tech. 2019, 14, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Research on gait rehabilitation based on neuromodulation technologies. AIP Conf. Proc. 2022, 2589, 020023. [Google Scholar]
- Lee, S.; Wang, H.; Shi, Q.; Dhakar, L.; Wang, J.; Thakor, N.V.; Yen, S.-C.; Lee, C. Development of battery-free neural interface and modulated control of tibialis anterior muscle via common peroneal nerve based on triboelectric nanogenerators (TENGs). Nano Energy 2017, 33, 1–11. [Google Scholar] [CrossRef]
- Lee, S.; Wang, H.; Xian Peh, W.Y.; He, T.; Yen, S.-C.; Thakor, N.V.; Lee, C. Mechano-neuromodulation of autonomic pelvic nerve for underactive bladder: A triboelectric neurostimulator integrated with flexible neural clip interface. Nano Energy 2019, 60, 449–456. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, J.; Long, Y.; Wei, H.; Ferreira, C.A.; Jeffery, J.J.; Lin, Y.; Cai, W.; Wang, X. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 2018, 9, 5349. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Yang, B.D.; Su, Y.; Tran, P.L.; Joe, P.; Anderson, E.; Xia, J.; Doraiswamy, V.; Dehdashti, B.; Feng, X.; et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. USA 2014, 111, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Deterre, M.; Lefeuvre, E.; Zhu, Y.; Woytasik, M.; Boutaud, B.; Dal Molin, R. Micro Blood Pressure Energy Harvester for Intracardiac Pacemaker. J. Microelectromech. Syst. 2014, 23, 651–660. [Google Scholar] [CrossRef]
- Qiang, Z.; Bojing, S.; Fengru, F.; Xinxin, W.; Ling, Y.; Weiwei, Y.; Sihong, W.; Hong, L.; Zhou, L.; Lin, W.Z. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 2014, 26, 5851–5856. [Google Scholar]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Ryu, H.; Park, H.-M.; Kim, M.-K.; Kim, B.; Myoung, H.S.; Kim, T.Y.; Yoon, H.-J.; Kwak, S.S.; Kim, J.; Hwang, T.H.; et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 2021, 12, 4374. [Google Scholar] [CrossRef]
- Kim, B.; Yoon, H.J.; Kim, Y.J.; Park, B.-J.; Jung, J.H.; Kim, S.W. Ultrasound-Driven Triboelectric Nanogenerator with Biocompatible 2-Hydroxyethyl Methacrylate. ACS Energy Lett. 2023, 8, 3412–3419. [Google Scholar] [CrossRef]
- Imani, I.M.; Kim, B.; Xiao, X.; Rubab, N.; Park, B.J.; Kim, Y.J.; Zhao, P.; Kang, M.; Kim, S.A.-O. Ultrasound-Driven On-Demand Transient Triboelectric Nanogenerator for Subcutaneous Antibacterial Activity. Adv. Sci. 2023, 10, 2204801. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, X.; Jeon, S.; Kim, D.; Park, B.J.; Kim, Y.J.; Rubab, N.; Kim, S.; Kim, S.W. An Ultrasound-Driven Bioadhesive Triboelectric Nanogenerator for Instant Wound Sealing and Electrically Accelerated Healing in Emergencies. Adv. Mater. 2022, 35, 2209054. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).