Modeling to Correct the Effect of Soil Moisture for Predicting Soil Total Nitrogen by Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
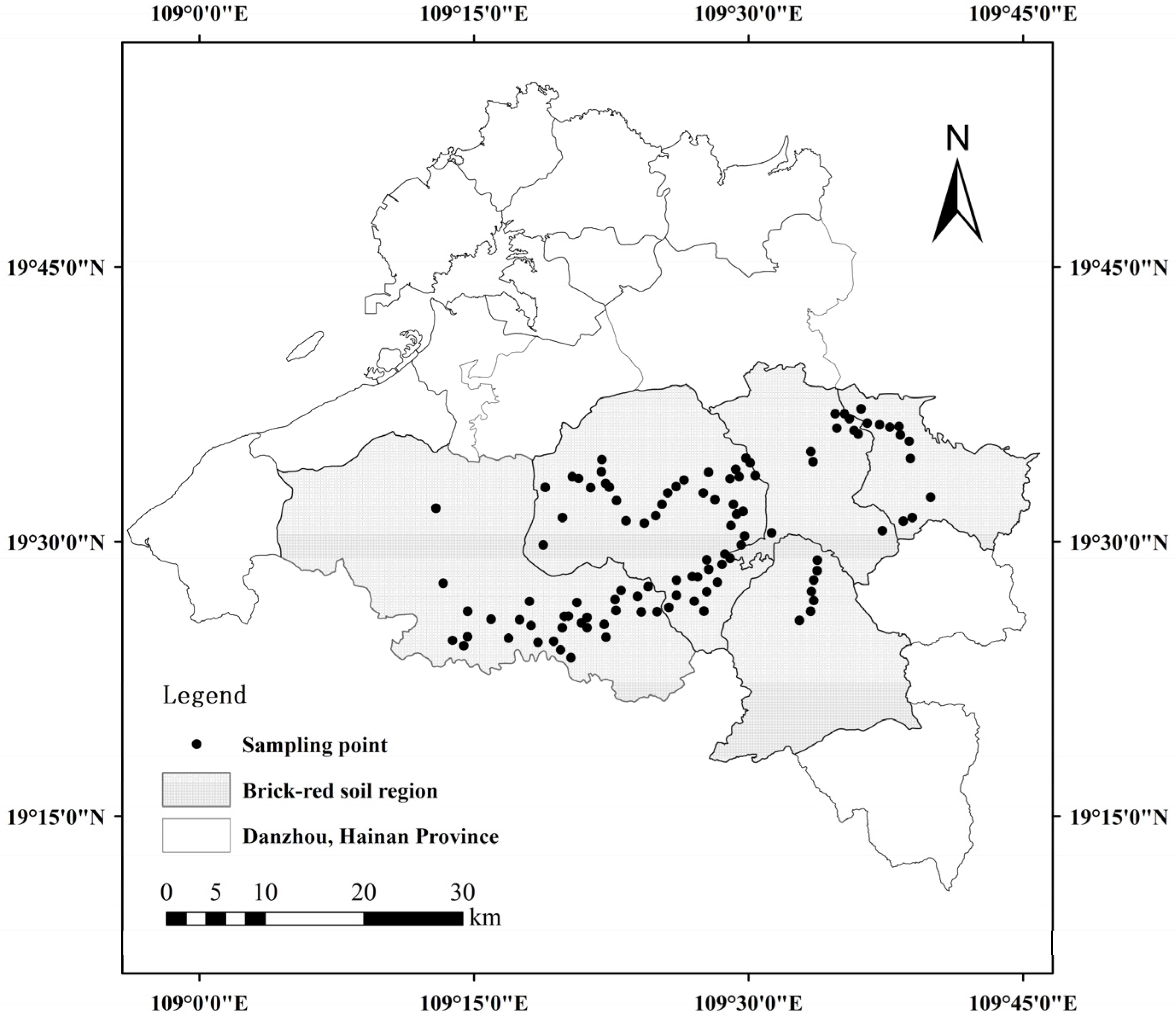
2.1. Study Area and Soil Collection
2.2. Acquisition of Soil Spectra
2.3. Wet Soil Sample Preparation
2.4. Methods to Remove Moisture Effects
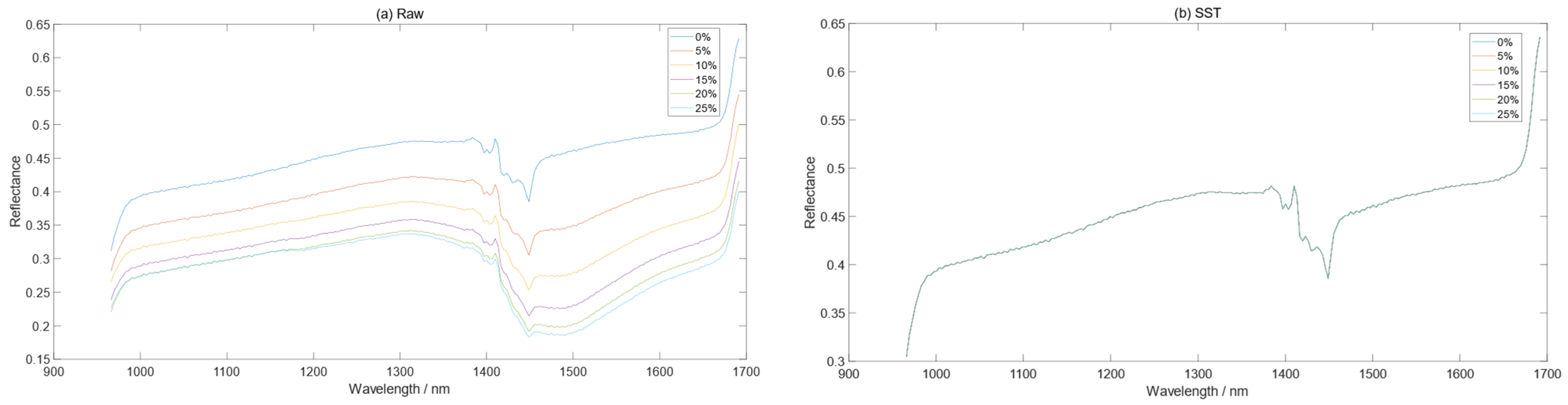
2.4.1. Spectral Space Transformation
2.4.2. Direct Standardization
2.4.3. Piecewise Direct Standardization
2.4.4. External Parameter Orthogonalization
- (1)
- Calculate the spectral difference matrix D between the wet soil spectra and the dry soil spectra;
- (2)
- Perform SVD on the spectral difference combination to obtain the matrix V;
- (3)
- Define the dimension as 4 and compute the subset of V, and;
- (4)
- Calculate the projection matrix P, P = I − Q; I represents the identity matrix;
2.4.5. Slope/Bias
2.5. Model Establishment and Evaluation
2.6. Flowchart
3. Results
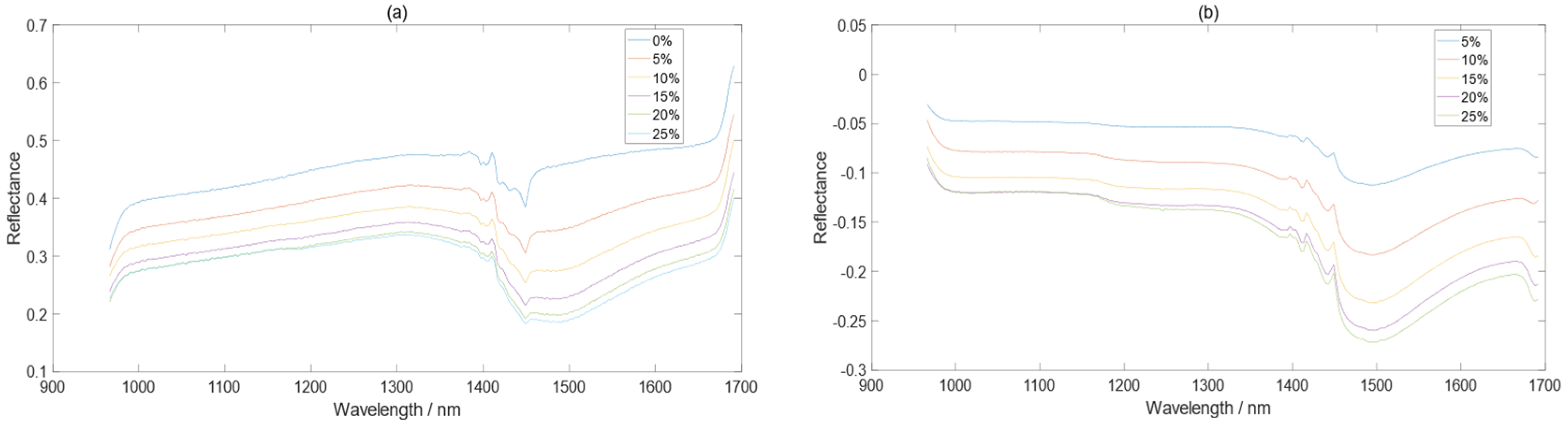
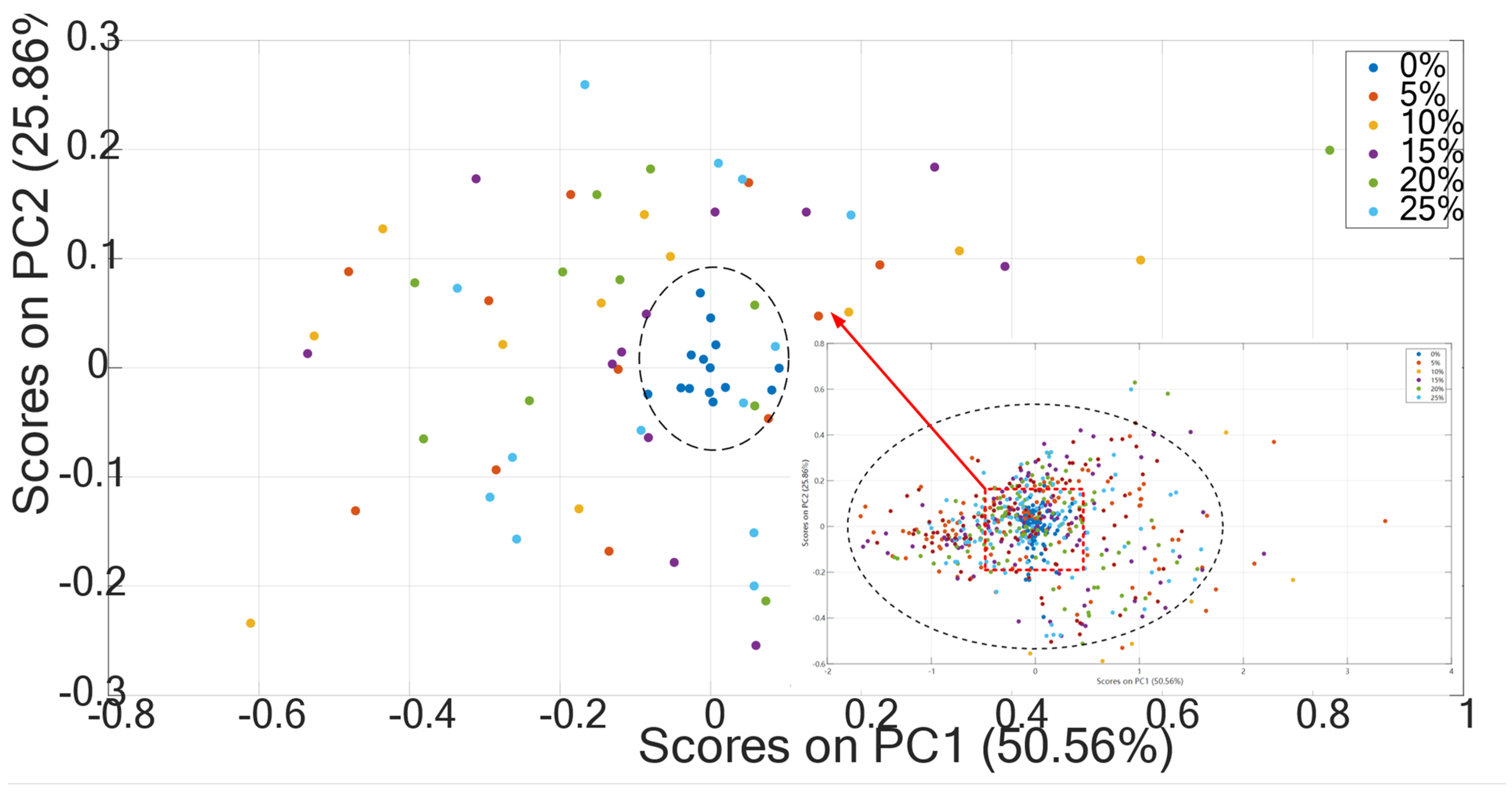
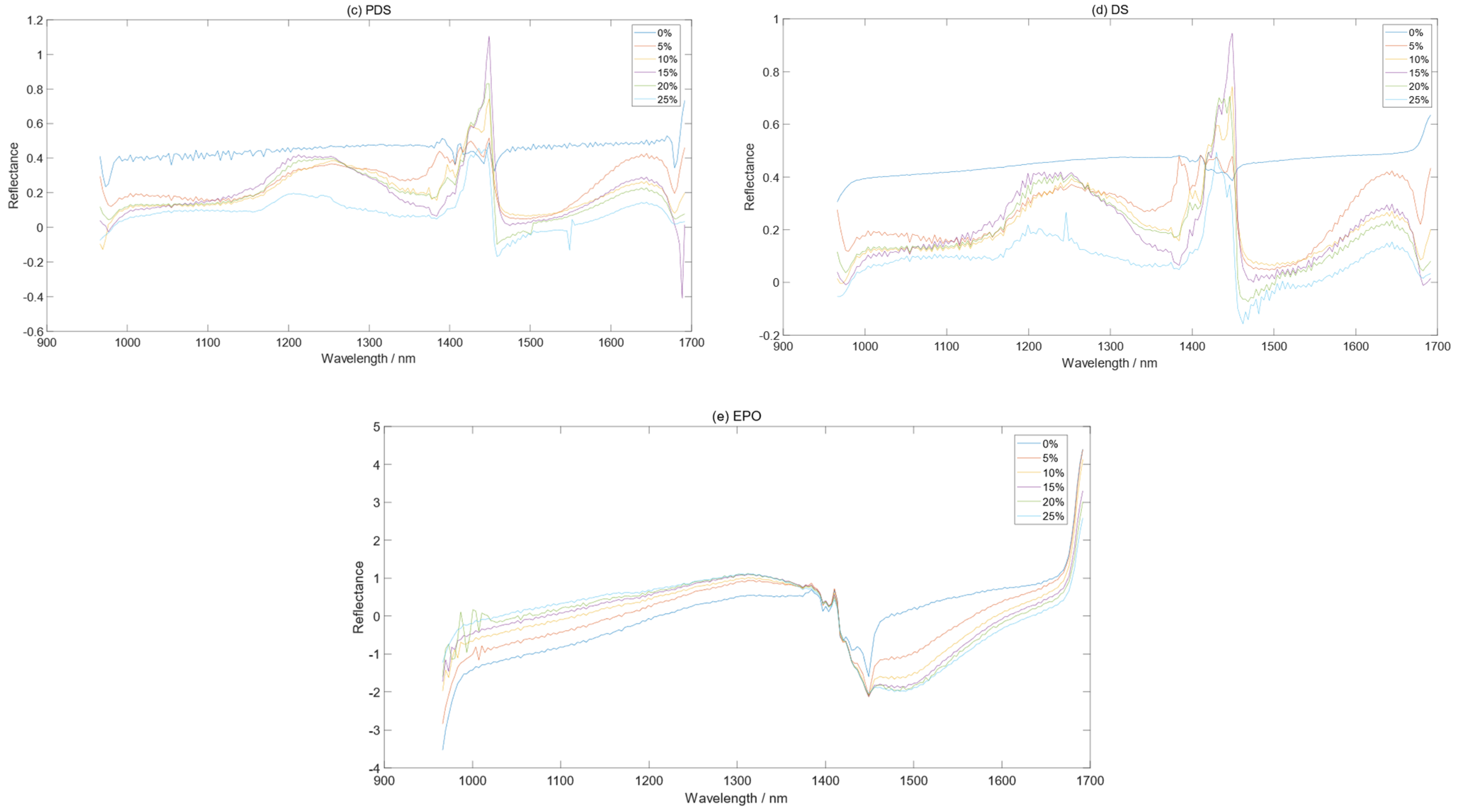
3.1. The Effect of Moisture on the Soil Spectra
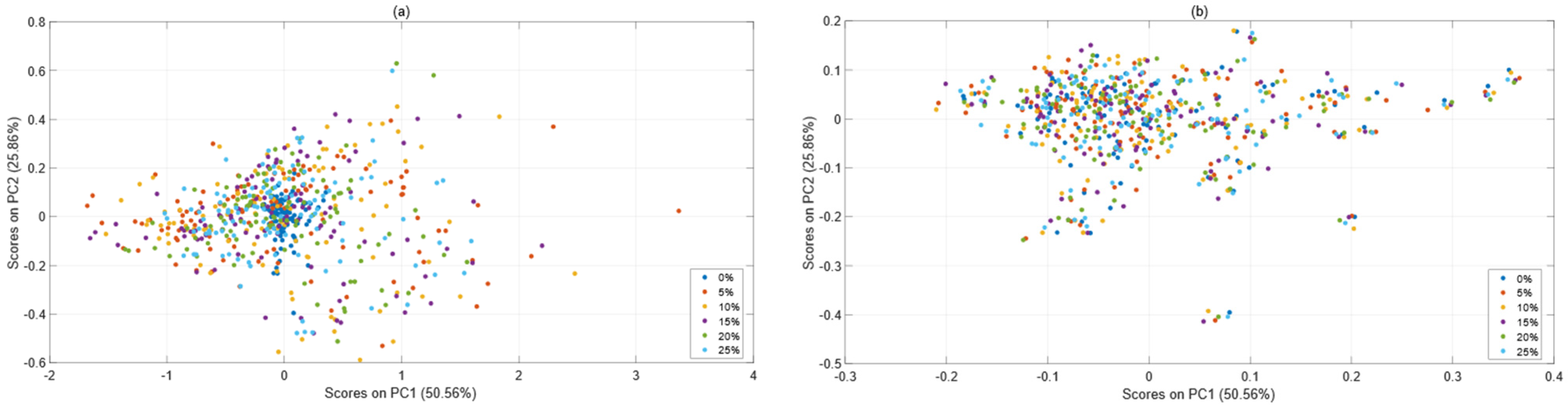
3.2. Comparison before and after Spectral Correction
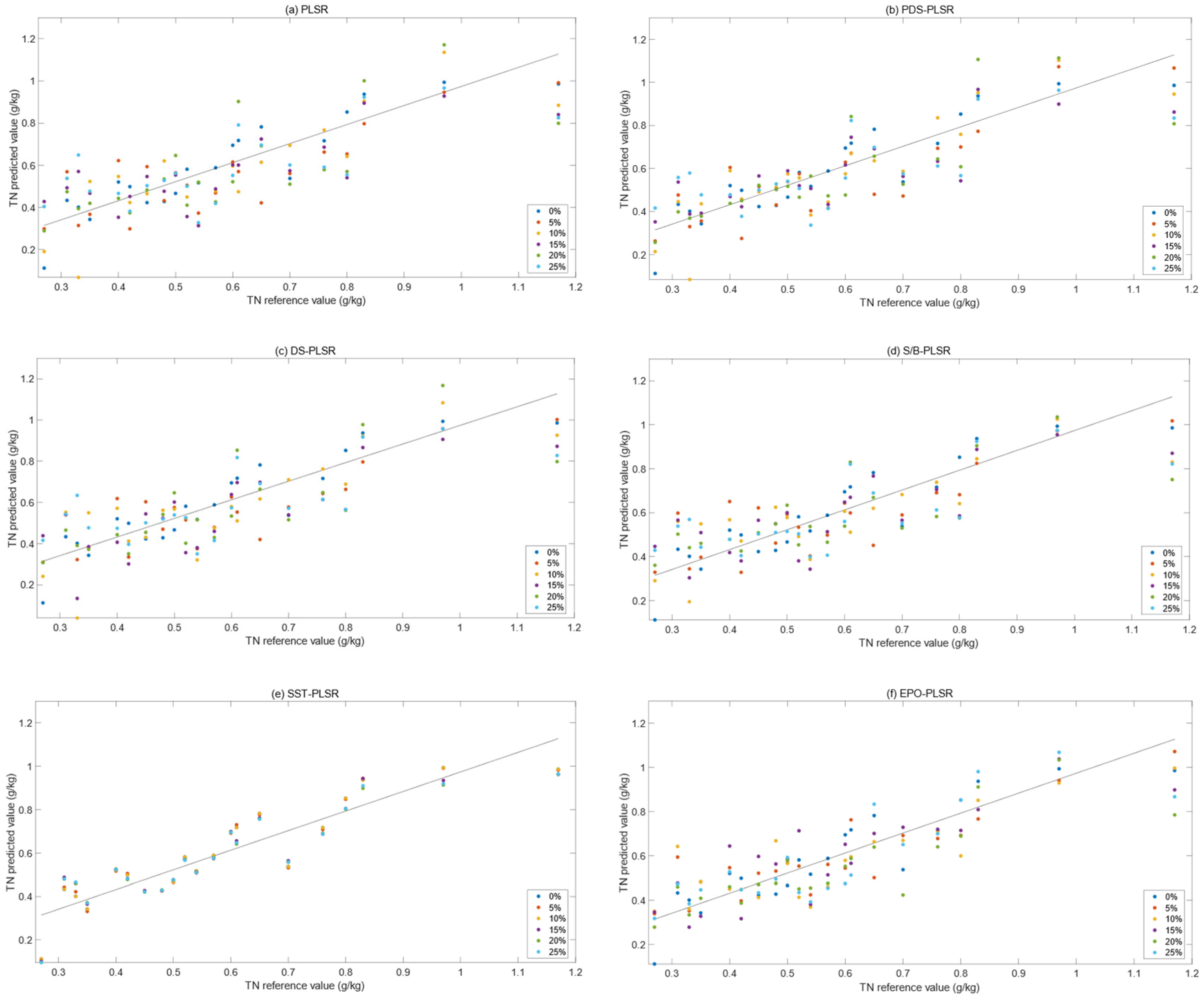
3.3. Comparison of Results of Different PLSR Models
3.3.1. PLSR Model for Dry Soil
3.3.2. The Corrected PLSR Model
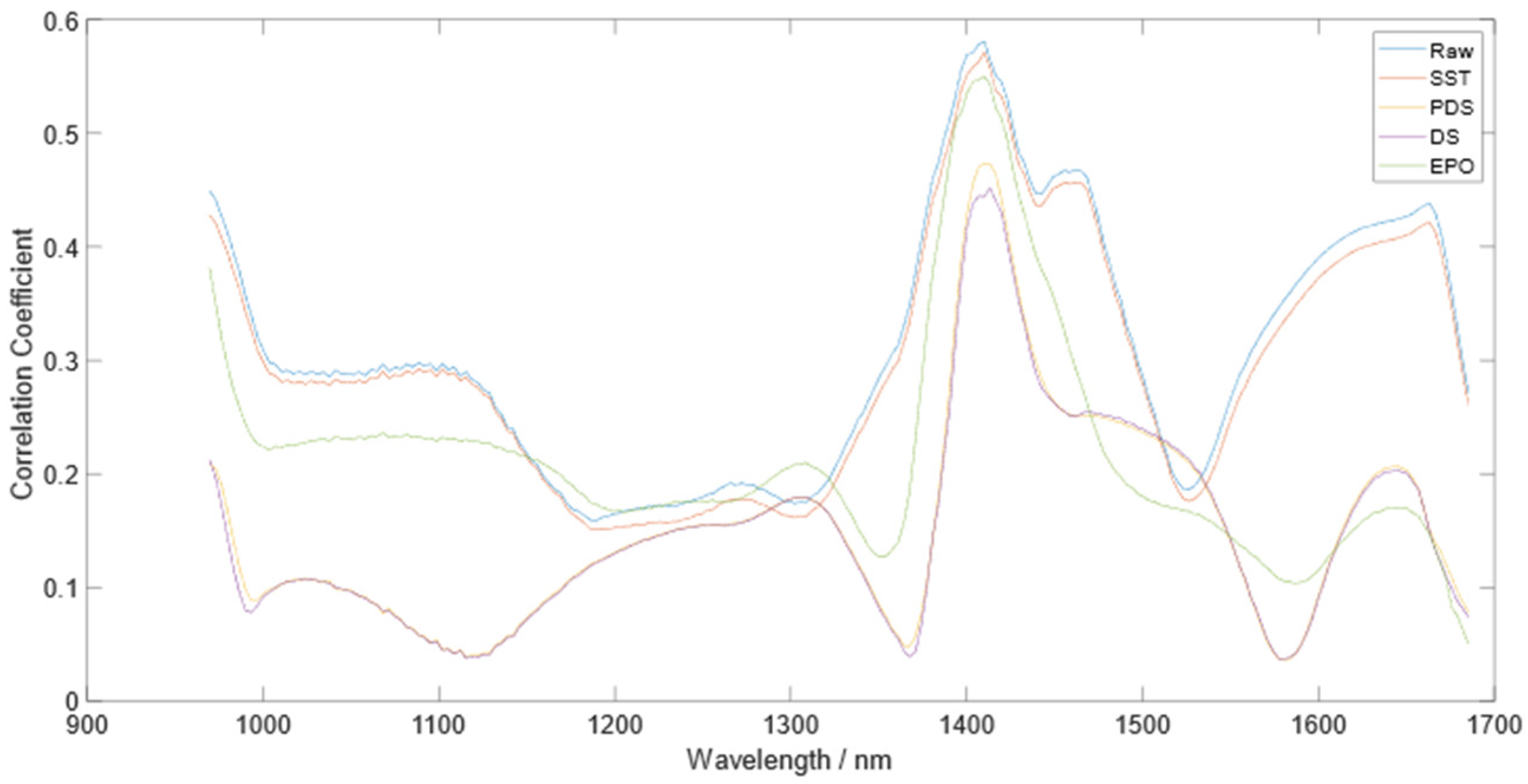
3.4. Correlation Coefficients between Dry Soil and Corrected Wet Soil and TN Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahrends, A.; Hollingsworth, P.M.; Ziegler, A.D.; Fox, J.M.; Chen, H.; Su, Y.; Xu, J. Current trends of rubber plantation expansion may threaten biodiversity and livelihoods. Glob. Environ. Chang. 2015, 34, 48–58. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef]
- Reddy, K.S.; Singh, M.; Tripathi, A.K.; Singh, M.; Saha, M.N. Changes in amount of organic and inorganic fractions of nitrogen in an Eutrochrept soil after long-term cropping with different fertilizer and organic manure inputs. J. Plant Nutr. Soil Sci. 2003, 166, 232–238. [Google Scholar] [CrossRef]
- Li, X.; Ge, T.; Chen, Z.; Wang, S.; Ou, X.; Wu, Y.; Chen, H.; Wu, J. Enhancement of soil carbon and nitrogen stocks by abiotic and microbial pathways in three rubber-based agroforestry systems in Southwest China. Land Degrad. Dev. 2020, 31, 2507–2515. [Google Scholar] [CrossRef]
- Etheridge, R.; Pesti, G.; Foster, E. A comparison of nitrogen values obtained utilizing the Kjeldahl nitrogen and Dumas combustion methodologies (Leco CNS 2000) on samples typical of an animal nutrition analytical laboratory. Anim. Feed. Sci. Technol. 1998, 73, 21–28. [Google Scholar] [CrossRef]
- de Santana, F.B.; de Giuseppe, L.O.; de Souza, A.M.; Poppi, R.J. Removing the moisture effect in soil organic matter determination using NIR spectroscopy and PLSR with external parameter orthogonalization. Microchem. J. 2018, 145, 1094–1101. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Du, Y. Combining multivariate method and spectral variable selection for soil total nitrogen estimation by Vis–NIR spectroscopy. Arch. Agron. Soil Sci. 2020, 67, 1665–1678. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, W.; Li, M.; Wang, W. A New Coupled Elimination Method of Soil Moisture and Particle Size Interferences on Predicting Soil Total Nitrogen Concentration through Discrete NIR Spectral Band Data. Remote. Sens. 2021, 13, 762. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Zhou, P.; Cui, Y.; Wang, D.; Li, M. Development and performance test of a vehicle-mounted total nitrogen content prediction system based on the fusion of near-infrared spectroscopy and image information. Comput. Electron. Agric. 2021, 192, 106613. [Google Scholar] [CrossRef]
- Tamburini, E.; Vincenzi, F.; Costa, S.; Mantovi, P.; Pedrini, P.; Castaldelli, G. Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy. Sensors 2017, 17, 2366. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, B. Effects of soil sample pretreatments and standardised rewetting as interacted with sand classes on Vis-NIR predictions of clay and soil organic carbon. Geoderma 2010, 158, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Kuang, B.; Mouazen, A.M. Non-biased prediction of soil organic carbon and total nitrogen with vis–NIR spectroscopy, as affected by soil moisture content and texture. Biosyst. Eng. 2013, 114, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Barthès, B.G.; Brunet, D.; Ferrer, H.; Chotte, J.-L.; Feller, C. Determination of Total Carbon and Nitrogen Content in a Range of Tropical Soils Using near Infrared Spectroscopy: Influence of Replication and Sample Grinding and Drying. J. Near Infrared Spectrosc. 2006, 14, 341–348. [Google Scholar] [CrossRef]
- Nawar, S.; Munnaf, M.A.; Mouazen, A.M. Machine Learning Based On-Line Prediction of Soil Organic Carbon after Removal of Soil Moisture Effect. Remote Sens. 2020, 12, 1308. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Cheng, X.; Zheng, D.; Song, H.; Han, P.; Yuen, P. Application of Slope/Bias and Direct Standardization Algorithms to Correct the Effect of Soil Moisture for the Prediction of Soil Organic Matter Content Based on the Near Infrared Spectroscopy (English). Spectrosc. Spectr. Anal. 2019, 39, 1986–1992. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Y.; Guo, L.; Fei, T.; Qi, K. Estimating Soil Organic Carbon of Cropland Soil at Different Levels of Soil Moisture Using VIS-NIR Spectroscopy. Remote. Sens. 2016, 8, 755. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-P.; Shao, M.-A.; Wang, Y.-Q. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China. Geoderma 2013, 197–198, 67–78. [Google Scholar] [CrossRef]
- Chu, X.L.; Yuan, H.F.; Lu, W.Z. Model Transfer in Multivariate CalibrationModel Transfer in Spectral Multivariate Correction. Spectrosc. Spectr. Anal. 2001, 21, 881–885. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, C.; Lu, Y.; Shen, Q.; Zhao, H.; Tao, Y.; Pan, X. Evaluating the characteristics of soil vis-NIR spectra after the removal of moisture effect using external parameter orthogonalization. Geoderma 2020, 376, 114568. [Google Scholar] [CrossRef]
- Du, W.; Chen, Z.-P.; Zhong, L.-J.; Wang, S.-X.; Yu, R.-Q.; Nordon, A.; Littlejohn, D.; Holden, M. Maintaining the predictive abilities of multivariate calibration models by spectral space transformation. Anal. Chim. Acta 2011, 690, 64–70. [Google Scholar] [CrossRef]
- Rehman, T.U.; Zhang, L.; Ma, D.; Wang, L.; Jin, J. Calibration transfer across multiple hyperspectral imaging-based plant phenotyping systems: I-Spectral space adjustment. Comput. Electron. Agric. 2020, 176, 105685. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Lin, W. Calla lily intercropping in rubber tree plantations changes the nutrient content, microbial abundance, and enzyme activity of both rhizosphere and non-rhizosphere soil and calla lily growth. Ind. Crop. Prod. 2019, 132, 344–351. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Nawar, S.; Mouazen, A. On-line vis-NIR spectroscopy prediction of soil organic carbon using machine learning. Soil Tillage Res. 2019, 190, 120–127. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, S.; Liu, J.; Liu, B.; Tao, L.; Wu, J.; Hu, S.; Zhao, L.; Wang, Q.; Wu, Y. A calibration transfer optimized single kernel near-infrared spectroscopic method. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019, 220, 117098. [Google Scholar] [CrossRef] [PubMed]
- Fonollosa, J.; Fernández, L.; Gutiérrez-Gálvez, A.; Huerta, R.; Marco, S. Calibration transfer and drift counteraction in chemical sensor arrays using Direct Standardization. Sensors Actuators Chem. 2016, 236, 1044–1053. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Chen, S.; Hu, J.; Xia, F.; Xu, J.; Li, Y.; Shi, Z. Application of portable XRF and VNIR sensors for rapid assessment of soil heavy metal pollution. PLoS ONE 2017, 12, e0172438. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-Infrared Reflectance Spectroscopy-Principal Components Regression Analyses of Soil Properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Kuang, B.; Mouazen, A.M. Calibration of visible and near infrared spectroscopy for soil analysis at the field scale on three European farms. Eur. J. Soil Sci. 2011, 62, 629–636. [Google Scholar] [CrossRef]
- Mouazen, A.M.; De Baerdemaeker, J.; Ramon, H. Towards development of on-line soil moisture content sensor using a fibre-type NIR spectrophotometer. Soil Tillage Res. 2005, 80, 171–183. [Google Scholar] [CrossRef]
- Li, S.; Shi, Z.; Chen, S.; Ji, W.; Zhou, L.; Yu, W.; Webster, R. In Situ Measurements of Organic Carbon in Soil Profiles Using vis-NIR Spectroscopy on the Qinghai–Tibet Plateau. Environ. Sci. Technol. 2015, 49, 4980–4987. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Asner, G.P. Moisture Effects on Soil Reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Demattê, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhao, Y.; Wang, M.; Shi, X. Comparison of multivariate methods for estimating selected soil properties from intact soil cores of paddy fields by Vis–NIR spectroscopy. Geoderma 2018, 310, 29–43. [Google Scholar] [CrossRef]
- Cao, Y.; Bao, N.; Liu, S.; Zhao, W.; Li, S. Reducing moisture effects on soil organic carbon content prediction in visible and near-infrared spectra with an external parameter othogonalization algorithm. Can. J. Soil Sci. 2020, 100, 253–262. [Google Scholar] [CrossRef]
- Rossel, R.V.; Cattle, S.; Ortega, A.; Fouad, Y. In situ measurements of soil colour, mineral composition and clay content by vis–NIR spectroscopy. Geoderma 2009, 150, 253–266. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra-Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zheng, L.; An, X.; Sun, H. Fast Measurement and Advanced Sensors of Soil Parameters with NIR Spectroscopy. J. Agric. Mach. 2013, 44, 73–87. [Google Scholar] [CrossRef]

| Type of Samples | Number of Samples | Max | Min | Mean | Median | Standard Deviation | |
|---|---|---|---|---|---|---|---|
| TN (g/kg) | Total samples | 107 | 1.74 | 0.13 | 0.58 | 0.53 | 0.25 |
| Calibration samples | 86 | 1.74 | 0.13 | 0.58 | 0.53 | 0.25 | |
| Prediction samples | 21 | 1.17 | 0.27 | 0.58 | 0.54 | 0.23 |
| Model | Moisture Content % | Calibration Set | Prediction Set | ||||
|---|---|---|---|---|---|---|---|
| RMSEC (g/kg) | RPD | RMSEP (g/kg) | RPD | ||||
| PLSR | 0 | 0.74 | 0.13 | 1.71 | 0.82 | 0.09 | 2.40 |
| PLSR | 5–25 | 0.38–0.70 | 0.15–0.20 | 0.80–1.55 | 0.49–0.68 | 0.13–0.17 | 1.43–1.80 |
| SST–PLSR | 5–25 | 0.74 | 0.13 | 1.71 | 0.81–0.82 | 0.09–0.10 | 2.32–2.40 |
| EPO–PLSR | 5–25 | 0.65–0.71 | 0.14–0.15 | 1.40–1.61 | 0.69–0.78 | 0.10–0.12 | 1.84–2.17 |
| PDS–PLSR | 5–25 | 0.43–0.66 | 0.14–0.19 | 0.93–1.41 | 0.52–0.74 | 0.11–0.15 | 1.46–2.02 |
| DS–PLSR | 5–25 | 0.43–0.69 | 0.14–0.19 | 0.93–1.45 | 0.50–0.70 | 0.12–0.16 | 1.45–1.89 |
| S/B–PLSR | 5–25 | 0.42–0.66 | 0.14–0.19 | 0.92–1.41 | 0.55–0.69 | 0.12–0.15 | 1.53–1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Jiang, K.; Li, C.; Li, X.; Wu, J. Modeling to Correct the Effect of Soil Moisture for Predicting Soil Total Nitrogen by Near-Infrared Spectroscopy. Electronics 2023, 12, 1271. https://doi.org/10.3390/electronics12061271
Tang R, Jiang K, Li C, Li X, Wu J. Modeling to Correct the Effect of Soil Moisture for Predicting Soil Total Nitrogen by Near-Infrared Spectroscopy. Electronics. 2023; 12(6):1271. https://doi.org/10.3390/electronics12061271
Chicago/Turabian StyleTang, Rongnian, Kaixuan Jiang, Chuang Li, Xiaowei Li, and Jingjin Wu. 2023. "Modeling to Correct the Effect of Soil Moisture for Predicting Soil Total Nitrogen by Near-Infrared Spectroscopy" Electronics 12, no. 6: 1271. https://doi.org/10.3390/electronics12061271





