Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications
Abstract
:1. Introduction
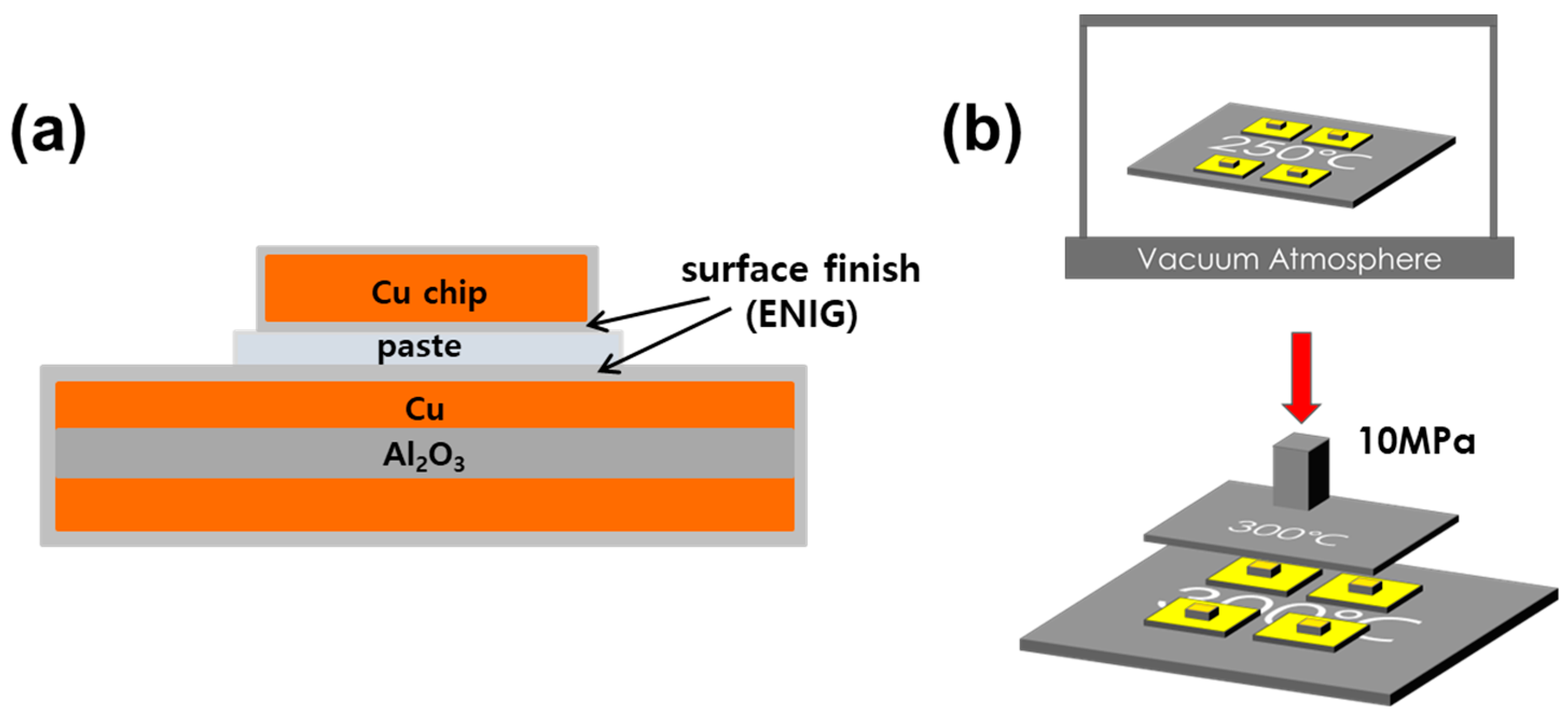
2. Materials and Methods
3. Results
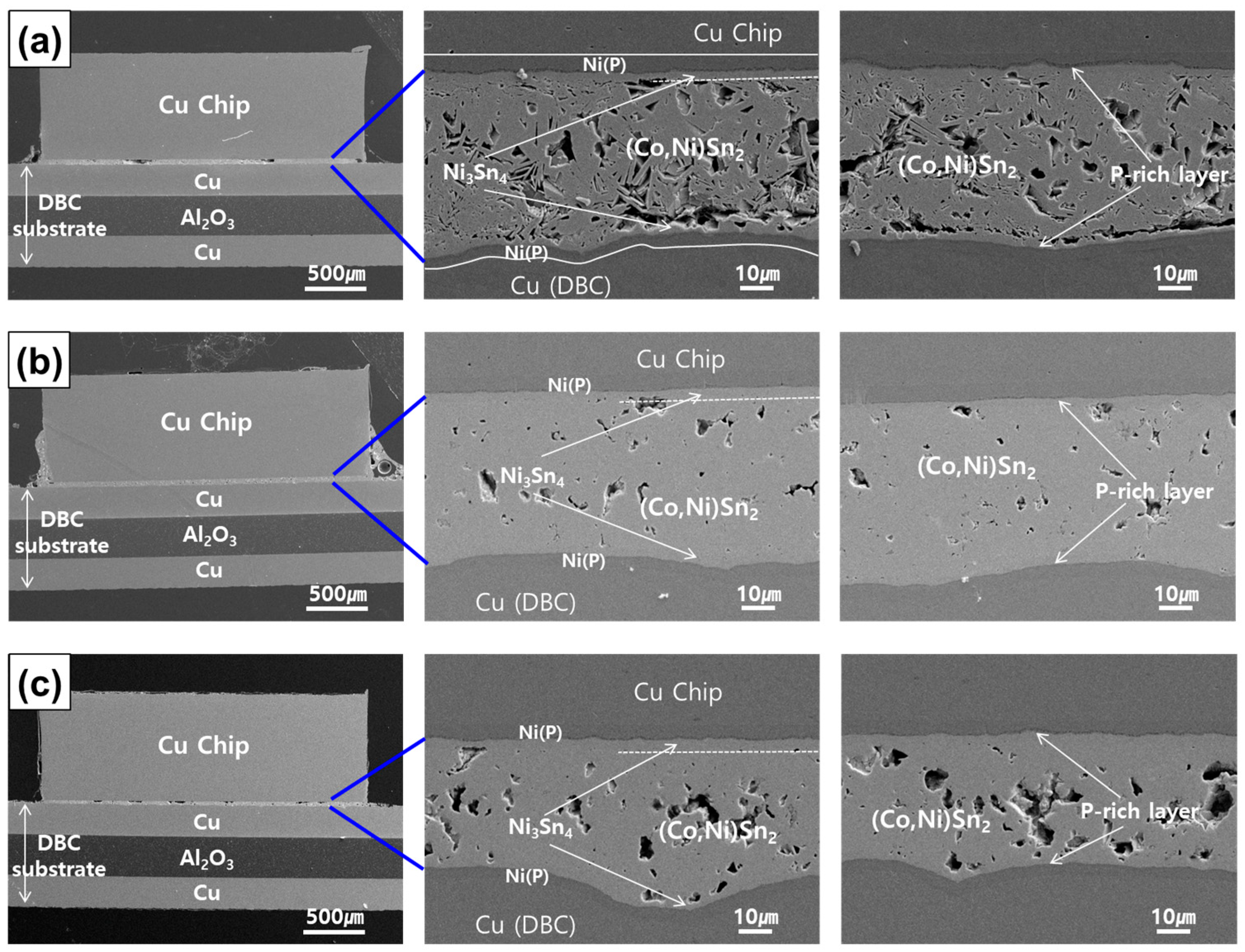
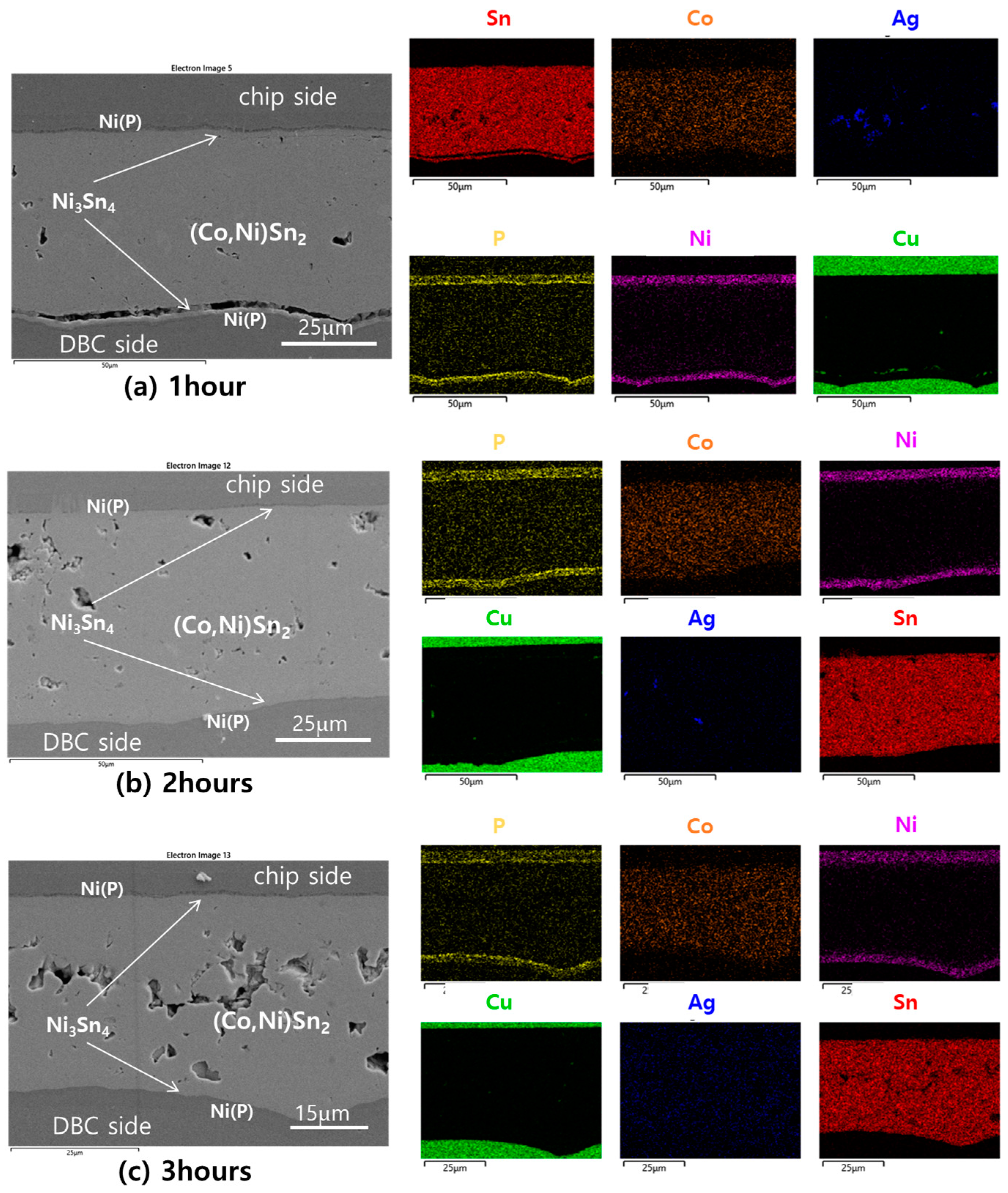
3.1. Phase and Microsturture Evolution during the TLP Bonding Process
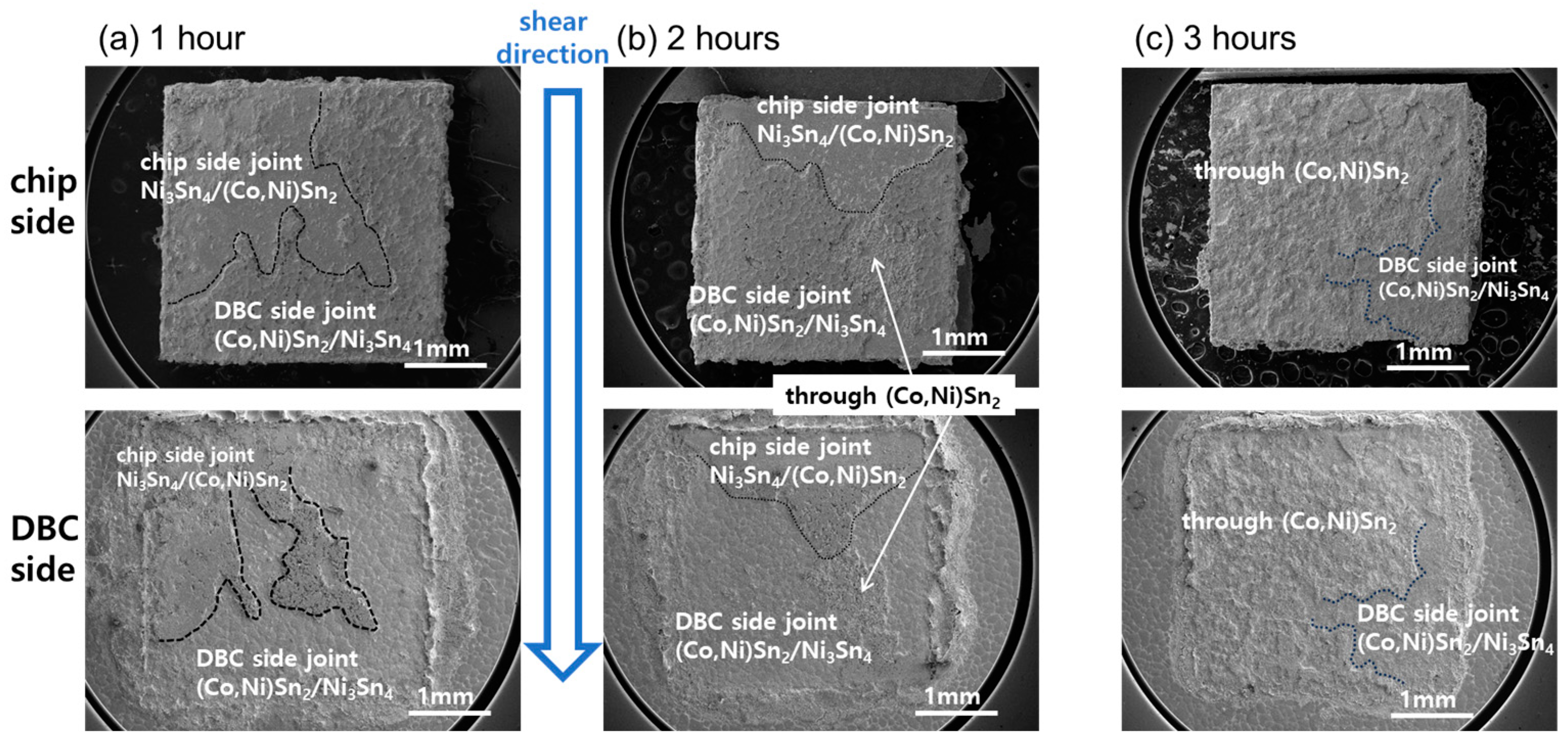
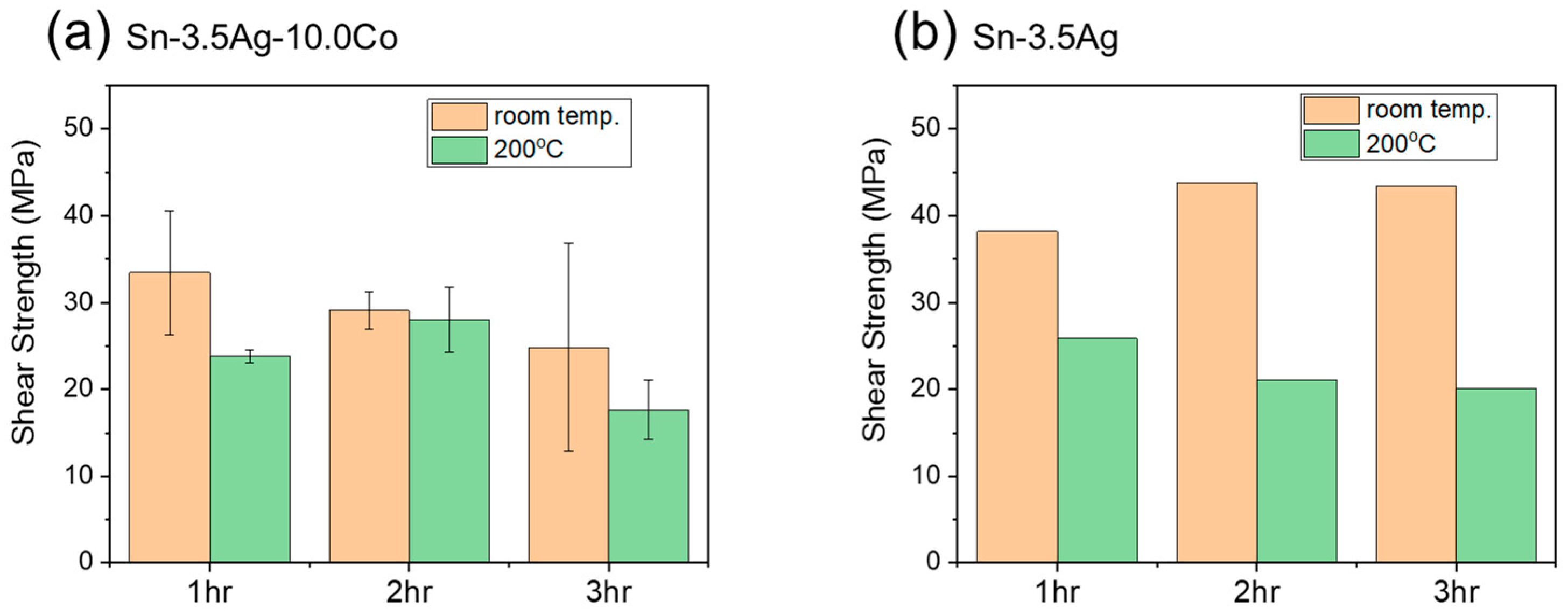
3.2. Shear Test Results of TLP-Bonded Specimens
4. Discussion
4.1. Correlation between Microstructure and Mechanical Reliability
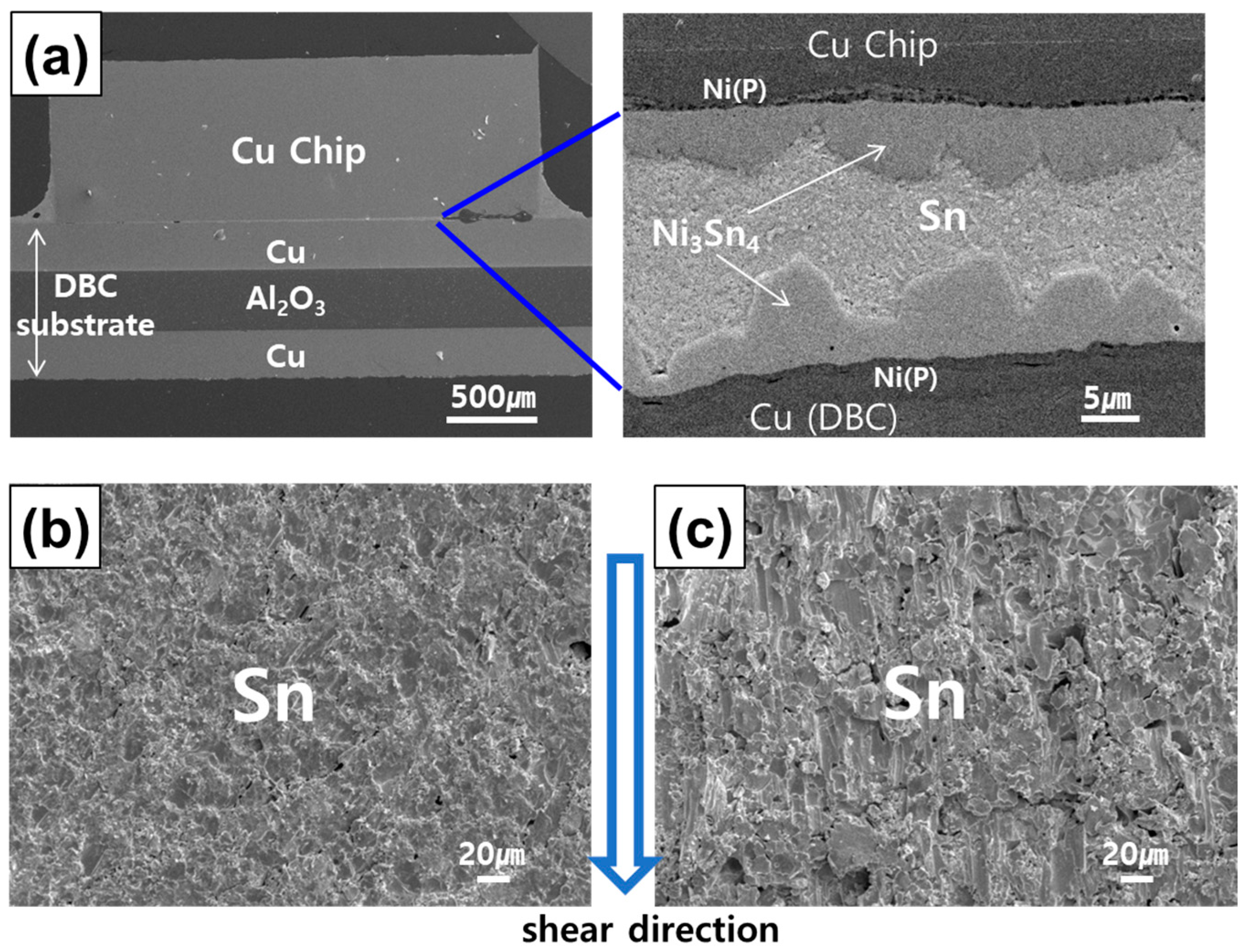
4.2. Void Formation in the TLP Joint Interfaces
5. Conclusions
- (1)
- After 1 h of TLP bonding, the Sn-3.5Ag-10.0Co paste transformed into IMCs, but voids persisted between them, particularly between (Co,Ni)Sn2 and Ni3Sn4. Voids significantly reduced after 2 h of bonding, with IMCs fully interconnected. The joint density further increased after 3 h of bonding, but voids tended to accumulate at the joint center.
- (2)
- Under TLP bonding conditions of 300 °C and 10 MPa, the entire Sn-3.5Ag-10.0Co solder underwent transformation into the high-melting point CoSn2 phase. This result corresponds to the observed minimum temperature required for CoSn2 formation during the reaction between liquid Sn and Co, possibly due to the extended reaction duration under elevated pressure.
- (3)
- In the 1 h bonded specimen, cracks propagated through the interface of Ni3Sn4 and (Co,Ni)Sn2, exhibiting separation, as well as within the loose interior of the (Co,Ni)Sn2 layer. In the 2 h bonded specimen, failure occurred at the interface of Ni3Sn4 and (Co,Ni)Sn2, present on both the chip and DBC sides. In the 3 h bonded specimen, failure was observed within the internal voids of the (Co,Ni)Sn2 IMC layer, partly involving the Ni3Sn4/(Co,Ni)Sn2 interface.
- (4)
- When utilizing the engineered Sn-Ag-Co TLP joint, no significant decrease in shear strength was observed, even under the 200 °C shear test. In contrast, the Sn-3.5Ag control specimen exhibited large reduction in shear strength. This decline can be attributed to the fact that the shear test temperature corresponds to 0.94 homologous temperature for the remaining Sn, evident by the presence of numerous shear bands.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Wang, Q.; Huang, X.; Muhammad, A.; Paramane, A.; Ren, N. Enhancement of electrical properties by including nano-aluminum nitride to micro-silicon carbide/silicone elastomer composites for potential power module packaging applications. J. Mater. Sci. Mater. Electron. 2022, 33, 18768–18785. [Google Scholar] [CrossRef]
- Zhang, Y.; Nee, H.P.; Hammam, T.; Belov, I.; Ranstad, P.; Bakowski, M. Multiphysics characterization of a novel SiC power module. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 9, 489–501. [Google Scholar] [CrossRef]
- He, S.; Que, L.; Lv, J.; Ang, S.S. Silicon carbide power electronic module packaging. In Proceedings of the 2015 16th International Conference on Electronic Packaging Technology (ICEPT), Changsha, China, 11–14 August 2015; pp. 483–486. [Google Scholar]
- Khazaka, R.; Mendizabal, L.; Henry, D.; Hanna, R. Survey of high-temperature reliability of power electronics packaging components. IEEE Trans. Power Electron. 2014, 30, 2456–2464. [Google Scholar] [CrossRef]
- Syed-Khaja, A. Diffusion Soldering for the High-Temperature Packaging of Power Electronics; FAU University Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Jung, D.H.; Sharma, A.; Mayer, M.; Jung, J.P. A review on recent advances in transient liquid phase (TLP) bonding for thermoelectric power module. Rev. Adv. Mater. Sci. 2018, 53, 147–160. [Google Scholar] [CrossRef]
- Kang, H.; Sharma, A.; Jung, J.P. Recent progress in transient liquid phase and wire bonding technologies for power electronics. Metals 2020, 10, 934. [Google Scholar] [CrossRef]
- Liu, Y.; Joshi, S.N.; Dede, E.M. Novel transient liquid phase bonding for high-temperature automotive power electronics systems. J. Electron. Packag. 2020, 142, 011003. [Google Scholar] [CrossRef]
- Baek, S.; Jeong, G.W.; Son, J.H.; Kim, M.S.; Lee, H.B.R.; Kim, J.; Ko, Y.H. Interfacial reactions and mechanical properties of transient liquid-phase bonding joints in Cu/Sn/Ni (P) and Ni/Sn/(OSP) Cu structures for power modules. J. Mater. Sci. Mater. Electron. 2021, 32, 3324–3333. [Google Scholar] [CrossRef]
- Yoon, S.W.; Glover, M.D.; Shiozaki, K. Nickel–tin transient liquid phase bonding toward high-temperature operational power electronics in electrified vehicles. IEEE Trans. Power Electron. 2012, 28, 2448–2456. [Google Scholar] [CrossRef]
- Lee, B.S.; Hyun, S.K.; Yoon, J.W. Cu–Sn and Ni–Sn transient liquid phase bonding for die-attach technology applications in high-temperature power electronics packaging. J. Mater. Sci. Mater. Electron. 2017, 28, 7827–7833. [Google Scholar] [CrossRef]
- Lis, A.; Kicin, S.; Brem, F.; Leinenbach, C. Thermal stress assessment for transient liquid-phase bonded Si chips in high-power modules using experimental and numerical methods. J. Electron. Mater. 2017, 46, 729–741. [Google Scholar] [CrossRef]
- Pahinkar, D.G.; Puckett, W.; Graham, S.; Boteler, L.; Ibitayo, D.; Narumanchi, S.; Major, J. Transient liquid phase bonding of AlN to AlSiC for durable power electronic packages. Adv. Eng. Mater. 2018, 20, 1800039. [Google Scholar] [CrossRef]
- Chidambaram, V.; Hattel, J.H. High-temperature lead-free solder alternatives. Microelectron. Eng. 2011, 88, 981–989. [Google Scholar] [CrossRef]
- Sharif, A.; Gan, C.L.; Chen, Z. Transient liquid phase Ag-based solder technology for high-temperature packaging applications. J. Alloys Compd. 2014, 587, 365–368. [Google Scholar] [CrossRef]
- Fujino, M.; Narusawa, H.; Kuramochi, Y.; Higurashi, E.; Suga, T.; Shiratori, T.; Mizukoshi, M. Transient liquid-phase sintering using tin and silver powder mixture for die bonding. In Proceedings of the International Conference on Solid State Devices and Materials, Sapporo, Japan, 27–30 September 2015; The Japan Society of Applied Physics: Tokyo, Japan; pp. 60–61. [Google Scholar]
- Bao, Y.D.; Wu, A.P.; Shao, H.K.; Zhao, Y.; Liu, L.; Zou, G.S. Microstructural evolution and mechanical reliability of transient liquid phase sintered joint during thermal aging. J. Mater. Sci. 2019, 54, 765–776. [Google Scholar] [CrossRef]
- Saud, N.; Said, R.M. Transient liquid phase bonding for solder-a short review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012050. [Google Scholar] [CrossRef]
- Greve, H.; Moeini, S.A.; McCluskey, P.; Joshi, S. Prediction and mitigation of vertical cracking in high-temperature transient liquid phase sintered joints by thermomechanical simulation. J. Electron. Packag. 2018, 140, 020903. [Google Scholar] [CrossRef]
- Lang, F.; Yamaguchi, H.; Nakagawa, H.; Sato, H. Thermally stable bonding of SiC devices with ceramic substrates: Transient liquid phase sintering using Cu/Sn powders. J. Electrochem. Soc. 2013, 160, 315–319. [Google Scholar] [CrossRef]
- Nishikawa, H.; Komatsu, A.; Takemoto, T. Interfacial reaction between Sn–Ag–Co solder and metals. Mater. Trans. 2005, 46, 2394–2399. [Google Scholar] [CrossRef]
- Nishikawa, H.; Komatsu, A.; Takemoto, T. Effect of Ni or Co addition to Sn-Ag solder on microstructure and joint strength at interface. Mater. Trans. 2008, 49, 1518–1523. [Google Scholar] [CrossRef]
- Tay, S.L.; Haseeb, A.S.M.A.; Johan, M.R. Effect of addition Cobalt nanoparticles on Sn-Ag-Cu lead-free solder. In Proceedings of the 2010 12th Electronics Packaging Technology Conference, Singapore, 8–10 December 2010; pp. 433–436. [Google Scholar]
- Lee, J.S.; Chu, K.M.; Patzelt, R.; Manessis, D.; Ostmann, A.; Jeon, D.Y. Effects of Co addition in eutectic Sn–3.5 Ag solder on shear strength and microstructural development. Microelectron. Eng. 2008, 85, 1577–1583. [Google Scholar] [CrossRef]
- Nishikawa, H.; Komatsu, A.; Takemoto, T. Morphology and pull strength of Sn-Ag(-Co) solder joint with copper pad. J. Electron. Mater. 2007, 36, 1137–1143. [Google Scholar] [CrossRef]
- Yakymovych, A.; Plevachuk, Y.; Švec, P.; Janičkovič, D.; Šebo, P.; Beronska, N.; Ipser, H. Nanocomposite SAC solders: Morphology, electrical and mechanical properties of Sn–3.8 Ag–0.7 Cu solders by adding Co nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 10965–10973. [Google Scholar] [CrossRef]
- Anderson, I.E.; Foley, J.C.; Cook, B.A.; Harringa, J.; Terpstra, R.L.; Unal, O. Alloying effects in near-eutectic Sn-Ag-Cu solder alloys for improved microstructural stability. J. Electron. Mater. 2001, 30, 1050–1059. [Google Scholar] [CrossRef]
- Kim, D.; Nagao, S.; Chen, C.; Wakasugi, N.; Yamamoto, Y.; Suetake, A.; Takemasa, T.; Sugahara, T.; Suganuma, K. Online thermal resistance and reliability characteristic monitoring of power modules with Ag sinter joining and Pb, Pb-free solders during power cycling test by SiC TEG chip. IEEE Trans. Power Electron. 2020, 36, 4977–4990. [Google Scholar] [CrossRef]
- Wang, M.; Mei, Y.H.; Jin, J.; Chen, S.; Li, X.; Lu, G.Q. Pressureless sintered-silver die-attach at 180 °C for power electronics packaging. IEEE Trans. Power Electron. 2021, 36, 12141–12145. [Google Scholar] [CrossRef]
- Yin, L.; Yang, F.; Bao, X.; Xue, W.; Du, Z.; Wang, X.; Cheng, J.; Ji, H.; Sui, J.; Liu, X.; et al. Low-temperature sintering of Ag nanoparticles for high-performance thermoelectric module design. Nat. Energy 2023, 8, 665–674. [Google Scholar] [CrossRef]
- Alam, M.O.; Chan, Y.C.; Hung, K.C. Reliability study of the electroless Ni–P layer against solder alloy. Microelectron. Reliab. 2002, 42, 1065–1073. [Google Scholar] [CrossRef]
- Yoon, J.W.; Park, J.H.; Shur, C.C.; Jung, S.B. Characteristic evaluation of electroless nickel–phosphorus deposits with different phosphorus contents. Microelectron. Eng. 2007, 84, 2552–2557. [Google Scholar] [CrossRef]
- Liu, P.L.; Xu, Z.; Shang, J.K. Thermal stability of electroless-nickel/solder interface: Part A. Interfacial chemistry and microstructure. Metall. Mater. Trans. A 2000, 31, 2857–2866. [Google Scholar] [CrossRef]
- Sohn, Y.C.; Yu, J.; Kang, S.K.; Shih, D.Y.; Choi, W.K. Effects of phosphorus content on the reaction of electroless Ni-P with Sn and crystallization of Ni-P. J. Electron. Mater. 2004, 33, 790–795. [Google Scholar] [CrossRef]
- Qi, L.; Huang, J.; Zhao, X.; Zhang, H. Effect of thermal-shearing cycling on Ag3Sn microstructural coarsening in SnAgCu solder. J. Alloys Compd. 2009, 469, 102–107. [Google Scholar] [CrossRef]
- Cui, Y.; Xian, J.W.; Zois, A.; Marquardt, K.; Yasuda, H.; Gourlay, C.M. Nucleation and growth of Ag3Sn in Sn-Ag and Sn-Ag-Cu solder alloys. Acta Mater. 2023, 249, 118831. [Google Scholar] [CrossRef]
- Shen, J.; Chan, Y.C.; Liu, S.Y. Growth mechanism of bulk Ag3Sn intermetallic compounds in Sn–Ag solder during solidification. Intermetallics 2008, 16, 1142–1148. [Google Scholar] [CrossRef]
- Wang, C.H.; Kuo, C.Y.; Huang, S.E.; Li, P.Y. Temperature effects on liquid-state Sn/Co interfacial reactions. Intermetallics 2013, 32, 57–63. [Google Scholar] [CrossRef]
- Odashima, N.; O, M.; Kajihara, M. Formation of intermetallic compounds and microstructure evolution due to isothermal reactive diffusion at the interface between solid Co and liquid Sn. J. Electron. Mater. 2020, 49, 1568–1576. [Google Scholar] [CrossRef]
- Gao, F.; Cheng, F.; Nishikawa, H.; Takemoto, T. Characterization of Co–Sn intermetallic compounds in Sn–3.0 Ag–0.5 Cu–0.5 Co lead-free solder alloy. Mater. Lett. 2008, 62, 2257–2259. [Google Scholar] [CrossRef]
- Vassilev, G.P.; Lilova, K.I.; Gachon, J.C. Calorimetric and phase diagram studies of the Co-Sn system. Intermetallics 2007, 15, 1156–1162. [Google Scholar] [CrossRef]
- Baheti, V.A. Phase evolutions and growth kinetics in the Co–Sn system. SN Appl. Sci. 2019, 1, 185. [Google Scholar] [CrossRef]
- Jeong, M.S.; Lee, D.H.; Yoon, J.W. Effect of temperature on shear properties of Sn-3.0 Ag-0.5 Cu and Sn-58Bi solder joints. J. Alloys Compd. 2022, 903, 163987. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mahmudi, R. Elevated-temperature mechanical properties of lead-free Sn-0.7Cu-xSiC nanocomposite solders. J. Electron. Mater. 2018, 47, 1721–1729. [Google Scholar] [CrossRef]
- Hang, C.; Tian, Y.; Zhang, R.; Yang, D. Phase transformation and grain orientation of Cu–Sn intermetallic compounds during low temperature bonding process. J. Mater. Sci. Mater. Electron. 2013, 24, 3905–3913. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Yu, J.J.; Kuo, M.S.; Tong, H.M.; Kao, C.R. Elimination of voids in reactions between Ni and Sn: A novel effect of silver. Scr. Mater. 2012, 66, 171–174. [Google Scholar] [CrossRef]
- Li, C.C.; Chung, C.K.; Shih, W.L.; Kao, C.R. Volume shrinkage induced by interfacial reaction in micro-Ni/Sn/Ni joints. Metall. Mater. Trans. A 2014, 45, 2343–2346. [Google Scholar] [CrossRef]
- Lin, S.K.; Cho, C.L.; Chang, H.M. Interfacial reactions in Cu/Ga and Cu/Ga/Cu couples. J. Electron. Mater. 2014, 43, 204–211. [Google Scholar] [CrossRef]
- Mokhtari, O.; Nishikawa, H. The shear strength of transient liquid phase bonded Sn–Bi solder joint with added Cu particles. Adv. Powd. Technol. 2016, 27, 1000–1005. [Google Scholar] [CrossRef]
- Shao, H.; Wu, A.; Bao, Y.; Zhao, Y.; Zou, G. Microstructure characterization and mechanical behavior for Ag3Sn joint produced by foil-based TLP bonding in air atmosphere. Mater. Sci. Eng. A 2017, 680, 221–231. [Google Scholar] [CrossRef]
- Villars, P. (Ed.) Ni3Sn4 Crystal Structure. In Pauling File in: Inorganic Solid Phases; SpringerMaterials (online database); Springer: Berlin/Heidelberg, Germany, 2023; Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0528976 (accessed on 1 May 2024).
- Armbrüster, M.; Schmidt, M.; Cardoso-Gil, R.; Borrmann, H.; Grin, Y. Crystal structures of iron distannide, FeSn2, and cobalt distannide, CoSn2. Z. Krist.-New Cryst. Struct. 2007, 222, 83–84. [Google Scholar] [CrossRef]



| Ni3Sn4 | CoSn2 | Sn | |
|---|---|---|---|
| Melting temp. (K) | 1071 | 843 | 505 |
| Test temp. 1 (K) Homologous temp. (T/Tm) | 293 0.27 | 293 0.35 | 293 0.58 |
| Test temp. 2 (K) Homologous temp. (T/Tm) | 473 0.44 | 473 0.56 | 473 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Cheon, G.; Ko, Y.-H.; Sohn, Y. Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications. Electronics 2024, 13, 2173. https://doi.org/10.3390/electronics13112173
Kim B, Cheon G, Ko Y-H, Sohn Y. Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications. Electronics. 2024; 13(11):2173. https://doi.org/10.3390/electronics13112173
Chicago/Turabian StyleKim, Byungwoo, Gyeongyeong Cheon, Yong-Ho Ko, and Yoonchul Sohn. 2024. "Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications" Electronics 13, no. 11: 2173. https://doi.org/10.3390/electronics13112173
APA StyleKim, B., Cheon, G., Ko, Y.-H., & Sohn, Y. (2024). Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications. Electronics, 13(11), 2173. https://doi.org/10.3390/electronics13112173







