Impact of H-Related Chemical Bonds on Physical Properties of SiNx:H Films Deposited via Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Film Deposition and Testing Methods
2.1. Film Deposition
2.2. Characterization Methods
2.2.1. H-Related Chemical Bonds
2.2.2. Refractive Index
2.2.3. Breakdown Field Strength
2.2.4. Stress
2.2.5. Wet Etching Rate Ratio
2.2.6. Deposition Rate and Non-Uniformity
2.2.7. Surface Roughness
3. Results and Discussion
3.1. Influence of Deposition Conditions on H-Related Chemical Bonds
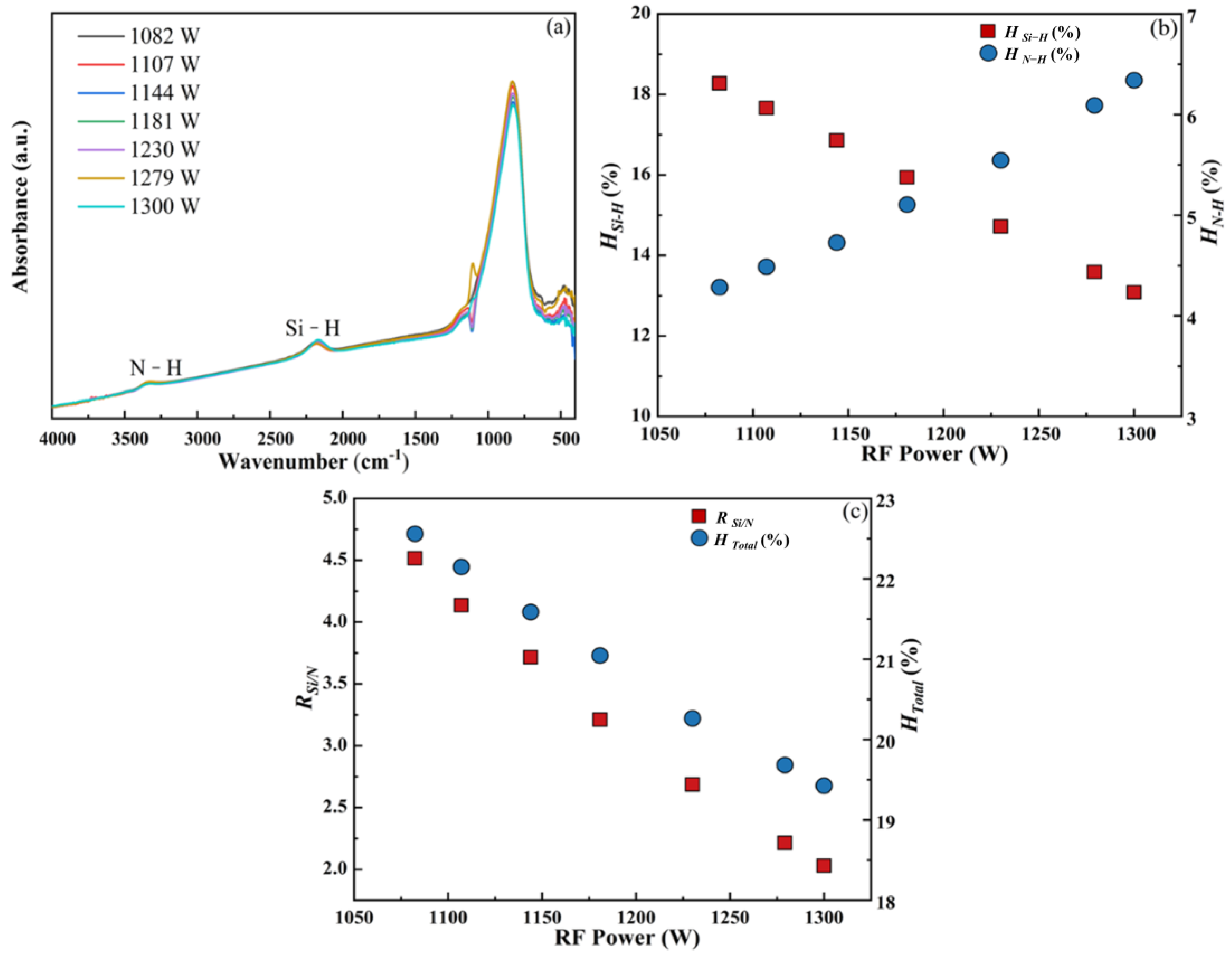
3.1.1. RF Power
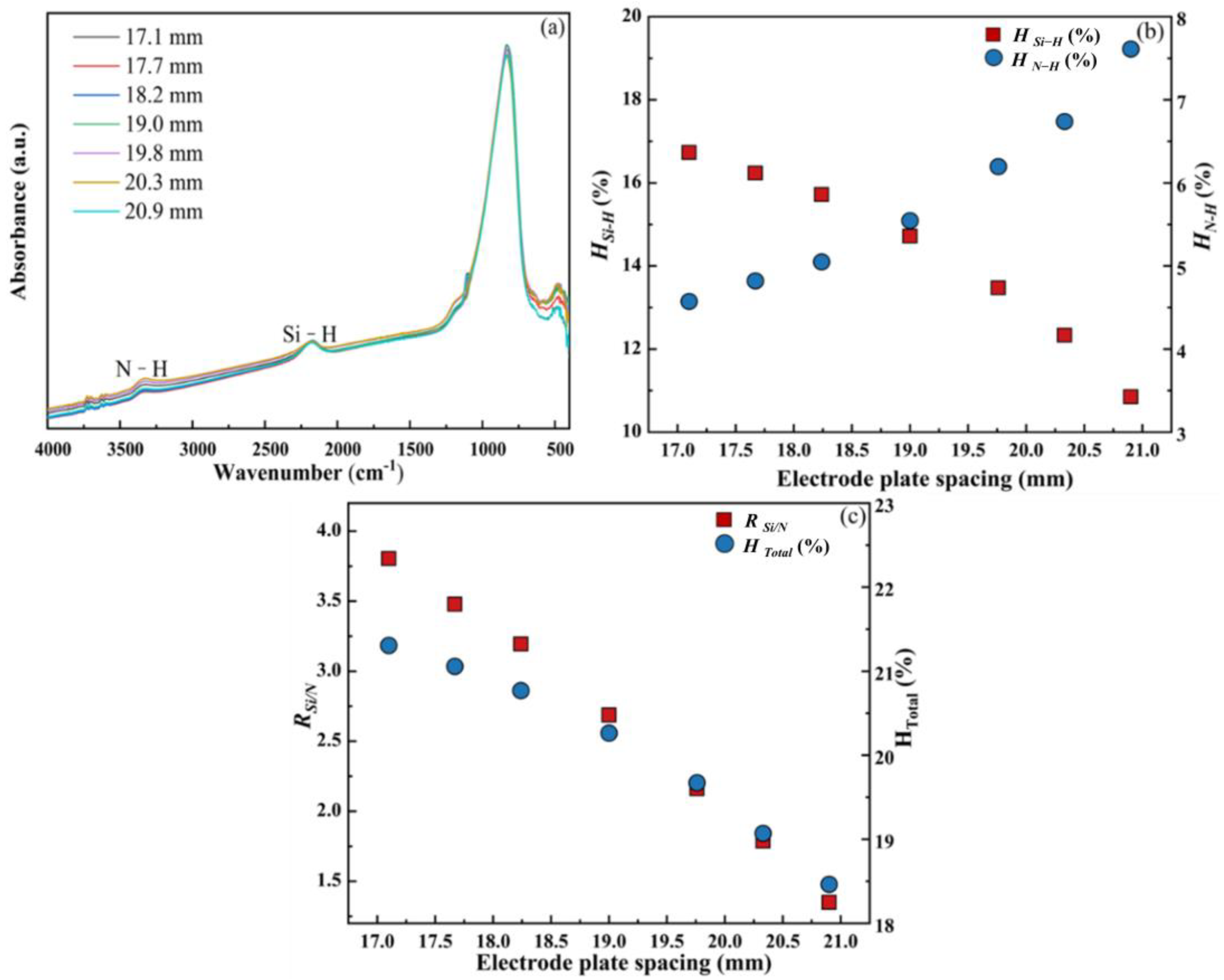
3.1.2. Electrode Plate Spacing
3.1.3. Temperature
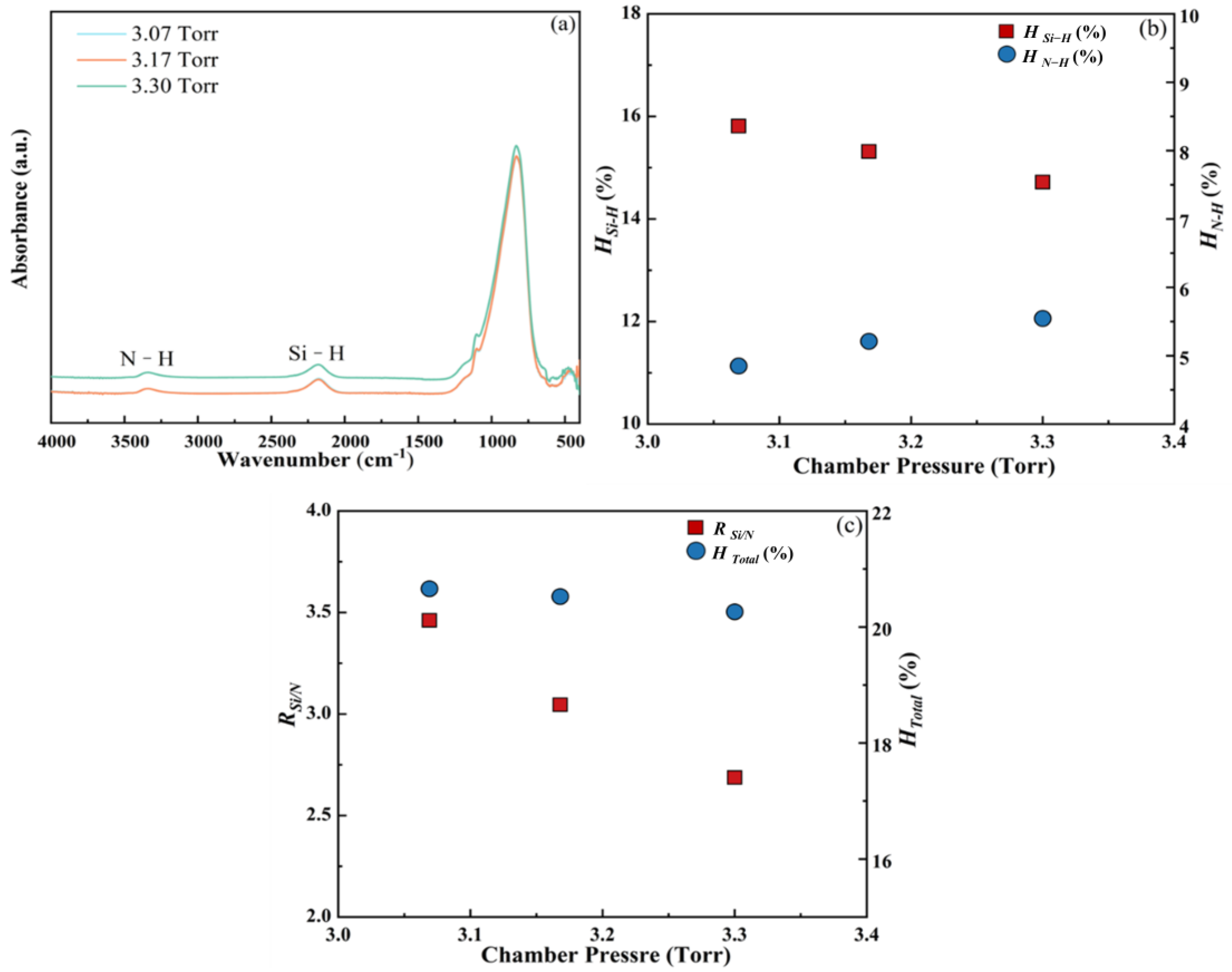
3.1.4. Chamber Pressure
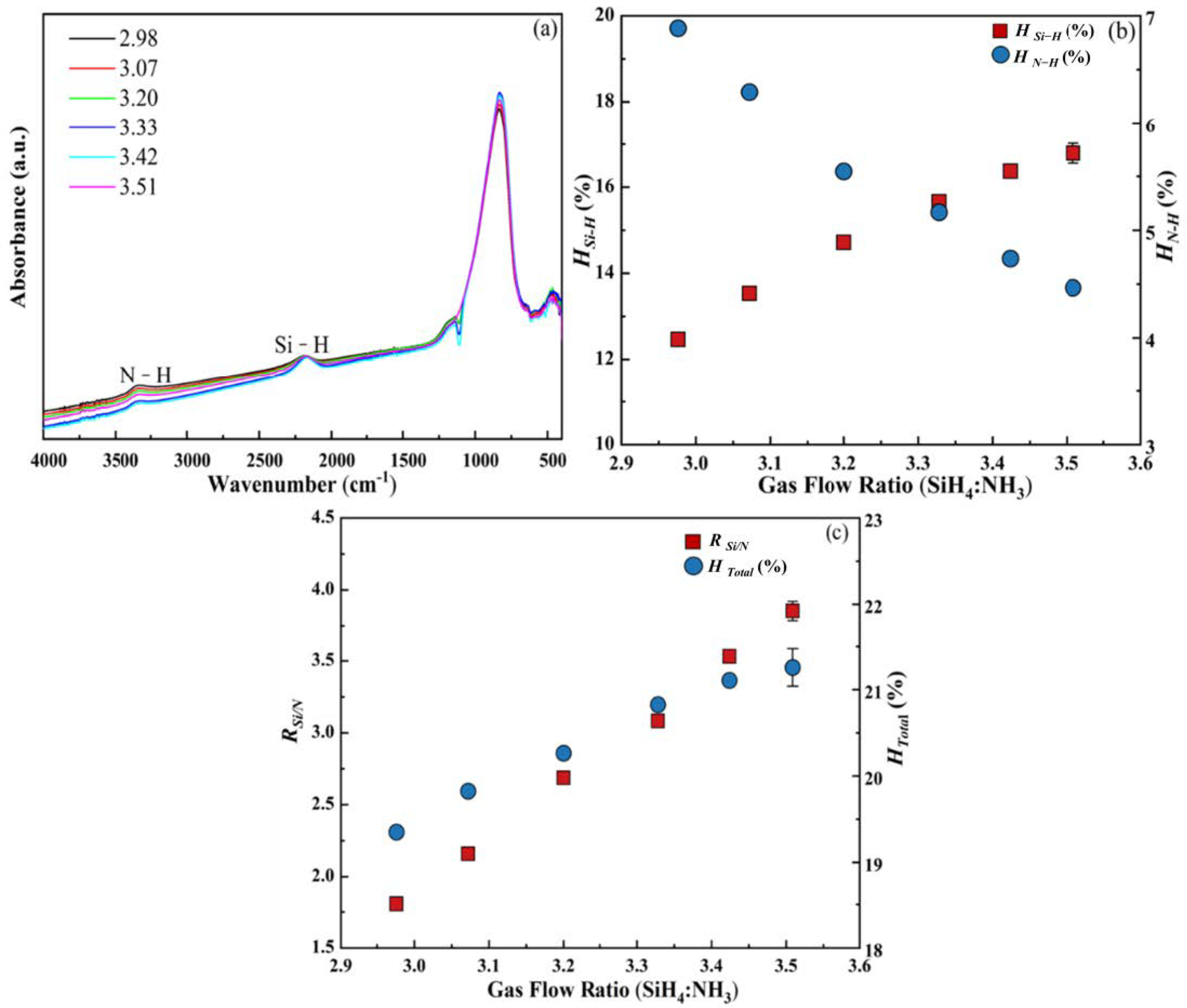
3.1.5. SiH4:NH3 Gas Flow Ratio
3.2. Influence of Deposition Conditions on SiNx:H Film Properties
3.2.1. Refractive Index
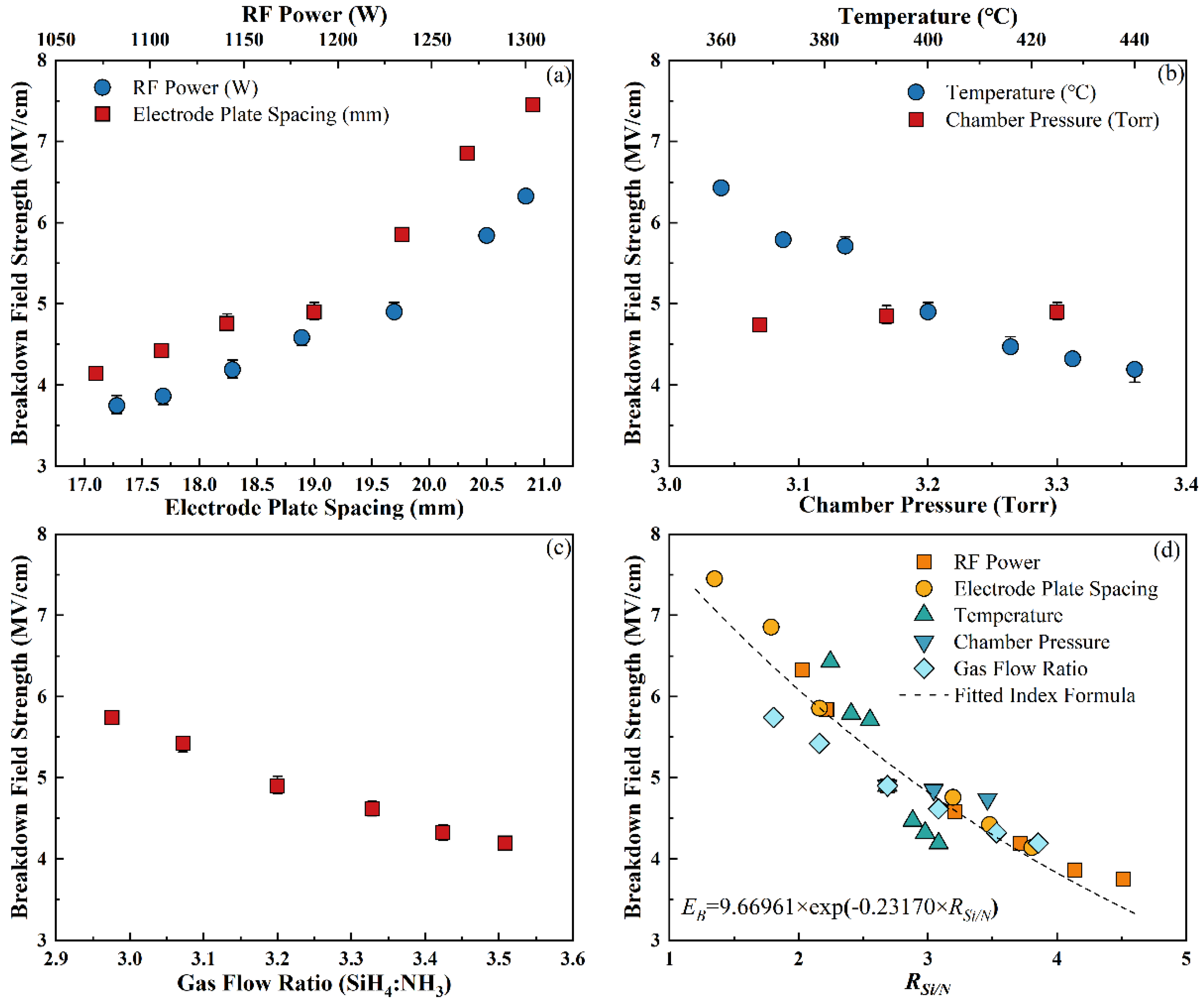
3.2.2. Breakdown Field Strength
3.2.3. Stress
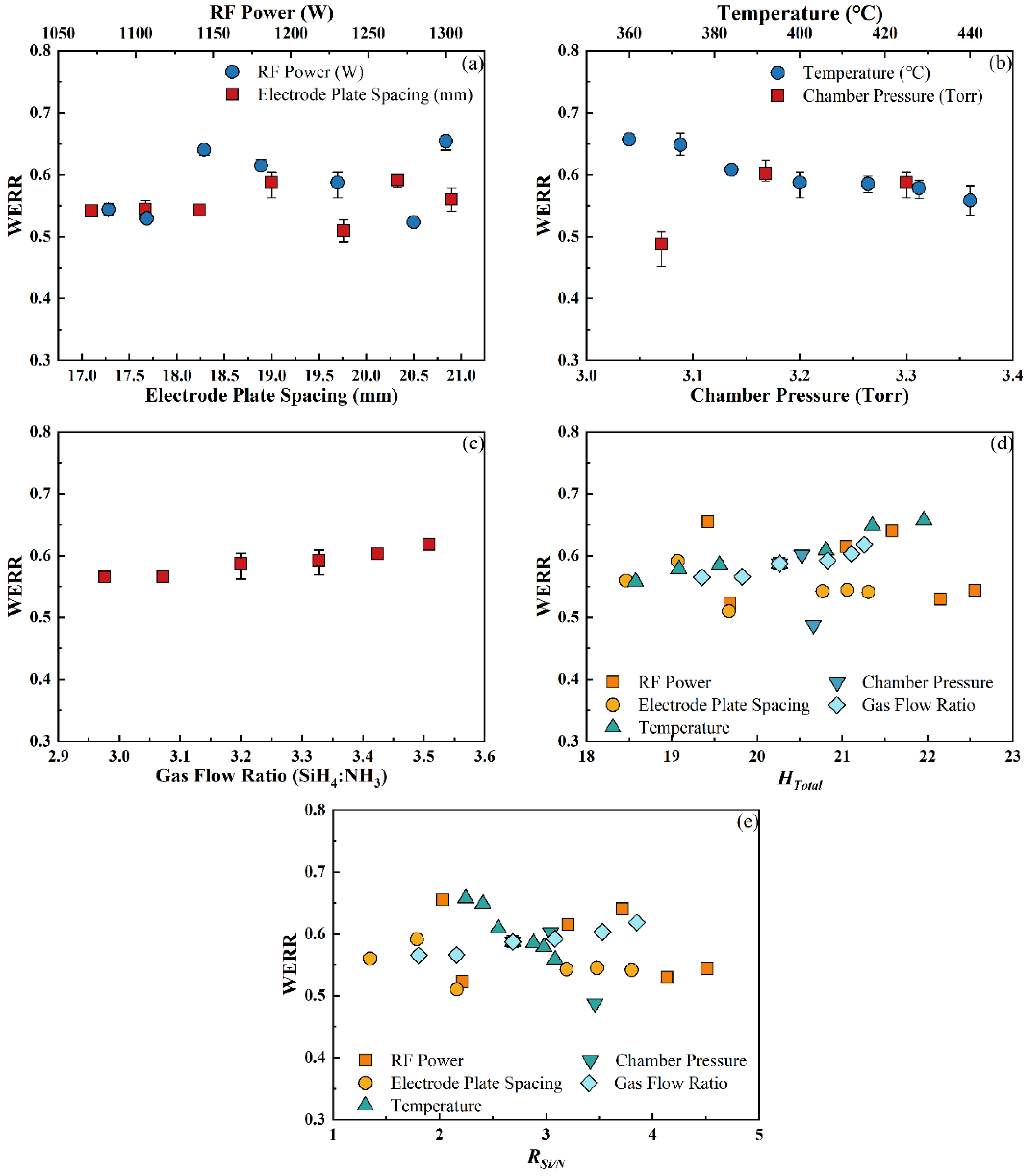
3.2.4. Wet Etching Rate Ratio
3.3. Influence of Deposition Conditions on Yield and Quality
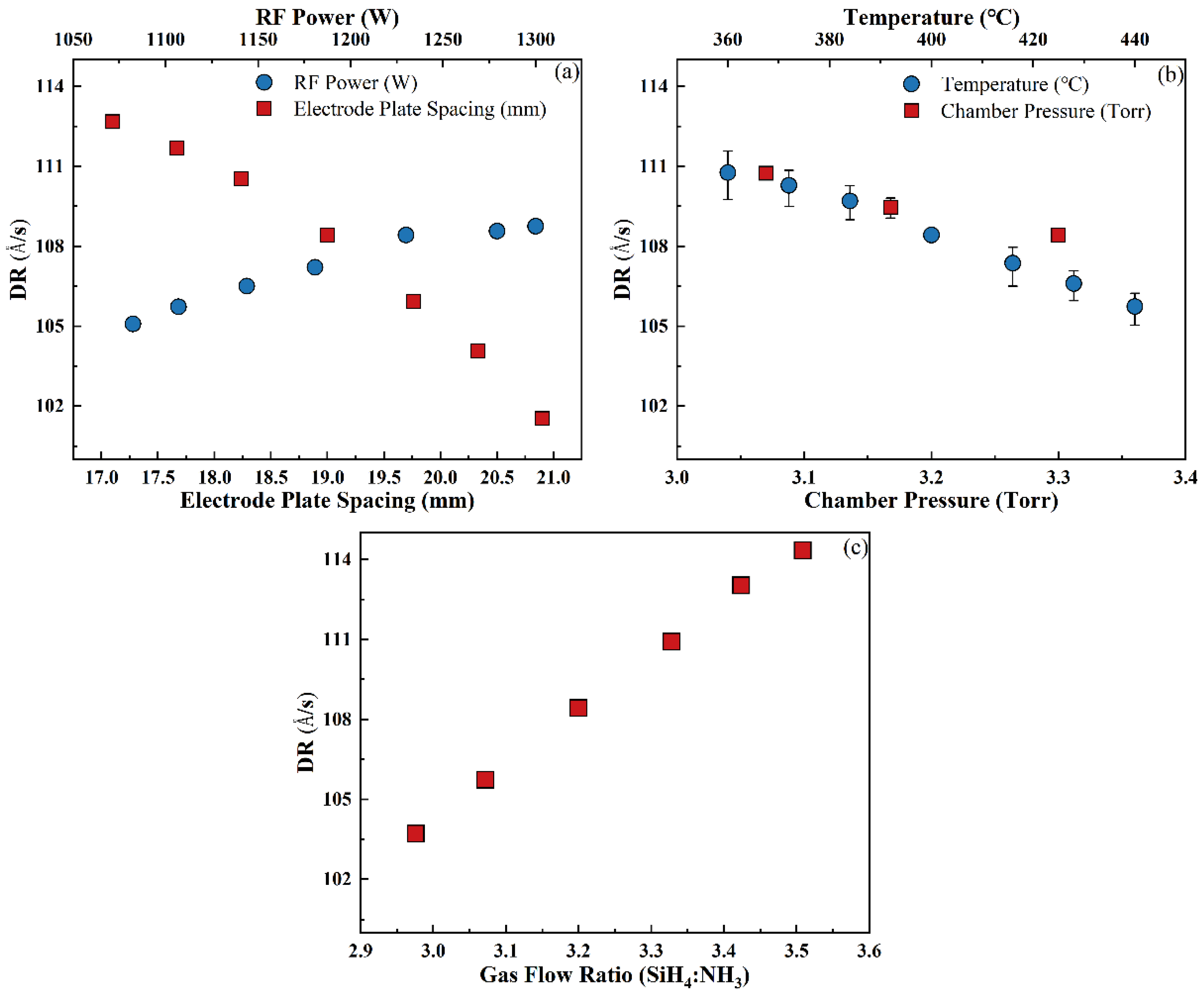
3.3.1. Deposition Rate
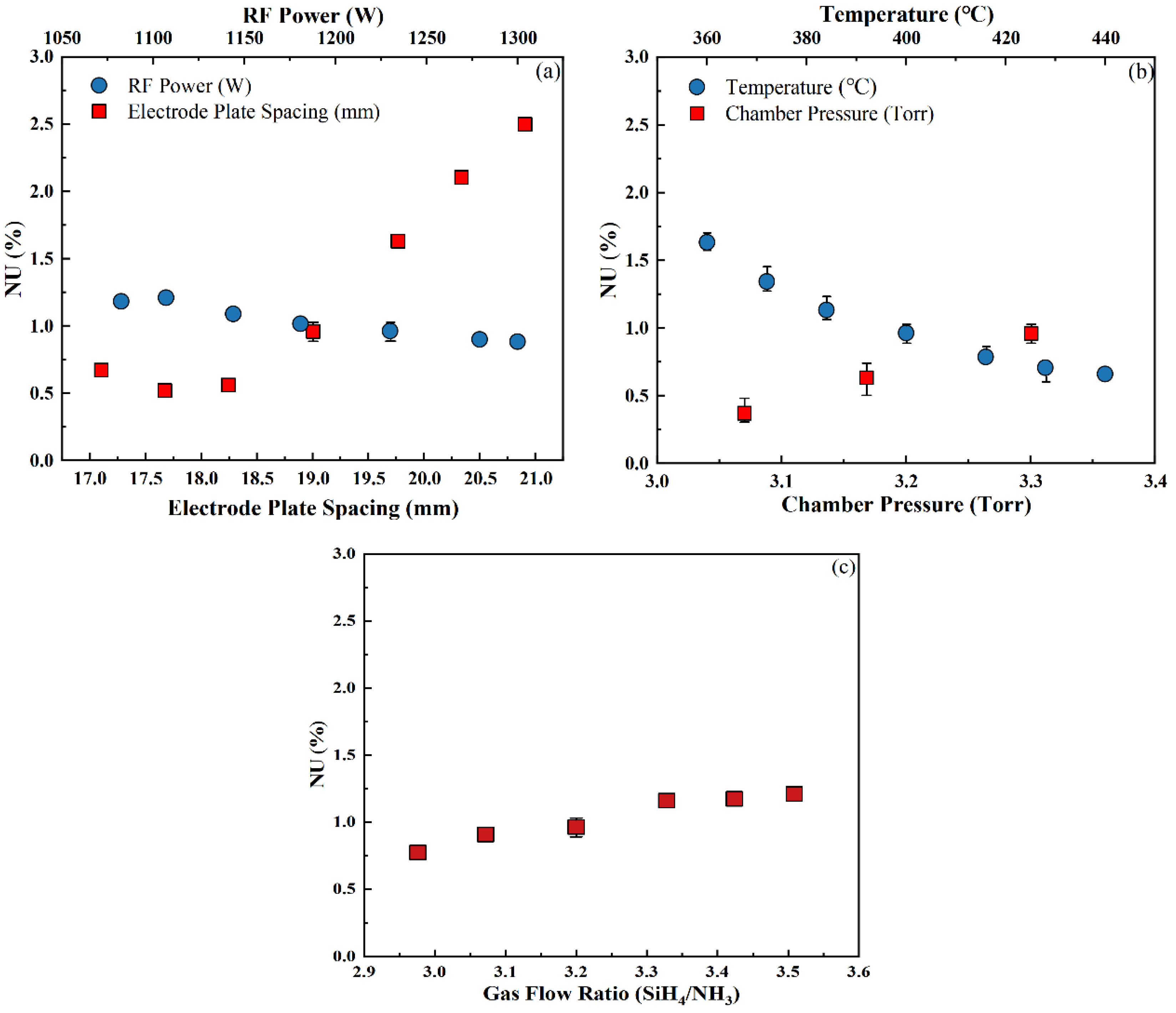
3.3.2. Non-Uniformity
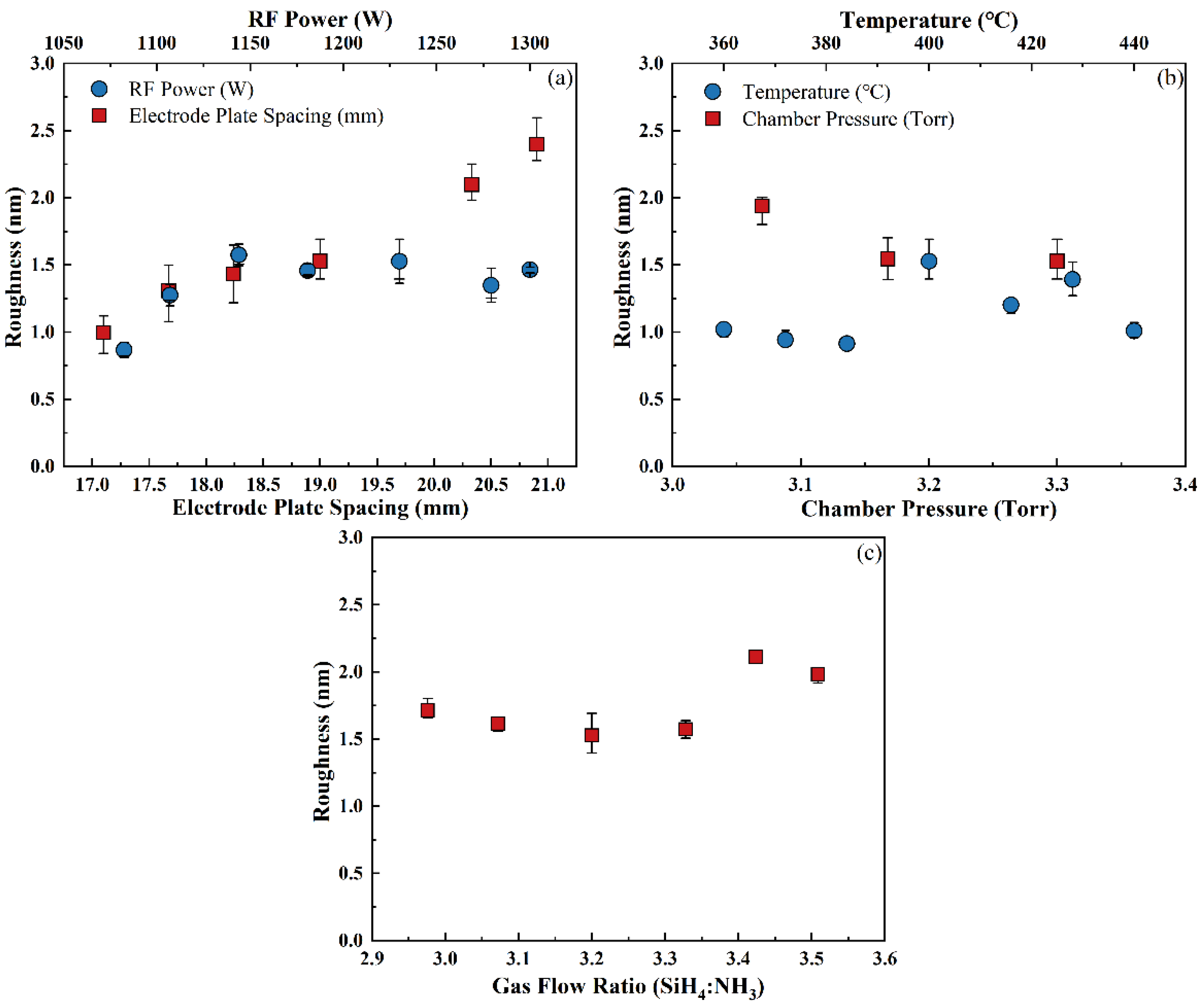
3.3.3. Surface Roughness
4. Conclusions
- Under the different deposition conditions, the differences in the RI and EB can be mainly attributed to the change in RSi/N. Meanwhile, HTotal had a greater impact on the WERR of the SiNx:H films, and the stress was not entirely dependent on HTotal.
- As RSi/N increased, the RI showed a linear growth trend. The reason for this is that the relationship between RSi/N and the Si:N atomic ratio is positive and linear, and the RI increases with an increase in the Si:N atomic ratio. EB shows an exponential downward trend with an increase in RSi/N, which is related to the decrease in the bandgap width.
- Excessive electrode plate spacing should be avoided in the development of the deposition process, as it can lead to significant increases in the NU and surface roughness, reducing the product quality.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schurink, B.; van den Beld, W.T.E.; Tiggelaar, R.M.; van de Kruijs, R.W.E.; Bijkerk, F. Synthesis and Characterization of Boron Thin Films Using Chemical and Physical Vapor Depositions. Coatings 2022, 12, 685. [Google Scholar] [CrossRef]
- Pecora, A.; Maiolo, L.; Fortunato, G.; Caligiore, C. A comparative analysis of silicon dioxide films deposited by ECR-PECVD, TEOS-PECVD and Vapox-APCVD. J. Non-Cryst. Solids 2006, 352, 1430–1433. [Google Scholar] [CrossRef]
- Takagi, T.; Takechi, K.; Nakagawa, Y.; Watabe, Y.; Nishida, S. High rate deposition of a-Si:H and a-SiNx:H by VHF PECVD. Vacuum 1998, 51, 751–755. [Google Scholar] [CrossRef]
- Park, H.K.; Song, W.S.; Hong, S.J. In Situ Plasma Impedance Monitoring of the Oxide Layer PECVD Process. Coatings 2023, 13, 559. [Google Scholar] [CrossRef]
- Tomankova, K.; Kubecka, M.; Rivolta, N.; Cornil, D.; Obrusnik, A. Simulation of a hollow-cathode PECVD process in O2/TMDSO for silicon dioxide deposition—Cross-code validation of 2D plasma model and global plasma model. Surf. Coat. Technol. 2023, 474, 130069. [Google Scholar] [CrossRef]
- Kaloyeros, A.E.; Pan, Y.; Goff, J.; Arkles, B. Review-Silicon Nitride and Silicon Nitride-Rich Thin Film Technologies: State-of-the-Art Processing Technologies, Properties, and Applications. ECS J. Solid State Sci. Technol. 2020, 9, 063006. [Google Scholar] [CrossRef]
- Jang, W.; Jeon, H.; Song, H.; Kim, H.; Park, J.; Kim, H.; Jeon, H. The effect of plasma power on the properties of low-temperature silicon nitride deposited by RPALD for a gate spacer. Phys. Status Solidi A 2015, 212, 2785–2790. [Google Scholar] [CrossRef]
- Cho, T.Y.; Lee, W.J.; Lee, S.J.; Lee, J.H.; Ryu, J.; Cho, S.K.; Choa, S.H. Moisture barrier and bending properties of silicon nitride films prepared by roll-to-roll plasma enhanced chemical vapor deposition. Thin Solid Film. 2018, 660, 101–107. [Google Scholar] [CrossRef]
- Chui, K.J.; Ang, K.W.; Chin, H.C.; Shen, C.; Wong, L.Y.; Tung, C.H.; Balasubramanian, N.; Li, M.F.; Samudra, G.S.; Yeo, Y.C. Strained-SOI n-Channel Transistor With Silicon–Carbon Source/Drain Regions for Carrier Transport Enhancement. IEEE Electron Device Lett. 2006, 27, 778–780. [Google Scholar] [CrossRef]
- Kaloyeros, A.E.; Jové, F.A.; Goff, J.; Arkles, B. Review—Silicon Nitride and Silicon Nitride-Rich Thin Film Technologies: Trends in Deposition Techniques and Related Applications. ECS J. Solid State Sci. Technol. 2017, 6, P691. [Google Scholar] [CrossRef]
- Oh, S.J.; Ma, B.S.; Yang, C.; Kim, T.-S. Intrinsic Mechanical Properties of Free-Standing SiNx Thin Films Depending on PECVD Conditions for Controlling Residual Stress. ACS Appl. Electron. Mater. 2022, 4, 3980–3987. [Google Scholar] [CrossRef]
- Subhani, K.N.; Remesh, N.; Niranjan, S.; Raghavan, S.; Muralidharan, R.; Nath, D.N.; Bhat, K.N. Nitrogen rich PECVD silicon nitride for passivation of Si and AlGaN/GaN HEMT devices. Solid-State Electron. 2021, 186, 108188. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, J.; Ren, Y.; Xu, W.; Liu, W.; Cai, X.; Zhao, B. Study on effect of process and structure parameters on SiNxHy growth by in-line PECVD. Sol. Energy 2020, 198, 469–478. [Google Scholar] [CrossRef]
- So, S.J.; Oh, D.S.; Sung, H.K.; Park, C.B. Fabrication of MIM capacitors with 1000 A silicon nitride layer deposited by PECVD for InGaP/GaAs HBT applications. J. Cryst. Growth 2005, 279, 341–348. [Google Scholar] [CrossRef]
- Huang, H.; Winchester, K.J.; Suvorova, A.; Lawn, B.R.; Liu, Y.; Hu, X.Z.; Dell, J.M.; Faraone, L. Effect of deposition conditions on mechanical properties of low-temperature PECVD silicon nitride films. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2006, 435, 453–459. [Google Scholar] [CrossRef]
- Seo, H.; Jung, J.-D. The Silicon Nitride Films according to The Frequency Conditions of Plasma Enhanced Chemical Vapor Deposition. J. Semicond. Disp. Technol. 2014, 13, 21–25. [Google Scholar]
- Beliaev, L.Y.; Shkondin, E.; Lavrinenko, A.V.; Takayama, O. Optical, structural and composition properties of silicon nitride films deposited by reactive radio-frequency sputtering, low pressure and plasma-enhanced chemical vapor deposition. Thin Solid Film. 2022, 763, 139568. [Google Scholar] [CrossRef]
- Barrera-Mendivelso, E.S.; Rodriguez-Gomez, A. Thin films of silicon nitride deposited at room temperature by non-reactive magnetron sputtering: Radiofrequency power and deposition time influence on the formation of a-Si3N4 and its optical properties. Front. Phys. 2023, 11, 1260579. [Google Scholar] [CrossRef]
- Ahn, S.; Hong, S.J.; Yang, H.S.; Cho, S.M. Effect of 2 MHz frequency power applied to the substrate for low-temperature silicon nitride thin film deposition. Mater. Sci. Semicond. Process. 2022, 143, 106538. [Google Scholar] [CrossRef]
- Bucio, T.D.; Khokhar, A.Z.; Lacava, C.; Stankovic, S.; Mashanovich, G.Z.; Petropoulos, P.; Gardes, F.Y. Material and optical properties of low-temperature NH3-free PECVD SiNx layers for photonic applications. J. Phys. D-Appl. Phys. 2017, 50, 025106. [Google Scholar] [CrossRef]
- Huang, W.D.; Wang, X.H.; Sheng, M.; Xu, L.Q.; Stubhan, F.; Luo, L.; Feng, T.; Wang, X.; Zhang, F.M.; Zou, S.C. Low temperature PECVD SiNx films applied in OLED packaging. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2003, 98, 248–254. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Zhang, C.; Li, W.-L.; Liu, G.-H.; Wu, M.; Liu, J.-Q.; Zhang, J.-H. Global optimization of process parameters for low-temperature SiNx based on orthogonal experiments. Adv. Manuf. 2022, 11, 181–190. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liou, Y.-Y.; Chang, C.-P.; Huang, P.-C.; Huang, C.-Y.; Chen, K.-C.; Lin, Y.-J. Estimated approach development and experimental validation of residual stress-induced warpage under the SiNx PECVD coating process. Surf. Coat. Technol. 2022, 434, 128225. [Google Scholar] [CrossRef]
- Hsiao, S.-N.; Britun, N.; Nguyen, T.-T.-N.; Sekine, M.; Hori, M. Etching Mechanism Based on Hydrogen Fluoride Interactions with Hydrogenated SiN Films Using HF/H2 and CF4/H2 Plasmas. ACS Appl. Electron. Mater. 2023, 5, 6797–6804. [Google Scholar] [CrossRef]
- Yang, X.; Wu, M.; Jian, M.; Zhu, S.; Jiang, J.; Yang, L. Feasibility of molecular dynamics simulation for process parameter guidance of silicon nitride thin films by PECVD. Appl. Surf. Sci. 2024, 654, 159401. [Google Scholar] [CrossRef]
- Kovačević, G.; Pivac, B. Reactions in silicon–nitrogen plasma. Phys. Chem. Chem. Phys. 2017, 19, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Benoit, D.; Clément, L.; Morin, P.; Cooper, D.; Bertin, F. Characterization of Strain Induced by PECVD Silicon Nitride Films in Transistor Channels. AIP Conf. Proc. 2011, 1395, 90–94. [Google Scholar] [CrossRef]
- Kamataki, K.; Sasaki, Y.; Nagao, I.; Yamashita, D.; Okumura, T.; Yamashita, N.; Itagaki, N.; Koga, K.; Shiratani, M. Low-temperature fabrication of silicon nitride thin films from a SiH4+N2 gas mixture by controlling SiNx nanoparticle growth in multi-hollow remote plasma chemical vapor deposition. Mater. Sci. Semicond. Process. 2023, 164, 107613. [Google Scholar] [CrossRef]
- Sciammarella, C.A.; Boccaccio, A.; Lamberti, L.; Pappalettere, C.; Rizzo, A.; Signore, M.A.; Valerini, D. Measurements of Deflection and Residual Stress in Thin Films Utilizing Coherent Light Reflection/Projection Moir, Interferometry. Exp. Mech. 2013, 53, 977–987. [Google Scholar] [CrossRef]
- Mao, S.C.; Tao, S.H.; Xu, Y.L.; Sun, X.W.; Yu, M.B.; Lo, G.Q.; Kwong, D.L. Low propagation loss SiN optical waveguide prepared by optimal low-hydrogen module. Opt. Express 2008, 16, 20809–20816. [Google Scholar] [CrossRef]
- Jehanathan, N. Thermal Stability of Plasma Enhanced Chemical Vapor Deposition Silicon Nitride Thin Films. Master’s Thesis, The University of Western Australia, Perth, Australia, 2007. [Google Scholar]
- Jiang, L.; Tian, H.; Li, J.; Xiang, P.; Peng, Y.; Wang, T.; Hou, P.; Xiao, T.; Tan, X. The influence of NH3 flow rate on the microstructure and oxidation properties of a-Si-C-N:H films prepared by PECVD technology. Appl. Surf. Sci. 2020, 513, 145861. [Google Scholar] [CrossRef]
- Xu, X.; He, Q.; Fan, T.; Jiang, Y.; Huang, L.; Ao, T.; Ma, C. Hard and relaxed a-SiNxHy films prepared by PECVD: Structure analysis and formation mechanism. Appl. Surf. Sci. 2013, 264, 823–831. [Google Scholar] [CrossRef]
- Bakardjieva, V.; Beshkova, G.; Vitanov, P.; Alexieva, Z. Effect of rapid thermal annealving on the properties of µPCVD and PECVD silicon nitride thin films. J. Optoelectron. Adv. Mater. 2005, 7, 377–380. [Google Scholar]
- Zhou, J.; Huang, J.; Liao, J.; Guo, Y.; Zhao, Z.; Liang, H. Multi-field simulation and optimization of SiNx:H thin-film deposition by large-size tubular LF-PECVD. Sol. Energy 2021, 228, 575–585. [Google Scholar] [CrossRef]
- Benoit, D.; Morin, P.; Regolini, J. Determination of silicon nitride film chemical composition to study hydrogen desorption mechanisms. Thin Solid Film. 2011, 519, 6550–6553. [Google Scholar] [CrossRef]
- Mäckel, H.; Lüdemann, R. Detailed study of the composition of hydrogenated SiNx layers for high-quality silicon surface passivation. J. Appl. Phys. 2002, 92, 2602–2609. [Google Scholar] [CrossRef]
- Ghosh, S.; Bose, D.N. Plasma-enhanced chemical-vapor-deposited silicon-nitride films for interface studies. J. Mater. Sci. Mater. Electron. 1994, 5, 193–198. [Google Scholar] [CrossRef]
- Koutsoureli, M.; Birmpiliotis, D.; Papaioannou, G. A study of material stoichiometry on charging properties of SiNx films for potential application in RF MEMS capacitive switches. Microelectron. Reliab. 2020, 114, 113759. [Google Scholar] [CrossRef]
- Inukai, T.I.T.; Ono, K.I.O.K.I. Optical Characteristics of Amorphous Silicon Nitride Thin Films Prepared by Electron Cyclotron Resonance Plasma Chemical Vapor Deposition. Jpn. J. Appl. Phys. 1994, 33, 2593. [Google Scholar] [CrossRef]
- Jang, W.; Jeon, H.; Kang, C.; Song, H.; Park, J.; Kim, H.; Seo, H.; Leskela, M.; Jeon, H. Temperature dependence of silicon nitride deposited by remote plasma atomic layer deposition. Phys. Status Solidi 2015, 211, 2166–2171. [Google Scholar] [CrossRef]
- Kim, J.G.; Yu, J. Behavior of residual stress on CVD diamond films. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 1998, 57, 24–27. [Google Scholar] [CrossRef]
- Peng, K.-P.; Kuo, Y.-H.; Chang, L.-H.; Hsiao, C.-N.; Chung, T.-F.; George, T.; Lin, H.-C.; Li, P.-W. Silicon nitride engineering: Role of hydrogen-bonding in Ge quantum dot formation. Semicond. Sci. Technol. 2020, 35, 105018. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Murata, T.; Nishida, Y.; Nakai, T.; Uedono, A.; Hattori, N.; Matsuura, M.; Asai, K.; Yoneda, M. Local bonding structure of high-stress silicon nitride film modified by UV curing for strained silicon technology beyond 45 nm node SoC devices. Jpn. J. Appl. Phys. Part 1-Regul. Pap. Brief Commun. Rev. Pap. 2007, 46, 1984–1988. [Google Scholar] [CrossRef]
- Barcellona, M.; Samperi, O.; Russo, D.; Battaglia, A.; Fischer, D.; Fragala, M.E. Differences in HF Wet Etching Resistance of PECVD SiNx:H thin films. Mater. Chem. Phys. 2023, 306, 128023. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Sun, Q. Process Optimization of Amorphous Carbon Hard Mask in Advanced 3D-NAND Flash Memory Applications. Electronics 2021, 10, 1374. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Kim, B.; Seo, Y.H.; Yoon, N.-G.; Han, D. Impact of Duty Ratio-Controlled Ion Energy on Surface Roughness of Silicon Nitride Films Deposited Using a SiH4-NH3 Plasma. J. Nanosci. Nanotechnol. 2011, 11, 5744–5748. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Kar, J.P.; Lee, T.I.; Lee, D.; Choi, D.-K.; Cho, J.-H.; Myoung, J.-M. Investigation of optical and compositional properties of thin SiNx:H films with an enhanced growth rate by high frequency PECVD method. Vacuum 2011, 85, 1032–1036. [Google Scholar] [CrossRef]
- El Amrani, A.; Bekhtari, A.; Mahmoudi, B.; Lefgoum, A.; Menari, H. Experimental study of the effect of process parameters on plasma-enhanced chemical vapour deposition of silicon nitride film. Vacuum 2011, 86, 386–390. [Google Scholar] [CrossRef]





| Deposition Conditions | RF Power | Electrode Plate Spacing | Temperature | Pressure | SiH4:NH3 Gas Flow Ratio |
|---|---|---|---|---|---|
| (W) | (mm) | (°C) | (Torr) | ||
| Value | 1230 | 19.0 | 400 | 3.30 | 3.20 |
| Deposition Conditions | RF Power | Electrode Plate Spacing | Temperature | Pressure | SiH4:NH3 Gas Flow Ratio |
|---|---|---|---|---|---|
| (W) | (mm) | (°C) | (Torr) | ||
| Basic | 1230 | 19.0 | 400 | 3.30 | 3.20 |
| RF Power | 1082 | 19.0 | 400 | 3.30 | 3.20 |
| 1180 | 19.0 | 400 | 3.30 | 3.20 | |
| 1144 | 19.0 | 400 | 3.30 | 3.20 | |
| 1107 | 19.0 | 400 | 3.30 | 3.20 | |
| 1279 | 19.0 | 400 | 3.30 | 3.20 | |
| 1300 | 19.0 | 400 | 3.30 | 3.20 | |
| Electrode Plate Spacing | 1230 | 17.1 | 400 | 3.30 | 3.20 |
| 1230 | 17.7 | 400 | 3.30 | 3.20 | |
| 1230 | 18.2 | 400 | 3.30 | 3.20 | |
| 1230 | 19.8 | 400 | 3.30 | 3.20 | |
| 1230 | 20.3 | 400 | 3.30 | 3.20 | |
| 1230 | 20.9 | 400 | 3.30 | 3.20 | |
| Temperature | 1230 | 19.0 | 360 | 3.30 | 3.20 |
| 1230 | 19.0 | 372 | 3.30 | 3.20 | |
| 1230 | 19.0 | 384 | 3.30 | 3.20 | |
| 1230 | 19.0 | 400 | 3.30 | 3.20 | |
| 1230 | 19.0 | 416 | 3.30 | 3.20 | |
| 1230 | 19.0 | 428 | 3.30 | 3.20 | |
| 1230 | 19.0 | 440 | 3.30 | 3.20 | |
| Pressure | 1230 | 19.0 | 400 | 3.07 | 3.20 |
| 1230 | 19.0 | 400 | 3.17 | 3.20 | |
| SiH4:NH3 Gas Flow Ratio | 1230 | 19.0 | 400 | 3.30 | 2.98 |
| 1230 | 19.0 | 400 | 3.30 | 3.07 | |
| 1230 | 19.0 | 400 | 3.30 | 3.33 | |
| 1230 | 19.0 | 400 | 3.30 | 3.42 | |
| 1230 | 19.0 | 400 | 3.30 | 3.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Tang, Z.; Chen, L.; Li, B.; Wu, Q.; Sun, Y.; Zhou, D. Impact of H-Related Chemical Bonds on Physical Properties of SiNx:H Films Deposited via Plasma-Enhanced Chemical Vapor Deposition. Electronics 2024, 13, 2779. https://doi.org/10.3390/electronics13142779
Ning J, Tang Z, Chen L, Li B, Wu Q, Sun Y, Zhou D. Impact of H-Related Chemical Bonds on Physical Properties of SiNx:H Films Deposited via Plasma-Enhanced Chemical Vapor Deposition. Electronics. 2024; 13(14):2779. https://doi.org/10.3390/electronics13142779
Chicago/Turabian StyleNing, Jianping, Zhen Tang, Lunqian Chen, Bowen Li, Qidi Wu, Yue Sun, and Dayu Zhou. 2024. "Impact of H-Related Chemical Bonds on Physical Properties of SiNx:H Films Deposited via Plasma-Enhanced Chemical Vapor Deposition" Electronics 13, no. 14: 2779. https://doi.org/10.3390/electronics13142779





