Advances in Robotic Surgery: A Review of New Surgical Platforms
Abstract
:1. Introduction
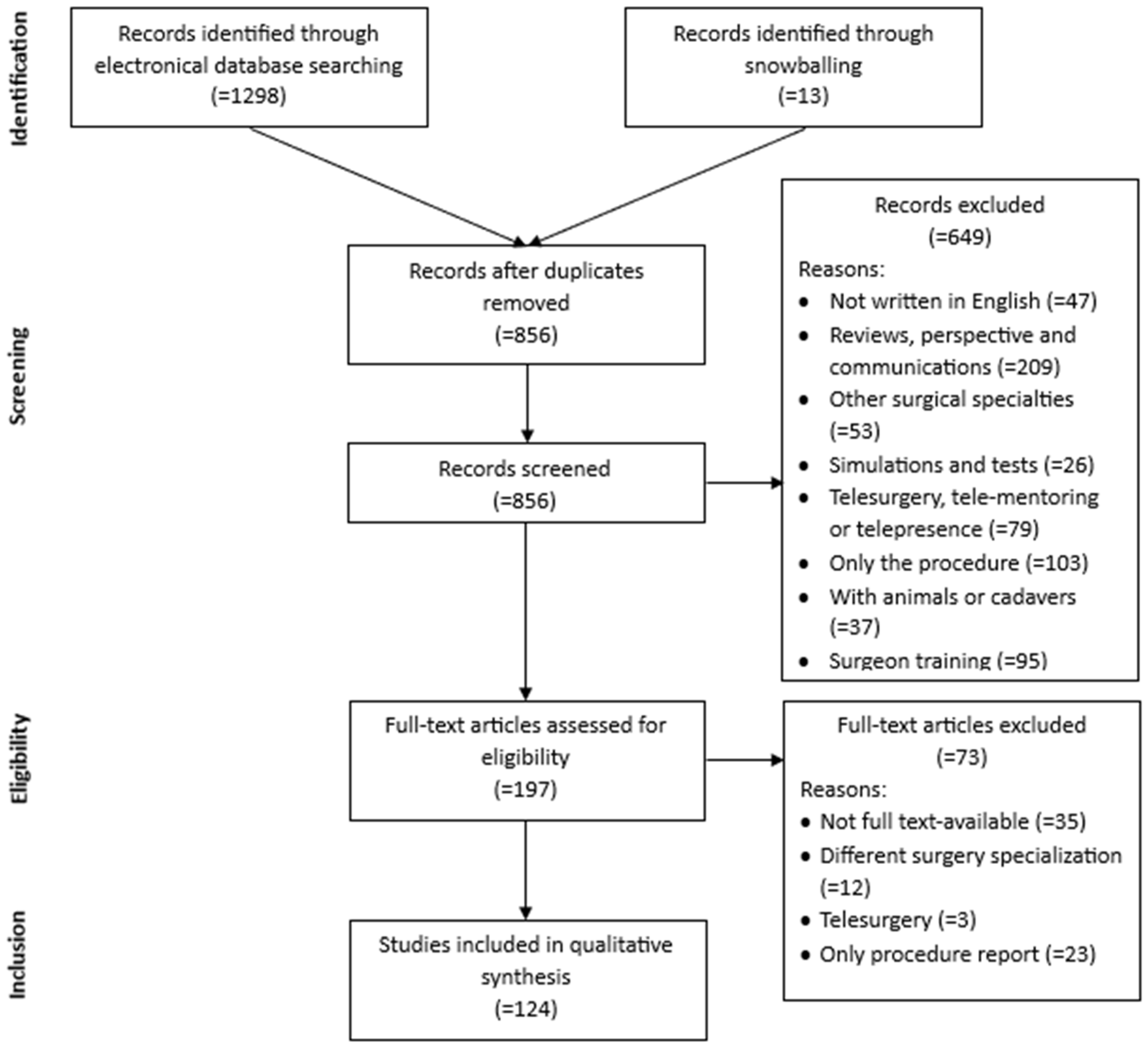
2. Materials and Methods
- Written in the English language.
- Full articles excluding reviews, perspectives, and communications.
- Full text available.
- Published from 2014 to June 2024.
- Any general surgery intervention performed in gynecology, urology, or general surgery.
- Any robotic system which has a console.
- Articles which contained simulations and tests.
- Papers centered on telesurgery, telementoring, or telepresence.
- Studies which report only the procedure.
- Papers related to studies on animals or cadavers.
- Articles concerned with surgeon training.
3. Results
3.1. Studies’ Characteristics
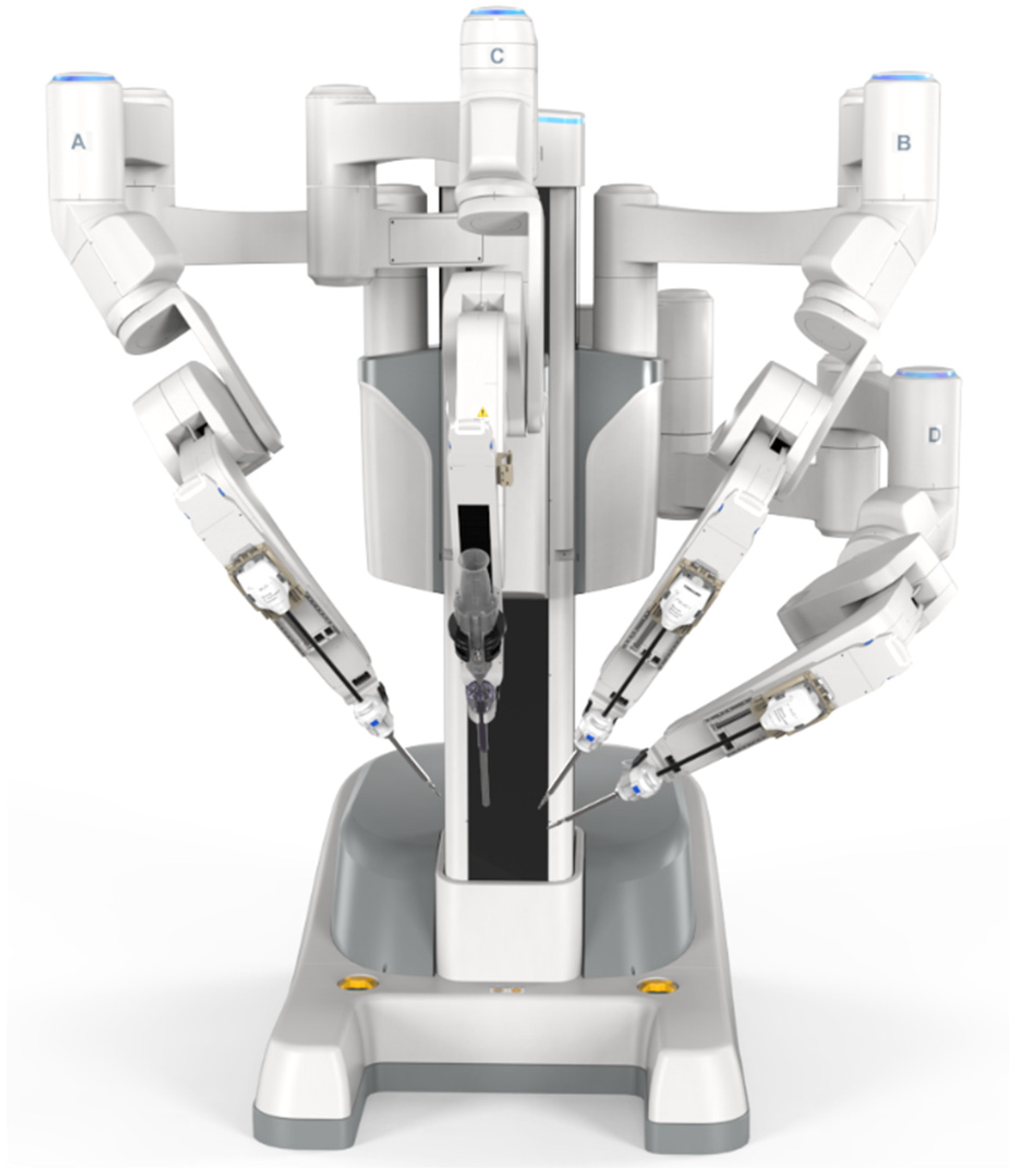
3.2. Surgical Robotic Platforms
3.2.1. Senhance®
3.2.2. Revo-i®
3.2.3. Micro Hand S
3.2.4. HugoTM
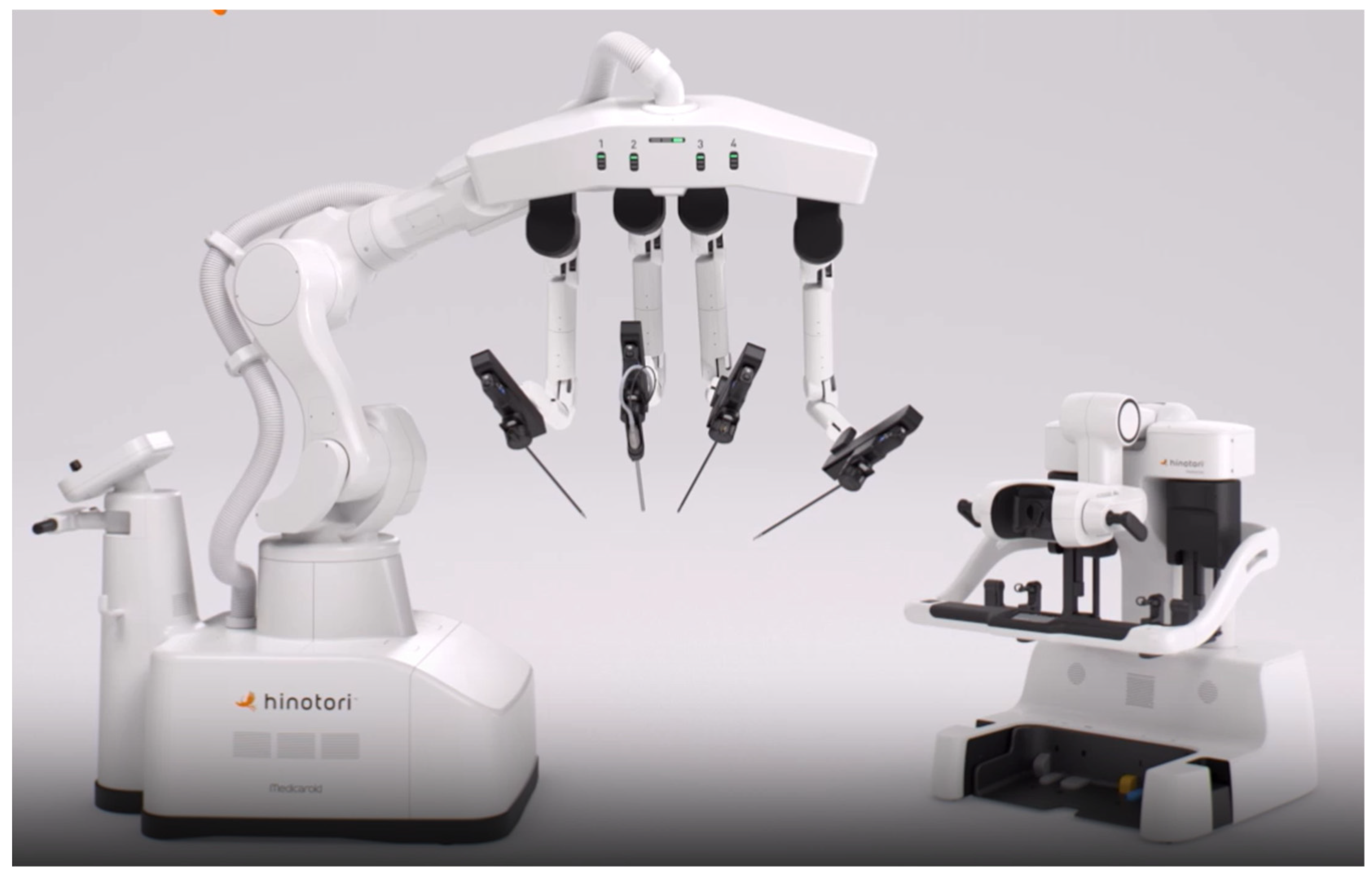
3.2.5. HinotoriTM
3.2.6. KangDuo
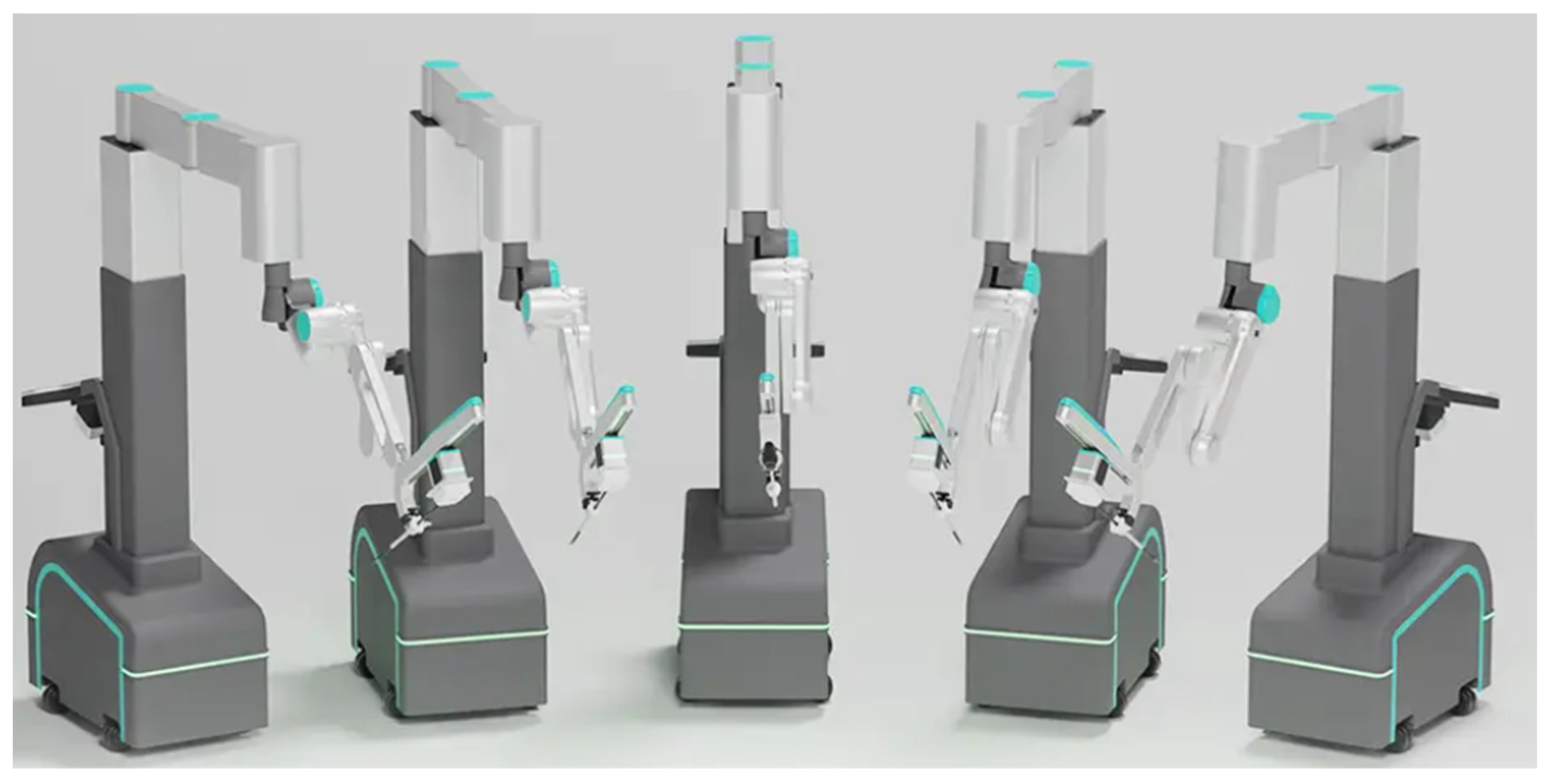
3.2.7. Versius®
3.2.8. Avatera
3.2.9. Dexter
3.2.10. Mantra
3.2.11. Toumai®
3.2.12. Technical Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Source | Year | Surgical Platform | Surgical Specialty | Country |
| Yi, B. et al. [19] | 2016 | Micro Hand S | General surgery | China |
| Ku, G. et al. [20] | 2020 | Revo-i | General surgery | Republic of Korea |
| Kang, I. et al. [21,22] | 2020 | Revo-i | General surgery | Republic of Korea |
| Kondo, H. et al. [22] | 2020 | Senhance | General surgery | Japan |
| Kanego, G. et al. [23] | 2021 | Senhance | Urology | Japan |
| Minagawa, Y. et al. [24] | 2021 | Senhance | General surgery | Japan |
| Sugita, H. et al. [25] | 2021 | Senhance | General surgery | Japan |
| Hirano, Y. et al. [26] | 2021 | Senhance | General surgery | Japan |
| Monterossi, G. et al. [27] | 2022 | Hugo | Gynecology | Italy |
| Böhlen, D. et al. [28] | 2023 | Dexter | Urology | Switzerland |
| Pavone, M. et al. [29] | 2023 | Hugo | Gynecology | Italy |
| Mottaran, A. et al. [30] | 2023 | Hugo | Urology | Belgium |
| Panico, G. et al. [31] | 2023 | Hugo | Urogynecology | Italy |
| Campagna, G. et al. [32] | 2023 | Hugo | Gynecology | Italy |
| Chen, S. et al. [33] | 2023 | KangDuo | Urology | China |
| Miura, R. et al. [34] | 2023 | Hinotori | General surgery | Japan |
| Miyo, M. et al. [35] | 2023 | Hinotori | General surgery | Japan |
| Alkatout, I. et al. [36] | 2024 | Dexter | Gynecology | Germany |
| Formisano, G. et al. [37] | 2024 | Hugo | General surgery | Italy |
| Komatsu, H. et al. [38] | 2024 | Hugo | Gynecology | Japan |
| Tomihara, K. et al. [39] | 2024 | Hinotori | General surgery | Japan |
| Hayashi, T. et al. [40] | 2024 | Hinotori | Urology | Japan |
| Spinelli, A. et al. [41] | 2017 | Senhance | General surgery | Italy |
| Stephan, D. et al. [42] | 2018 | Senhance | General surgery | Germany |
| Montlouis-Calixte, J. et al. [43] | 2019 | Senhance | Gynecology and general surgery | France |
| Melling, N. et al. [44] | 2019 | Senhance | General surgery | Germany |
| Yao, Y. et al. [45] | 2020 | Micro Hand S | General surgery | China |
| Li, J. et al. [46] | 2020 | Micro Hand S | General surgery | China |
| Samalavicius, N.E. et al. [47] | 2020 | Senhance | General Surgery, gynecology, and urology | Lithuania |
| Lim, J.H. et al. [48] | 2021 | Revo-I | General surgery | Republic of Korea |
| Fan, S. et al. [49] | 2021 | Kangduo | Urology | China |
| Puntamberkar, S.P. et al. [50] | 2021 | Versius | Gynecology | India |
| Collins, D. et al. [51] | 2021 | Versius | General surgery | UK |
| Kelkar, D. et al. [52] | 2021 | Versius | Gynecology and general surgery | India |
| Dixon, F. et al. [53] | 2021 | Versius | General surgery | UK |
| Kastelan, Z. et al. [49] | 2021 | Senhance | Urology | Croatia |
| Lin, C.C. at al. [55] | 2021 | Senhance | General surgery | Taiwan |
| Venckus, R. et al. [56] | 2021 | Senhance | Urology | Lithuania |
| Siaulys, R. et al. [57] | 2021 | Senhance | Gynecology | Lithuania |
| Bravi, C.A. et al. [58] | 2022 | Hugo | Urology | Belgium |
| Fan, S. et al. [59] | 2022 | Kangduo | Urology | China |
| Puntamberkar, S.P. et al. [60] | 2022 | Versius | General surgery | UK |
| Borse, M. et al. [61] | 2022 | Versius | Gynecology | India |
| Puntambekar, S. et al. [62] | 2022 | Versius | General surgery | India |
| Knežević, N. et al. [63] | 2022 | Senhance | Urology | Croatia |
| Sasaki, M. et al. [64] | 2022 | Senhance | General surgery | Japan |
| Samalavicius, N.E. et al. [65] | 2022 | Senhance | General surgery | Lithuania |
| Sassani, J.C. et al. [66] | 2022 | Senhance | Urology | USA |
| Samalavicius, N.E. et al. [67] | 2022 | Senhance | General surgery | Multiple (Europe: Germany, Belarus, Lithuania) |
| Kallidonis, P. et al. [68] | 2023 | Avatera | Urology | Grece |
| Hahnloser, D. et al. [69] | 2023 | Dexter | General surgery | Switzerland. |
| Monterossi, G. et al. [70] | 2023 | Hugo | Gynecology | Italy |
| Bravi, C.A. et al. [71] | 2023 | Hugo | Urology | Belgium |
| Gallioli, A. et al. [72] | 2023 | Hugo | Urology | Spain |
| Territo, A. et al. [73] | 2023 | Hugo | Urology | Spain |
| Bianchi, P.P. et al. [74] | 2023 | Hugo | General surgery | Italy |
| Paciotti, M. et al. [75] | 2023 | Hugo | Urology | Belgium |
| Marques-Monteiro, M. et al. [76] | 2023 | Hugo | Urology | Portugal |
| Ou, Y.C. et al. [77] | 2023 | Hugo | Urology | Taiwan |
| Elorrieta, V. et al. [78] | 2023 | Hugo | Urology | Chile |
| Belyaev, O. et al. [79] | 2023 | Hugo | General surgery | Germany |
| Alfano, C.G. et al. [80] | 2023 | Hugo | Urology | USA |
| Panico, G. et al. [81] | 2023 | Hugo | Urogynecology | Italy |
| Raffaelli, M. et al. [82] | 2023 | Hugo | General surgery | Italy |
| Xiong, S. et al. [83] | 2023 | Kangduo | Urology | China |
| Dong, J. et al. [84] | 2023 | Kangduo | General surgery | China |
| Kelkar, D.S. et al. [85] | 2023 | Versius | General surgery | UK |
| Wehrmann, S. et al. [86] | 2023 | Versius | General surgery | Germany |
| El Dahdad, J. et al. [87] | 2023 | Versius | General surgery | United Arab Emirates |
| Togami, S. et al. [88] | 2023 | Hinotori | Gynecological surgery | Japan |
| Motoyama, D. et al. [89] | 2023 | Hinotori | Urology | Japan |
| Hudolin, T. et al. [90] | 2023 | Senhance | Urology | Croatia |
| Sasaki, T. et al. [91] | 2023 | Senhance | General surgery | Japan |
| Thillou, D. et al. [92] | 2024 | Dexter | Urology | France |
| Mehrotra, M. et al. [93] | 2024 | Mantra | General surgery | India |
| Pokhrel, G. et al. [94] | 2024 | Toumai | Urology | China |
| Prata, F. et al. [95] | 2024 | Hugo | Urology | Italy |
| Dell’Oglio, P. et al. [96] | 2024 | Hugo | Urology | Italy |
| Totaro, A. et al. [97] | 2024 | Hugo | Urology | Italy |
| Takahara, K. et al. [98] | 2024 | Hugo | Urology | Japan |
| Prata, F. et al. [99] | 2024 | Hugo | Urology | Italy |
| Prata, F. et al. [142] | 2024 | Hugo | Urology | Italy |
| Caputo, D. et al. [100] | 2024 | Hugo | General surgery | Italy |
| Belyaev, O. et al. [101] | 2024 | Hugo | General surgery | Germany |
| Jebakumar, S.G.S. et al. [102] | 2024 | Hugo | General surgery | India |
| Caputo, D. et al. [103] | 2024 | Hugo | General surgery | Italy |
| Andrede, G.M. et al. [104] | 2024 | Hugo | Urology | Brazil |
| Salem, S.A. et al. [105] | 2024 | Hugo | General surgery | Israel |
| Gioè, A. et al. [106] | 2024 | Hugo | Gynecology | Italy |
| Quezada, N. et al. [107] | 2024 | Hugo | General surgery | Chile |
| Pavone, M. et al. [108] | 2024 | Hugo | Gynecology | Italy |
| Dibitetto, F. et al. [109] | 2024 | Versius | Urology | Italy |
| Meneghetti, I. et al. [110] | 2024 | Versius | Urology | Italy |
| De Maria, M. et al. [111] | 2024 | Versius | Urology | Italy |
| Inoue, S. et al. [112] | 2024 | Hinotori | General surgery | Japan |
| Kulis, T. et al. [113] | 2024 | Senhance | Urology | Lithuania, Croatia |
| Chang, K.D. et al. [114] | 2018 | Revo-I | Urology | Republic of Korea |
| Aggarwal, R. et al. [115] | 2020 | Senhance | General surgery | UK |
| Zeng, Y. et al. [116] | 2021 | Micro Hand S | General surgery | China |
| Wang, Y. et al. [118] | 2021 | Micro Hand S | General surgery | China |
| Jiang, J. et al. [117] | 2021 | Micro Hand S | General surgery | China |
| Wang, Y. et al. [120] | 2022 | Micro Hand S | General surgery | China |
| Lei, Y. et al. [119] | 2022 | Micro Hand S | General surgery | China |
| Kulis, T. at al. [121] | 2022 | Senhance | Urology | Croatia |
| Collà Ruvolo, C. et al. [122] | 2023 | Hugo | Gynecology | Belgium |
| Li, X. et al. [123] | 2023 | Kangduo | Urology | China |
| Motoyama, D. et al. [124] | 2023 | Hinotori | General surgery | Japan |
| Motoyama, D. et al. [125] | 2023 | Hinotori | Urology | Japan |
| Motoyama, D. et al. [126] | 2023 | Hinotori | Urology | Japan |
| Glass Clark, S. et al. [127] | 2023 | Senhance | Urology | USA |
| Kim, J.S. et al. [128] | 2024 | Revo-I | General surgery | Republic of Korea |
| Bravi, C.A. et al. [129] | 2024 | Hugo | Urology | Belgium |
| Balestrazzi, E. et al. [130] | 2024 | Hugo | Urology | Belgium |
| Brime Menendez, R. et al. [131] | 2024 | Hugo | Urology | Spain |
| Ou, H.C. et al. [132] | 2024 | Hugo | Urology | Taiwan |
| Prata, F. et al. [133] | 2024 | Hugo | Urology | Italy |
| Grandi, C. et al. [134] | 2024 | Hugo | Urology | Italy |
| Antonelli, A. et al. [135] | 2024 | Hugo | Urology | Italy |
| Shen, C. et al. [136] | 2024 | Kangduo | Urology | China |
| Sun, Z. et al. [137] | 2024 | Kangduo | General surgery | China |
| Liu, Y. et al. [138] | 2024 | Kangduo | General surgery | China |
| Halabi, M. et al. [139] | 2024 | Versius | General surgery | United Arab Emirates |
| Kohjimoto, Y. et al. [140] | 2024 | Hinotori | Urology | Japan |
| Lin, Y.C. et al. [141] | 2024 | Senhance | Urology | Taiwan |
References
- Zhang, X.; Ma, X.; Zhou, J.; Zhou, Q. Summary of medical robot technology development. In Proceedings of the 2018 IEEE International Conference on Mechatronics and Automation, ICMA 2018, Changchun, China, 5–8 August 2018; pp. 443–448. [Google Scholar] [CrossRef]
- Cepolina, F.; Razzoli, R.P. An introductory review of robotically assisted surgical systems. Int. J. Med. Robot. 2022, 18, e2409. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, S.; Ganguly, K.; Mandal, D. Advancements in robotic surgery: Innovations, challenges and future prospects. J. Robot. Surg. 2024, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in Robotic Surgery: A Comprehensive Overview of Current Utilizations and Upcoming Frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Clancy, O.; Grover, V.; Darzi, A. The evolution of robotic surgery: Surgical and anaesthetic aspects. Br. J. Anaesth. 2017, 119, i72–i84. [Google Scholar] [CrossRef] [PubMed]
- Kutana, S.; Bitner, D.P.; Addison, P.; Chung, P.J.; Talamini, M.A.; Filicori, F. Objective assessment of robotic surgical skills: Review of literature and future directions. Surg. Endosc. 2022, 36, 3698–3707. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Sikander, S.; Kulkarni, P. Recent advances in robot-assisted surgical systems. Biomed. Eng. Adv. 2023, 6, 100109. [Google Scholar] [CrossRef]
- Almujalhem, A.; Rha, K.H. Surgical robotic systems: What we have now? A urological perspective. BJUI Compass 2020, 1, 152–159. [Google Scholar] [CrossRef]
- Rao, R.; Nayyar, R.; Panda, S.; Hemal, A.K. Surgical techniques: Robotic bladder diverticulectomy with the da Vinci-S surgical system. J. Robot. Surg. 2007, 1, 217–220. [Google Scholar] [CrossRef]
- Guthart, G.J.P. Morgan Healthcare Conference 2019. Available online: https://investor.intuitivesurgical.com/static-files/ae564df0-fff1-42d9-a85f-5ef54ba22419 (accessed on 7 September 2024).
- Trute, R.J.; Zapico, C.S.; Christou, A.; Layeghi, D.; Craig, S.; Erden, M.S. Development of a Robotic Surgery Training System. Front. Robot. AI 2022, 8, 773830. [Google Scholar] [CrossRef]
- Rivero-Moreno, Y.; Echevarria, S.; Vidal-Valderrama, C.; Stefano-Pianetti, L.; Cordova-Guilarte, J.; Navarro-Gonzalez, J.; Acevedo-Rodríguez, J.; Dorado-Avila, G.; Osorio-Romero, L.; Chavez-Campos, C.; et al. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus 2023, 15, e42370. [Google Scholar] [CrossRef]
- Fairag, M.; Almahdi, R.H.; Siddiqi, A.; Alharthi, F.K.; Alqurashi, B.S.; Alzahrani, N.G.; Alsulami, A.; Alshehri, R. Robotic Revolution in Surgery: Diverse Applications Across Specialties and Future Prospects Review Article. Cureus 2024, 16, e52148. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Ng, J.C.; Awuah, W.A.; Huang, H.; Kalmanovich, J.; Agrawal, A.; Abdul-Rahman, T.; Hasan, M.M.; Sikora, V.; Isik, A. Embracing robotic surgery in low- and middle-income countries: Potential benefits, challenges, and scope in the future. Ann. Med. Surg. 2022, 84, 104803. [Google Scholar] [CrossRef] [PubMed]
- Simorov, A.; Otte, R.S.; Kopietz, C.M.; Oleynikov, D. Review of surgical robotics user interface: What is the best way to control robotic surgery? Surg. Endosc. 2012, 26, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Guthart, P.G. JPMorgan Healthcare Conference 2023. Available online: https://isrg.intuitive.com/static-files/6683d2bb-75e2-4fa0-b0cd-463ead7c30a4 (accessed on 7 September 2024).
- Hughes, T.; Rai, B.; Madaan, S.; Chedgy, E.; Somani, B. The Availability, Cost, Limitations, Learning Curve and Future of Robotic Systems in Urology and Prostate Cancer Surgery. J. Clin. Med. 2023, 12, 2268. [Google Scholar] [CrossRef]
- Rassweiler, J.J.; Autorino, R.; Klein, J.; Mottrie, A.; Goezen, A.S.; Stolzenburg, J.; Rha, K.H.; Schurr, M.; Kaouk, J.; Patel, V.; et al. Future of robotic surgery in urology. BJU Int. 2017, 120, 822–841. [Google Scholar] [CrossRef]
- Yi, B.; Wang, G.; Li, J.; Jiang, J.; Son, Z.; Su, H.; Zhu, S. The first clinical use of domestically produced Chinese minimally invasive surgical robot system “Micro Hand S”. Surg. Endosc. 2016, 30, 2649–2655. [Google Scholar] [CrossRef]
- Ku, G.; Kang, I.; Lee, W.J.; Kang, C.M. Revo-i assisted robotic central pancreatectomy. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 547–550. [Google Scholar] [CrossRef]
- Kang, I.; Hwang, H.K.; Lee, W.J.; Kang, C.M. First experience of pancreaticoduodenectomy using Revo-i in a patient with insulinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 104–108. [Google Scholar] [CrossRef]
- Kondo, H.; Yamaguchi, S.; Hirano, Y.; Ishii, T.; Obara, N.; Wang, L.; Asari, M.; Kato, T.; Takayama, T.; Sugita, H.; et al. A first case of ileocecal resection using a Senhance Surgical System in Japan. Surg. Case Rep. 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Kaneko, G.; Shirotake, S.; Oyama, M.; Koyama, I. Initial experience of laparoscopic radical nephrectomy using the Senhance® robotic system for renal cell carcinoma. Int. Cancer Conf. J. 2021, 10, 228–232. [Google Scholar] [CrossRef]
- Minagawa, Y.; Hirano, Y.; Kataoka, A.; Shimamura, S.; Kataoka, M.; Asari, M.; Fujii, T.; Ishikawa, S.; Ishii, T.; Sato, H.; et al. The first single-incision plus one-port transverse colon resection using Senhance Digital Laparoscopy System: A case report. Surg. Case Rep. 2021, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sugita, H.; Sakuramoto, S.; Aoyama, J.; Ito, S.; Oya, S.; Watanabe, K.; Fujiwara, N.; Kondo, H.; Miyawaki, Y.; Hirano, Y.; et al. First experience using the Senhance surgical system in laparoscopic local gastrectomy for gastrointestinal stromal tumor. Asian J. Endosc. Surg. 2021, 14, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Kondo, H.; Miyawaki, Y.; Sugita, H.; Sakuramoto, S.; Yamaguchi, S. Single-incision plus two-port robotic surgery for sigmoid colon cancer using the Senhance robotic system. Asian J. Endosc. Surg. 2021, 14, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Monterossi, G.; Anchora, L.P.; Alletti, S.G.; Fagotti, A.; Fanfani, F.; Scambia, G. The first European gynaecological procedure with the new surgical robot Hugo™ RAS. A total hysterectomy and salpingo-oophorectomy in a woman affected by BRCA-1 mutation. Facts Views Vis. Obgyn 2022, 14, 91–94. [Google Scholar] [CrossRef]
- Böhlen, D.; Gerber, R. First Ever Radical Prostatectomy Performed with the New Dexter Robotic System™. Eur. Urol. 2023, 83, 479–480. [Google Scholar] [CrossRef]
- Pavone, M.; Goglia, M.; Campolo, F.; Scambia, G.; Ianieri, M.M. En-block butterfly excision of posterior compartment deep endometrio-sis: The first experience with the new surgical robot HugoTM RAS. Facts Views Vis. Obgyn 2023, 15, 359. [Google Scholar] [CrossRef]
- Mottaran, A.; Paciotti, M.; Bravi, C.A.; Sarchi, L.; Nocera, L.; Piro, A.; Farinha, R.; DE Backer, P.; Piazza, P.; Pauwaert, K.; et al. Robot-assisted simple prostatectomy with the novel HUGO™ RAS System: Feasibility, setting, and perioperative outcomes. Minerva Urol. Nephrol. 2023, 75, 235–239. [Google Scholar] [CrossRef]
- Panico, G.; Campagna, G.; Caramazza, D.; Vacca, L.; Mastrovito, S.; Ercoli, A.; Scambia, G. HUGO(TM) RAS System in urogynaecology: The first nerve sparing Sacral Colpopexy for Pelvic Organ Pro-lapse. Facts Views Vis. Obgyn. 2023, 15, 83–87. [Google Scholar] [CrossRef]
- Campagna, G.; Panico, G.; Vacca, L.; Caramazza, D.; Mastrovito, S.; Lombisani, A.; Ercoli, A.; Scambia, G. Robotic sacrocolpopexy plus ventral rectopexy as combined treatment for multicompartment pelvic organ prolapse using the new Hugo RAS system. Tech. Coloproctol. 2023, 27, 499–500. [Google Scholar] [CrossRef]
- Chen, S.; Fan, S.; Guan, H.; Yang, K.; Li, Z.; Xiong, S.; Wang, X.; Li, Z.; Shen, C.; Zhou, L.; et al. The application of internal suspension technique in retroperitoneal robot-assisted laparoscopic partial nephrectomy with a new robotic system KangDuo Surgical Robot-01: Initial experience. Asian J. Urol. 2023, 10, 482–487. [Google Scholar] [CrossRef]
- Miura, R.; Okuya, K.; Akizuki, E.; Miyo, M.; Noda, A.; Ishii, M.; Ichihara, M.; Korai, T.; Toyota, M.; Ito, T.; et al. World-first report of low anterior resection for rectal cancer with the hinotori™ Surgical Robot System: A case report. Surg. Case Rep. 2023, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Miyo, M.; Okita, K.; Okuya, K.; Ito, T.; Akizuki, E.; Ogawa, T.; Ishii, M.; Miura, R.; Ichihara, M.; Korai, T.; et al. Right hemicolectomy for ascending colon cancer using the hinotori surgical robot system: The first ever case report for colon cancer. Asian J. Endosc. Surg. 2023, 16, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Becker, T.; Nuhn, P.; Pochhammer, J.; Peters, G.; Donald, K.; Mettler, L.; Ackermann, J. The first robotic-assisted hysterectomy below the bikini line with the Dexter robotic system™. Facts Views Vis. Obgyn 2024, 16, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Formisano, G.; Ferraro, L.; Salaj, A.; Bianchi, P.P. First report of robotic retromuscular incisional hernia repair with Hugo Ras™ surgical system. Updat. Surg. 2024, 76, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Wada, I.; Harada, T.; Taniguchi, F. First report of robotic-assisted total hysterectomy using the Hugo™ RAS system. Updat. Surg. 2023, 76, 315–318. [Google Scholar] [CrossRef]
- Tomihara, K.; Ide, T.; Ito, K.; Tanaka, T.; Noshiro, H. Robotic spleen-preserving distal pancreatectomy using the first domestic surgical robot platform (the hinotori™ Surgical Robot System): A case report. Surg. Case Rep. 2024, 10, 22. [Google Scholar] [CrossRef]
- Hayashi, T.; Kitano, H.; Hieda, K.; Hinata, N. First case report of robot-assisted radical cystectomy and intracorporeal urinary diversion using the hinotori Surgical Robot System. Transl. Cancer Res. 2024, 13, 471–479. [Google Scholar] [CrossRef]
- Spinelli, A.; David, G.; Gidaro, S.; Carvello, M.; Sacchi, M.; Montorsi, M.; Montroni, I. First experience in colorectal surgery with a new robotic platform with haptic feedback. Color. Dis. 2018, 20, 228–235. [Google Scholar] [CrossRef]
- Stephan, D.; Sälzer, H.; Willeke, F. First Experiences with the New Senhance® Telerobotic System in Visceral Surgery. Visc. Med. 2018, 34, 31–36. [Google Scholar] [CrossRef]
- Montlouis-Calixte, J.; Ripamonti, B.; Barabino, G.; Corsini, T.; Chauleur, C. Senhance 3-mm robot-assisted surgery: Experience on first 14 patients in France. J. Robot. Surg. 2019, 13, 643–647. [Google Scholar] [CrossRef]
- Melling, N.; Barr, J.; Schmitz, R.; Polonski, A.; Miro, J.; Ghadban, T.; Wodack, K.; Izbicki, J.; Zani, S.; Perez, D. Robotic cholecystectomy: First experience with the new Senhance robotic system. J. Robot. Surg. 2019, 13, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Y.; Li, Z.; Yi, B.; Wang, G.; Zhu, S. Chinese surgical robot micro hand S: A consecutive case series in general surgery. Int. J. Surg. 2020, 75, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, S.; Juan, J.; Yi, B. Preliminary exploration of robotic complete mesocolic excision for colon cancer with the domestically produced Chinese minimally invasive Micro Hand S surgical robot system. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Samalavicius, N.E.; Janusonis, V.; Siaulys, R.; Jasėnas, M.; Deduchovas, O.; Venckus, R.; Ezerskiene, V.; Paskeviciute, R.; Klimaviciute, G. Robotic surgery using Senhance® robotic platform: Single center experience with first 100 cases. J. Robot. Surg. 2020, 14, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, W.J.; Choi, S.H.; Kang, C.M. Cholecystectomy using the Revo-i robotic surgical system from Korea: The first clinical study. Updat. Surg. 2020, 73, 1029–1035. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Wang, J.; Xiong, S.; Dai, X.; Chen, X.; Li, Z.; Han, G.; Zhu, J.; Hao, H.; et al. Robot-Assisted Radical Prostatectomy Using the KangDuo Surgical Robot-01 System: A Prospective, Single-Center, Single-Arm Clinical Study. J. Urol. 2022, 208, 119–127. [Google Scholar] [CrossRef]
- Puntambekar, S.P.; Goel, A.; Chandak, S.; Chitale, M.; Hivre, M.; Chahal, H.; Rajesh, K.N.; Manerikar, K. Feasibility of robotic radical hysterectomy (RRH) with a new robotic system. Experience at Galaxy Care Laparoscopy Institute. J. Robot. Surg. 2021, 15, 451–456. [Google Scholar] [CrossRef]
- Collins, D.; Paterson, H.M.; Skipworth, R.J.E.; Speake, D. Implementation of the Versius robotic surgical system for colorectal cancer surgery: First clinical experience. Color. Dis. 2021, 23, 1233–1238. [Google Scholar] [CrossRef]
- Kelkar, D.; Borse, M.A.; Godbole, G.P.; Kurlekar, U.; Slack, M. Interim safety analysis of the first-in-human clinical trial of the Versius surgical system, a new robot-assisted device for use in minimal access surgery. Surg. Endosc. 2021, 35, 5193–5202. [Google Scholar] [CrossRef]
- Dixon, F.; O’hara, R.; Ghuman, N.; Strachan, J.; Khanna, A.; Keeler, B.D. Major colorectal resection is feasible using a new robotic surgical platform: The first report of a case series. Tech. Coloproctol. 2021, 25, 285–289. [Google Scholar] [CrossRef]
- Kastelan, Z.; Hudolin, T.; Kulis, T.; Knezevic, N.; Penezic, L.; Maric, M.; Zekulic, T. Upper urinary tract surgery and radical prostatectomy with Senhance® robotic system: Single center experience—First 100 cases. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2269. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Huang, S.; Lin, H.; Chang, S.; Chen, W.; Jiang, J. An early experience with the Senhance surgical robotic system in colorectal surgery: A single-institute study. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2206. [Google Scholar] [CrossRef] [PubMed]
- Venckus, R.; Jasenas, M.; Telksnys, T.; Venckus, M.; Janusonis, V.; Dulskas, A.; Samalavicius, N.E. Robotic-assisted radical prostatectomy with the Senhance® robotic platform: Single center experience. World J. Urol. 2021, 39, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
- Siaulys, R.; Klimasauskiene, V.; Janusonis, V.; Ezerskiene, V.; Dulskas, A.; Samalavicius, N.E. Robotic gynaecological surgery using Senhance® robotic platform: Single centre experience with 100 cases. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102031. [Google Scholar] [CrossRef] [PubMed]
- Bravi, C.A.; Paciotti, M.; Sarchi, L.; Mottaran, A.; Nocera, L.; Farinha, R.; De Backer, P.; Vinckier, M.-H.; De Naeyer, G.; D’Hondt, F.; et al. Robot-assisted Radical Prostatectomy with the Novel Hugo Robotic System: Initial Experience and Optimal Surgical Set-up at a Tertiary Referral Robotic Center. Eur. Urol. 2022, 82, 233–237. [Google Scholar] [CrossRef]
- Fan, S.; Dai, X.; Yang, K.; Xiong, S.; Xiong, G.; Li, Z.; Cheng, S.; Li, X.; Meng, C.; Guan, H.; et al. Robot-assisted pyeloplasty using a new robotic system, the KangDuo-Surgical Robot-01: A prospective, single-centre, single-arm clinical study. BJU Int. 2021, 128, 162–165. [Google Scholar] [CrossRef]
- Puntambekar, S.P.; Rajesh, K.N.; Goel, A.; Hivre, M.; Bharambe, S.; Chitale, M.; Panse, M. Colorectal cancer surgery: By Cambridge Medical Robotics Versius Surgical Robot System—A single-institution study. Our experience. J. Robot. Surg. 2022, 16, 587–596. [Google Scholar] [CrossRef]
- Borse, M.; Godbole, G.; Kelkar, D.; Bahulikar, M.; Dinneen, E.; Slack, M. Early evaluation of a next-generation surgical system in robot-assisted total laparoscopic hysterectomy: A prospective clinical cohort study. Acta Obstet. Gynecol. Scand. 2022, 101, 978–986. [Google Scholar] [CrossRef]
- Puntambekar, S.; Bharambe, S.; Pawar, S.; Chitale, M.; Panse, M. Feasibility of transthoracic esophagectomy with a next-generation surgical robot. Sci. Rep. 2022, 12, 17925. [Google Scholar] [CrossRef]
- Knežević, N.; Penezić, L.; Kuliš, T.; Zekulić, T.; Saić, H.; Hudolin, T.; Kaštelan, Ž. Senhance robot-assisted adrenalectomy: A case series. Croat. Med. J. 2022, 63, 197–201. [Google Scholar] [CrossRef]
- Sasaki, M.; Hirano, Y.; Yonezawa, H.; Shimamura, S.; Kataoka, A.; Fujii, T.; Okazaki, N.; Ishikawa, S.; Ishii, T.; Deguchi, K.; et al. Short-term results of robot-assisted colorectal cancer surgery using Senhance Digital Laparoscopy System. Asian J. Endosc. Surg. 2022, 15, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Samalavicius, N.E.; Dulskas, A.; Janusonis, V.; Klimasauskiene, V.; Eismontas, V.; Deduchovas, O.; Janusonis, T.; Markelis, R.; Smolskas, E. Robotic colorectal surgery using the Senhance® robotic system: A single center experience. Tech. Coloproctol. 2022, 26, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Sassani, J.C.; Clark, S.G.; McGough, C.E.; Shepherd, J.P.; Bonidie, M. Sacrocolpopexy experience with a novel robotic surgical platform. Int. Urogynecol. J. 2022, 33, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Samalavicius, N.E.; Dulskas, A.; Sirvys, A.; Klimasauskiene, V.; Janusonis, V.; Janusonis, T.; Eismontas, V.; Deduchovas, O.; Stephan, D.; Darwich, I.; et al. Inguinal hernia TAPP repair using Senhance® robotic platform: First multicenter report from the TRUST registry. Hernia 2022, 26, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Kallidonis, P.; Tatanis, V.; Peteinaris, A.; Katsakiori, P.; Gkeka, K.; Faitatziadis, S.; Vagionis, A.; Vrettos, T.; Stolzenburg, J.-U.; Liatsikos, E. Robot-assisted pyeloplasty for ureteropelvic junction obstruction: Initial experience with the novel avatera system. World J. Urol. 2023, 41, 3155–3160. [Google Scholar] [CrossRef]
- Hahnloser, D.; Rrupa, D.; Grass, F. Feasibility of on-demand robotics in colorectal surgery: First cases. Surg. Endosc. 2023, 37, 8594–8600. [Google Scholar] [CrossRef]
- Monterossi, G.; Anchora, L.P.; Oliva, R.; Fagotti, A.; Fanfani, F.; Costantini, B.; Naldini, A.; Giannarelli, D.; Scambia, G. The new surgical robot HugoTM RAS for total hysterectomy: A pilot study. Facts. Views Vis. Obgyn. 2023, 15, 331–337. [Google Scholar] [CrossRef]
- Bravi, C.A.; Paciotti, M.; Balestrazzi, E.; Piro, A.; Piramide, F.; Peraire, M.; Sarchi, L.; Mottaran, A.; Nocera, L.; De Backer, P.; et al. Outcomes of Robot-assisted Radical Prostatectomy with the Hugo RAS Surgical System: Initial Experience at a High-volume Robotic Center. Eur. Urol. Focus 2023, 9, 642–644. [Google Scholar] [CrossRef]
- Gallioli, A.; Uleri, A.; Gaya, J.M.; Territo, A.; Aumatell, J.; Verri, P.; Basile, G.; Fontanet, S.; Tedde, A.; Diana, P.; et al. Initial experience of robot-assisted partial nephrectomy with Hugo™ RAS system: Implications for surgical setting. World J. Urol. 2023, 41, 1085–1091. [Google Scholar] [CrossRef]
- Territo, A.; Uleri, A.; Gallioli, A.; Gaya, J.M.; Verri, P.; Basile, G.; Farré, A.; Bravo, A.; Tedde, A.; Faba, R.; et al. Robot-assisted oncologic pelvic surgery with Hugo™ robot-assisted surgery system: A single-center experience. Asian J. Urol. 2023, 10, 461–466. [Google Scholar] [CrossRef]
- Bianchi, P.P.; Salaj, A.; Rocco, B.; Formisano, G. First worldwide report on Hugo RAS™ surgical platform in right and left colectomy. Updat. Surg. 2023, 75, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, M.; Bravi, C.A.; Mottaran, A.; Nocera, L.; Sarchi, L.; Piro, A.; Farinha, R.; Lores, M.P.; Balestrazzi, E.; Piramide, F.; et al. Nerve-sparing robot-assisted radical prostatectomy with the HUGO™ robot-assisted surgery system using the ‘Aalst technique’. BJU Int. 2023, 132, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Marques-Monteiro, M.; Teixeira, B.; Mendes, G.; Rocha, A.; Madanelo, M.; Mesquita, S.; Vital, J.; Vinagre, N.; Magalhães, M.; Oliveira, B.; et al. Extraperitoneal robot-assisted radical prostatectomy with the Hugo™ RAS system: Initial experience of a tertiary center with a high background in extraperitoneal laparoscopy surgery. World J. Urol. 2023, 41, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Ou, H.; Juan, Y.; Narasimhan, R.; Mottrie, A.; Weng, W.; Huang, L.; Lin, Y.; Hsu, C.; Yang, C.; et al. Robot-assisted radical prostatectomy using hugo RAS system: The pioneer experience in Taiwan and Northeast Asia. Int. J. Med. Robot. Comput. Assist. Surg. 2024, 20, e2577. [Google Scholar] [CrossRef] [PubMed]
- Elorrieta, V.; Villena, J.; Kompatzki, Á.; Velasco, A.; Salvadó, J.A. ROBOT Assisted Laparoscopic Surgeries For Nononcological Urologic Disease: Initial Experience With Hugo Ras System. Urology 2023, 174, 118–125. [Google Scholar] [CrossRef]
- Belyaev, O.; Fahlbusch, T.; Slobodkin, I.; Uhl, W. Safety and Feasibility of Cholecystectomy with the HugoTM RAS: Proof of Setup Guides and First-In-Human German Experience. Visc. Med. 2023, 39, 76–86. [Google Scholar] [CrossRef]
- Alfano, C.G.; Moschovas, M.C.; Montagne, V.; Soto, I.; Porter, J.; Patel, V.; Ureña, R.; Bodden, E. Implementation and outcomes of HugoTM RAS System in robotic-assisted radical prostatectomy. Int. Braz. J. Urol. 2023, 49, 211–220. [Google Scholar] [CrossRef]
- Panico, G.; Vacca, L.; Campagna, G.; Caramazza, D.; Mastrovito, S.; Lombisani, A.; Ercoli, A.; Scambia, G. The first 60 cases of robotic sacrocolpopexy with the novel HUGO RAS system: Feasibility, setting and perioperative outcomes. Front. Surg. 2023, 10, 1181824. [Google Scholar] [CrossRef]
- Raffaelli, M.; Gallucci, P.; Voloudakis, N.; Pennestrì, F.; De Cicco, R.; Arcuri, G.; De Crea, C.; Bellantone, R. The new robotic platform Hugo™ RAS for lateral transabdominal adrenalectomy: A first world report of a series of five cases. Updat. Surg. 2023, 75, 217–225. [Google Scholar] [CrossRef]
- Xiong, S.; Fan, S.; Chen, S.; Wang, X.; Han, G.; Li, Z.; Zuo, W.; Li, Z.; Yang, K.; Zhang, Z.; et al. Robotic urologic surgery using the KangDuo-Surgical Robot-01 system: A single-center prospective analysis. Chin. Med. J. 2023, 136, 2960–2966. [Google Scholar] [CrossRef]
- Dong, J.; Ji, R.; Liu, G.; Zhou, J.; Wang, H.; Xu, W.; Ji, Z.; Cui, L. Feasibility, safety and effectiveness of robot-assisted retroperitoneal partial adrenalectomy with a new robotic surgical system: A prospective clinical study. Front. Surg. 2023, 10, 1071321. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, D.S.F.; Kurlekar, U.; Stevens, L.M.; Wagholikar, G.D.M.; Slack, M.F. An Early Prospective Clinical Study to Evaluate the Safety and Performance of the Versius Surgical System in Robot-Assisted Cholecystectomy. Ann. Surg. 2023, 277, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wehrmann, S.; Tischendorf, K.; Mehlhorn, T.; Lorenz, A.; Gündel, M.; Rudolph, H.; Mirow, L. Clinical implementation of the Versius robotic surgical system in visceral surgery-A single centre experience and review of the first 175 patients. Surg. Endosc. 2023, 37, 528–534. [Google Scholar] [CrossRef] [PubMed]
- El Dahdah, J.; Halabi, M.; Kamal, J.; Zenilman, M.E.; Moussa, H. Initial experience with a novel robotic surgical system in abdominal surgery. J. Robot. Surg. 2023, 17, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Togami, S.; Higashi, T.; Tokudome, A.; Fukuda, M.; Mizuno, M.; Yanazume, S.; Kobayashi, H. The first report of surgery for gynecological diseases using the hinotori™ surgical robot system. Ultrasound Med. Biol. 2023, 53, 1034–1037. [Google Scholar] [CrossRef]
- Nakayama, A.; Izumi, K.; Ikezoe, E.; Inoue, M.; Tsujioka, H.; Nirazuka, A.; Hasegawa, K.; Osaka, A.; Yasuda, Y.; Fukuda, Y.; et al. Robot-assisted radical prostatectomy using the novel hinotoriTM surgical robot system: Initial experience and operation learning curve at a single institution. Transl. Cancer Res. 2024, 13, 57–64. [Google Scholar] [CrossRef]
- Hudolin, T.; Kuliš, T.; Penezić, L.; Zekulić, T.; Knežević, N.; Čikić, B.; Jurić, I.; Anđelić, J.; Saić, H.; Kaštelan, Ž. Senhance robotic radical prostatectomy: A single-centre, 3-year experience. Int. J. Med. Robot. Comput. Assist. Surg. 2023, 19, e2549. [Google Scholar] [CrossRef]
- Sasaki, T.; Tomohisa, F.; Nishimura, M.; Arifuku, H.; Ono, T.; Noda, A.; Otsubo, T. Initial 30 cholecystectomy procedures performed with the Senhance digital laparoscopy system. Asian J. Endosc. Surg. 2023, 16, 225–232. [Google Scholar] [CrossRef]
- Thillou, D.; Robin, H.; Ricolleau, C.; Benali, N.A.; Forgues, A.; Emeriau, D.; Mignot, H.; Hugues, G. Robot-assisted Radical Prostatectomy with the Dexter Robotic System: Initial Experience and Insights into On-demand Robotics. Eur. Urol. 2024, 85, 185–189. [Google Scholar] [CrossRef]
- Mehrotra, M.; Kumar, C.G. Initial experience of SSI Mantra robot-assisted Transabdominal pre-peritoneal repair of primary ventral hernias. J. Minimal Access Surg. 2024, 10, 4103. [Google Scholar] [CrossRef]
- Pokhrel, G.; Zheng, H.; Tao, J.; Cui, J.; Fan, Y.; Li, Z.; Dong, B.; Yu, S.; Zhang, X. Assessing the Feasibility and Safety of the Toumaiò Robotic System in Urologic Sur-Gery: Initial Experience. J. Endourol. 2024, 38, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Prata, F.; Iannuzzi, A.; Tedesco, F.; Ragusa, A.; Civitella, A.; Pira, M.; Fantozzi, M.; Sica, L.; Scarpa, R.M.; Papalia, R. Surgical Outcomes of Hugo™ RAS Robot-Assisted Partial Nephrectomy for Cystic Renal Masses: Technique and Initial Experience. J. Clin. Med. 2024, 13, 3595. [Google Scholar] [CrossRef] [PubMed]
- Dell’oglio, P.; Chierigo, F.; Cellini, V.; Tappero, S.; Olivero, A.; Maltzman, O.; Caviglia, A.; Piccione, A.; Buratto, C.; Barbieri, M.; et al. Retzius-sparing robot-assisted radical prostatectomy with the Hugo™ robot-assisted surgery system: Feasibility, operative setup and surgical outcomes. BJU Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Scarciglia, E.; Marino, F.; Campetella, M.; Gandi, C.; Ragonese, M.; Bientinesi, R.; Palermo, G.; Bizzarri, F.P.; Cretì, A.; et al. Robot-Assisted Radical Prostatectomy Performed with the Novel Surgical Robotic Platform Hugo™ RAS: Monocentric First Series of 132 Cases Reporting Surgical, and Early Functional and Oncological Outcomes at a Tertiary Referral Robotic Center. Cancers 2024, 16, 1602. [Google Scholar] [CrossRef]
- Takahara, K.; Motonaga, T.; Nakamura, W.; Saruta, M.; Nukaya, T.; Takenaka, M.; Zennami, K.; Ichino, M.; Sasaki, H.; Shiroki, R. Robot-assisted radical prostatectomy with the Hugo™ robot-assisted surgery system: A single-center initial experience in Japan. Asian J. Endosc. Surg. 2024, 17, e13342. [Google Scholar] [CrossRef]
- Prata, F.; Ragusa, A.; Anceschi, U.; Iannuzzi, A.; Tedesco, F.; Cacciatore, L.; Civitella, A.; Tuzzolo, P.; Cirillo, R.; Callè, P.; et al. Three-arms off-clamp robot-assisted partial nephrectomy with the new Hugo robot-assisted surgery system. BJU Int. 2024, 133, 48–52. [Google Scholar] [CrossRef]
- Caputo, D.; Farolfi, T.; Molina, C.; Coppola, R. Full robotic cholecystectomy: First worldwide experiences with HUGO RAS surgical platform. ANZ J. Surg. 2024, 94, 387–390. [Google Scholar] [CrossRef]
- Belyaev, O.; Fahlbusch, T.; Slobodkin, I.; Uhl, W. Use of HugoTM RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature. J. Clin. Med. 2024, 13, 3678. [Google Scholar] [CrossRef]
- Jebakumar, S.G.S.; Swain, S.K.; Munikrishnan, V.; Jayapal, L.; Kumar, R.S.; Baskaran, A.; Tasgaonkar, S.; Srivatsan, S. Robotic hernia repair with the novel HUGO robot system—An initial experience from a tertiary centre. J. Minimal Access Surg. 2024, 10, 4103. [Google Scholar] [CrossRef]
- Caputo, D.; Cammarata, R.; Farolfi, T.; Coppola, R.; La Vaccara, V. First worldwide report on rectal resections with Hugo™ surgical system: Description of docking angles and tips for an effective setup. ANZ J. Surg. 2024, 94, 1299–1304. [Google Scholar] [CrossRef]
- Andrade, G.M.; Lau, C.; Olivares, R.; Duarte, I.K.; Teles, S.B.; Gavassa, F.P.; Pereira, H.M.J.; Kayano, P.P.; Barbosa, A.R.G.; Bianco, B.; et al. Implementation of Robot-assisted Urologic Surgeries Using Hugo RAS System in a High-volume Robotic “Da Vinci Xi” Center: Outcomes and Initial Experience. Urology 2024, 192, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Abu Salem, S.; Marom, G.; Shein, G.S.; Fishman, Y.; Helou, B.; Brodie, R.; Elazary, R.; Pikarsky, A.J.; Mintz, Y. Robotic Heller’s myotomy using the new Hugo™ RAS system: First worldwide report. Surg. Endosc. 2023, 38, 1180–1190. [Google Scholar] [CrossRef]
- Gioè, A.; Monterossi, G.; Alletti, S.G.; Panico, G.; Campagna, G.; Costantini, B.; Naldini, A.; Anchora, L.P.; Oliva, R.; Mastrovito, S.; et al. The new robotic system HUGO RAS for gynecologic surgery: First European experience from Gemelli Hospital. Int. J. Gynecol. Obstet. 2024, 166, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Quezada, N.; Irarrazaval, M.J.; Chen, D.C.; Grimoldi, M.; Pimentel, F.; Crovari, F. Robotic transversus abdominis release using HUGO RAS system: Our initial experience. Surg. Endosc. 2024, 38, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Pavone, M.; Seeliger, B.; Alesi, M.V.; Goglia, M.; Marescaux, J.; Scambia, G.; Ianieri, M.M. Initial experience of robotically assisted endometriosis surgery with a novel robotic system: First case series in a tertiary care center. Updat. Surg. 2024, 76, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Dibitetto, F.; Spicchiale, C.F.; Castellucci, R.; Sansalone, S.; Akhundov, A.; Defidio, L.; De Dominicis, M. Extraperitoneal robot assisted laparoscopic prostatectomy with Versius system: Single centre experience. Prostate Cancer Prostatic Dis. 2024, 27, 323–326. [Google Scholar] [CrossRef]
- Meneghetti, I.; Sighinolfi, M.C.; Dibitetto, F.; Collins, J.W.; Mosillo, L.; Catalano, C.; Rocco, B.; De Dominicis, M.; De Maria, M. Partial nephrectomy series using Versius robotic surgical system: Technique and outcomes of an initial experience. J. Robot. Surg. 2024, 18, 73. [Google Scholar] [CrossRef]
- De Maria, M.; Meneghetti, I.; Mosillo, L.; Collins, J.W.; Catalano, C. Versius robotic surgical system: Case series of 18 robot-assisted radical prostatectomies. BJU Int. 2024, 133, 197–205. [Google Scholar] [CrossRef]
- Inoue, S.; Nakauchi, M.; Umeki, Y.; Suzuki, K.; Serizawa, A.; Akimoto, S.; Watanabe, Y.; Tanaka, T.; Shibasaki, S.; Inaba, K.; et al. First clinical experiences of robotic gastrectomy for gastric cancer using the hinotori™ surgical robot system. Surg. Endosc. 2024, 38, 1626–1636. [Google Scholar] [CrossRef]
- Kulis, T.; Samalavicius, N.E.; Hudolin, T.; Venckus, R.; Penezic, L.; Nausediene, V.; Willeke, F.; Kastelan, Z.; The TransEnterix European Patient Registry (TRUST). Robotic-assisted radical prostatectomy: A multicenter experience with the Senhance Surgical System. World J. Urol. 2024, 42, 39. [Google Scholar] [CrossRef]
- Alip, S.K.L.; Koukourikis, P.; Han, W.K.; Rha, K.H.; Na, J.C. Comparing Revo-i and da Vinci in Retzius-Sparing Robot-Assisted Radical Prostatectomy: A Preliminary Propensity Score Analysis of Outcomes. J. Endourol. 2022, 36, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Beatty, J.W.; Kinross, J.; von Roon, A.; Darzi, A.; Purkayastha, S. Initial Experience with a New Robotic Surgical System for Cholecystectomy. Surg. Innov. 2020, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, G.; Li, Z.; Lin, H.; Zhu, S.; Yi, B. The Micro Hand S vs. da Vinci Surgical Robot-Assisted Surgery on Total Mesorectal Excision: Short-Term Outcomes Using Propensity Score Matching Analysis. Front. Surg. 2021, 8, 656270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, S.; Yi, B.; Li, J. Comparison of the short-term operative, Oncological, and Functional Outcomes between two types of robot-assisted total mesorectal excision for rectal cancer: Da Vinci versus Micro Hand S surgical robot. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Li, Z.; Ling, H.; Yi, B.; Zhu, S. Comparison of the operative outcomes and learning curves between laparoscopic and “Micro Hand S” robot-assisted total mesorectal excision for rectal cancer: A retrospective study. BMC Gastroenterol. 2021, 21, 251. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Jiang, J.; Zhu, S.; Yi, B.; Li, J. Comparison of the short-term efficacy of two types of robotic total mesorectal excision for rectal cancer. Tech. Coloproctol. 2022, 26, 19–28. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Yi, B.; Zhu, S. Initial experience of Chinese surgical robot “Micro Hand S″-assisted versus open and laparoscopic total mesorectal excision for rectal cancer: Short-term outcomes in a single center. Asian J. Surg. 2022, 45, 299–306. [Google Scholar] [CrossRef]
- Kulis, T.; Hudolin, T.; Penezic, L.; Zekulic, T.; Saic, H.; Knezevic, N.; Kastelan, Z. Comparison of extraperitoneal laparoscopic and extraperitoneal Senhance radical prostatectomy. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2344. [Google Scholar] [CrossRef]
- Ruvolo, C.C.; Afonina, M.; Balestrazzi, E.; Paciotti, M.; Piro, A.; Piramide, F.; Bravi, C.A.; Lores, M.P.; Sorce, G.; Belmonte, M.; et al. A comparative analysis of the HUGOTM robot-assisted surgery system and the Da Vinci® Xi surgical system for robot-assisted sacrocolpopexy for pelvic organ prolapse treatment. Int. J. Med. Robot. Comput. Assist. Surg. 2023, 20, e2587. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Fan, S.; Xiong, S.; Dong, J.; Wang, J.; Dai, X.; Yang, K.; Xie, Y.; Liu, G.; et al. Robot-assisted Partial Nephrectomy with the Newly Developed KangDuo Surgical Robot Versus the da Vinci Si Surgical System: A Double-center Prospective Randomized Controlled Noninferiority Trial. Eur. Urol. Focus 2022, 9, 133–140. [Google Scholar] [CrossRef]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Robot-assisted adrenalectomy using a hinotori surgical robot system: Report of first series of six cases. Asian J. Endosc. Surg. 2023, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Robot-assisted radical nephrectomy using novel surgical robot platform, hinotori: Report of initial series of 13 cases. Int. J. Urol. 2023, 30, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Perioperative outcomes of robot-assisted partial nephrectomy using hinotori versus da Vinci surgical robot system: A propensity score-matched analysis. J. Robot. Surg. 2023, 17, 2435–2440. [Google Scholar] [CrossRef]
- Clark, S.G.; Shepherd, J.P.; Sassani, J.C.; Bonidie, M. Surgical cost of robotic-assisted sacrocolpopexy: A comparison of two robotic platforms. Int. Urogynecol. J. 2023, 34, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, M.; Hwang, H.S.; Lee, W.J.; Kang, C.M. The Revo-i Robotic Surgical System in Advanced Pancreatic Surgery: A Second Non-Randomized Clinical Trial and Comparative Analysis to the da Vinci™ System. Yonsei Med. J. 2024, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bravi, C.A.; Balestrazzi, E.; De Loof, M.; Rebuffo, S.; Piramide, F.; Mottaran, A.; Paciotti, M.; Sorce, G.; Nocera, L.; Sarchi, L.; et al. Robot-assisted Radical Prostatectomy Performed with Different Robotic Platforms: First Comparative Evidence Between Da Vinci and HUGO Robot-assisted Surgery Robots. Eur. Urol. Focus 2024, 10, 107–114. [Google Scholar] [CrossRef]
- Balestrazzi, E.; Paciotti, M.; Piro, A.; Piramide, F.; Bravi, C.A.; Lores, M.P.; Mottaran, A.; Sorce, G.; Ticonosco, M.; Frego, N.; et al. Comparative analysis of robot-assisted simple prostatectomy: The HUGO™ RAS system versus the DaVinci® Xi system. Prostate Cancer Prostatic Dis. 2024, 27, 122–128. [Google Scholar] [CrossRef]
- Menendez, R.B.; Rojo, E.G.; Palacios, V.H.; Ochoa, J.A.F.; Quintas, J.J.; Mateos, F.L.; Fraile, A.; Manfredi, C.; Belli, S.; Bozzini, G.; et al. Da Vinci vs. Hugo RAS for robot-assisted radical prostatectomy: A prospective comparative single-center study. World J. Urol. 2024, 42, 336. [Google Scholar] [CrossRef]
- Ou, H.-C.; Marian, L.; Li, C.-C.; Juan, Y.-S.; Tung, M.-C.; Shih, H.-J.; Chang, C.-P.; Chen, J.-T.; Yang, C.-H.; Ou, Y.-C. Robot-Assisted Radical Prostatectomy by the Hugo Robotic-Assisted Surgery (RAS) System and the da Vinci System: A Comparison between the Two Platforms. Cancers 2024, 16, 1207. [Google Scholar] [CrossRef]
- Prata, F.; Ragusa, A.; Tedesco, F.; Pira, M.; Iannuzzi, A.; Fantozzi, M.; Civitella, A.; Scarpa, R.M.; Papalia, R. Trifecta Outcomes of Robot-Assisted Partial Nephrectomy Using the New Hugo™ RAS System Versus Laparoscopic Partial Nephrectomy. J. Clin. Med. 2024, 13, 2138. [Google Scholar] [CrossRef]
- Gandi, C.; Marino, F.; Totaro, A.; Scarciglia, E.; Bellavia, F.; Bientinesi, R.; Gavi, F.; Russo, P.; Ragonese, M.; Palermo, G.; et al. Perioperative Outcomes of Robotic Radical Prostatectomy with Hugo™ RAS versus daVinci Surgical Platform: Propensity Score-Matched Comparative Analysis. J. Clin. Med. 2024, 13, 3157. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Veccia, A.; Malandra, S.; Rizzetto, R.; De Marco, V.; Baielli, A.; Franceschini, A.; Fumanelli, F.; Montanaro, F.; Palumbo, I.; et al. Intraoperative Performance of DaVinci Versus Hugo RAS During Radical Prostatectomy: Focus on Timing, Malfunctioning, Complications, and User Satisfaction in 100 Consecutive Cases (the COMPAR-P Trial). Eur. Urol. Open Sci. 2024, 63, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yan, W.; Chen, S.; Xu, W.; Wang, X.; Dong, J.; Zhang, Z.; Yang, K.; Fan, S.; Li, Z.; et al. Robot-assisted Radical Prostatectomy with the KangDuo Surgical System Versus the da Vinci Si System: A Prospective, Double-center, Randomized Controlled Trial. Eur. Urol. Focus 2024. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Huang, Z.; Lu, J.; Xu, L.; Wang, Y.; Li, X.; Wei, Z.; Wang, G.; Xiao, Y. Robot-assisted radical resection of colorectal cancer using the KangDuo surgical robot versus the da Vinci Xi robotic system: Short-term outcomes of a multicentre randomised controlled noninferiority trial. Surg. Endosc. 2024, 38, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Wang, C.; Wang, X.; Zhang, X.; Yang, Y.; Wei, Z.; Xiao, Y.; Wang, G. Comparison of short-term outcomes of robotic-assisted radical colon cancer surgery using the Kangduo Surgical Robotic System and the Da Vinci Si Robotic System: A prospective cohort study. Int. J. Surg. 2024, 110, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Halabi, M.; Khoury, K.; Alomar, A.; El Dahdah, J.; Hassan, O.; Hayyan, K.; Bishara, E.; Moussa, H. Operative efficiency: A comparative analysis of Versius and da Vinci robotic systems in abdominal surgery. J. Robot. Surg. 2024, 18, 132. [Google Scholar] [CrossRef]
- Kohjimoto, Y.; Yamashita, S.; Iwagami, S.; Muraoka, S.; Wakamiya, T.; Hara, I. hinotoriTM vs. da Vinci®: Propensity score-matched analysis of surgical outcomes of robot-assisted radical prostatectomy. J. Robot. Surg. 2024, 18, 130. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Yuan, L.-H.; Tseng, C.-S.; Hsieh, T.-Y.; Huang, Y.-W.; Huang, C.-Y.; Huang, S.-W. Comparison of senhance and da vinci robotic radical prostatectomy: Short-term outcomes, learning curve, and cost analysis. Prostate Cancer Prostatic Dis. 2024, 27, 116–121. [Google Scholar] [CrossRef]
- Sighinolfi, M.C.; Rocco, B.; Terzoni, S.; Morandi, A.; Afonina, M.; Assumma, S.; Calcagnile, T.; Turri, F.; Sangalli, M.; Panio, E.; et al. New robotic systems: First head-to-head comparison between Hugo RAS and Versius CMR in the pre-clinical setting. Minerva Urol. Nephrol. 2024, 76, 1–4. [Google Scholar] [CrossRef]
- Available online: https://www.asensus.com/senhance (accessed on 23 June 2024).
- Available online: http://revosurgical.com/render/view/revo_i/discover_revo_i.html (accessed on 23 June 2024).
- Available online: https://www.medtronic.com/covidien/en-ca/robotic-assisted-surgery/hugo-ras-system.html (accessed on 23 June 2024).
- Available online: https://www.medtronic.com/covidien/en-gb/robotic-assisted-surgery/hugo-ras-system/products-and-system.html (accessed on 23 June 2024).
- Available online: https://www.medicaroid.com/en/product/hinotori/ (accessed on 23 June 2024).
- Available online: https://us.cmrsurgical.com/news/cmr-receives-fda-marketing-authorization-for-versius (accessed on 23 June 2024).
- Available online: https://avatera.eu/en/avatera-system (accessed on 23 June 2024).
- Available online: https://www.distalmotion.com/Dexter (accessed on 23 June 2024).
- Available online: https://ssinnovations.com/ssi-mantra/ (accessed on 23 June 2024).
- Picozzi, P.; Nocco, U.; Puleo, G.; Labate, C.; Cimolin, V. Telemedicine and Robotic Surgery: A Narrative Review to Analyze Advantages, Limitations and Future Developments. Electronics 2023, 13, 124. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R.M. Artificial Intelligence in Surgery: Promises and Perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Jonmohamadi, Y.; Fontanarosa, D.; Crawford, R.; Pandey, A.K. One step surgical scene restoration for robot assisted minimally invasive surgery. Sci. Rep. 2023, 13, 3127. [Google Scholar] [CrossRef] [PubMed]
- Kumazu, Y.; Kobayashi, N.; Kitamura, N.; Rayan, E.; Neculoiu, P.; Misumi, T.; Hojo, Y.; Nakamura, T.; Kumamoto, T.; Kurahashi, Y.; et al. Automated segmentation by deep learning of loose connective tissue fibers to define safe dissection planes in robot-assisted gastrectomy. Sci. Rep. 2021, 11, 21198. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Wang, Z.; Yao, J.; Gao, J.; Yang, S.; Li, J.; Shi, J.; Wu, W.; Hua, S.; Wang, H. Application and evaluation of surgical tool and tool tip recognition based on Convolutional Neural Network in multiple endoscopic surgical scenarios. Surg. Endosc. 2023, 37, 7376–7384. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, S.; Reisner, L.A.; Pandya, A.K. Development and evaluation of an autonomous camera control algorithm on the da Vinci Surgical System. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, e2036. [Google Scholar] [CrossRef]
- Saeidi, H.; Opfermann, J.D.; Kam, M.; Wei, S.; Leonard, S.; Hsieh, M.H.; Kang, J.U.; Krieger, A. Autonomous robotic laparoscopic surgery for intestinal anastomosis. Sci. Robot. 2022, 7, eabj2908. [Google Scholar] [CrossRef]




| Robotic Platform | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgical Specialty | HugoTM | Versius® | Senhance® | Revo-i® | Micro Hand S | Avatera | Dexter | HinotoriTM | Mantra | KangDuo | Toumai® |
| General Surgery | 126 | 607 | 764 | 27 | 277 | - | 12 | 33 | 10 | 101 | - |
| Gynecology | 253 | 204 | 114 | - | - | - | 1 | 12 | - | - | - |
| Urology | 962 | 86 | 1036 | 48 | - | 9 | 11 | 105 | - | 175 | 20 |
| Surgical Platform | Company | Year | Country | CE Mark | FDA Approval | Approved in the Origin Nation |
|---|---|---|---|---|---|---|
| Senhance® | TransEnterix Surgical, which became Asensus Surgical in 2021, Durham, NC, USA | 2017 | USA | yes | yes | yes |
| Revo-i® | Meerecompany, Yongin, Gyeonggi-do, Republic of Korea | 2017 | Republic of Korea | no | no | yes |
| Micro Hand S | Shandon Wego Surgical Robot Co., Weihai, Shandong, China | 2017 | China | no | no | yes |
| Toumai® | Shanghai MicroPort MedBot (Group), Shanghai, China | 2018 | China | no | no | yes |
| Avatera | Avatera Medical, Jena Germany | 2019 | Germany | yes | NAI | yes |
| Versius® | CMR Surgical, Cambridge, UK | 2019 | UK | yes | no | yes |
| HinotoriTM | Medicaroid Inc., Kobe, Japan | 2020 | Japan | no | yes | yes |
| KangDuo | Suzhou KangDuo Robot Co., Suzhou, Jiangsu, China | 2020 | China | NAI | no | yes |
| HugoTM | Medtronic, Minneapolis, Minnesota, USA | 2021 | USA | yes | yes | yes |
| Dexter | Distalmotion, Epalinges, Switzerland | 2022 | Switzerland | yes | no | yes |
| Mantra | SS Innovation, Gurugram, Haryana, India | 2023 | India | ongoing | ongoing | yes |
| Surgical Platform | Single Port or Multiport | Chart | Number of Arms | Console | Vision | Fluorescence | Haptic Feedback | Eye Tracking | Instruments |
|---|---|---|---|---|---|---|---|---|---|
| Senhance® | Multiport | multiple | 4 | Semi-open | 3DHD | NAI | yes | NAI | wristed, 5 mm, disposable |
| Revo-i® | Multiport | single | 4 | Open | 3DHD | yes | yes | yes | rigid with a kit, wristed, unlimited uses, 5 mm |
| Micro Hand S | Multiport | single | 4 | Close | 3D HD | no | yes | no | wristed, multi-use (20) |
| Toumai® | Multiport | single | 4 | Open | 3DHD | yes | no | no | wristed, reusable |
| Avatera | Multiport | single | 4 | Open | 3D HD | no | no | yes | wristed, reusable |
| Versius® | Multiport | multiple | 4 | Open | 3D HD | yes | no | yes | wristed, disposable |
| HinotoriTM | Multiport | single | 4 | Semi-open | 3D HD | NAI | no | no | wristed, reusable used up to 10 times |
| Kangduo | Multiport | single | 3 | Open | 3D HD | yes | yes | NAI | wristed, reusable up to 10 uses |
| HugoTM | Multiport | multiple | 4 | Open | 3D 4k | NAI | NAI | Yes | NAI |
| Dexter | Multiport | multiple | 3 | Open | 3DHD | yes | No | NAI | reusable up to 10 times |
| Mantra | Multiport | multiple | 5 | Open | 3DHD | NA | NAI | NAI | NAI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picozzi, P.; Nocco, U.; Labate, C.; Gambini, I.; Puleo, G.; Silvi, F.; Pezzillo, A.; Mantione, R.; Cimolin, V. Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics 2024, 13, 4675. https://doi.org/10.3390/electronics13234675
Picozzi P, Nocco U, Labate C, Gambini I, Puleo G, Silvi F, Pezzillo A, Mantione R, Cimolin V. Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics. 2024; 13(23):4675. https://doi.org/10.3390/electronics13234675
Chicago/Turabian StylePicozzi, Paola, Umberto Nocco, Chiara Labate, Isabella Gambini, Greta Puleo, Federica Silvi, Andrea Pezzillo, Rocco Mantione, and Veronica Cimolin. 2024. "Advances in Robotic Surgery: A Review of New Surgical Platforms" Electronics 13, no. 23: 4675. https://doi.org/10.3390/electronics13234675
APA StylePicozzi, P., Nocco, U., Labate, C., Gambini, I., Puleo, G., Silvi, F., Pezzillo, A., Mantione, R., & Cimolin, V. (2024). Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics, 13(23), 4675. https://doi.org/10.3390/electronics13234675







