FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape
Abstract
1. Introduction
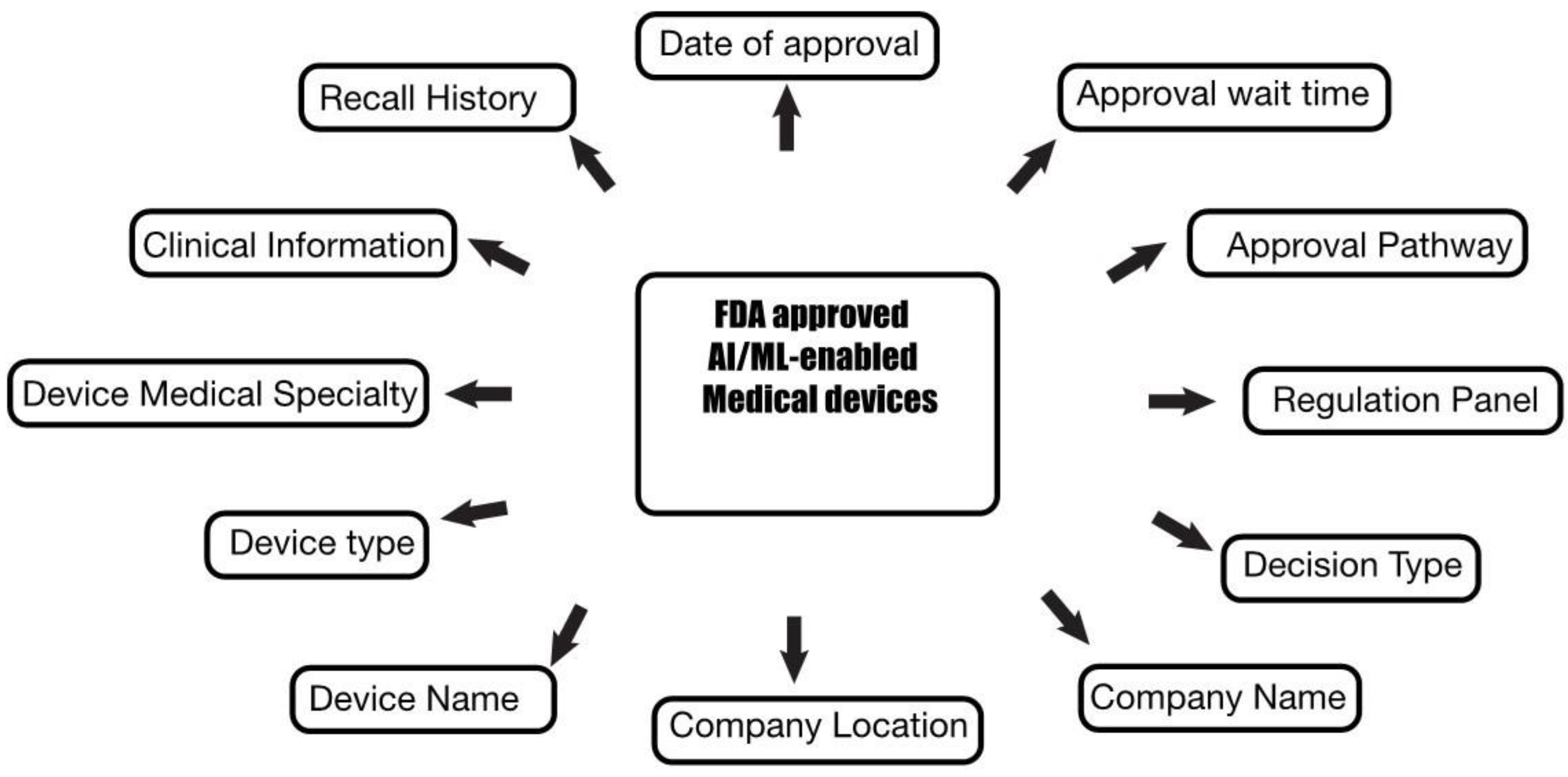
2. Materials and Methods
3. Results
3.1. Overall Trends
3.2. Medical Subspecialties
3.3. Device Classification
3.4. Clearance Pathway, Decision Type, and Recall Rate
3.5. Approval Wait Time
3.6. Applicant Company
3.7. Leading Countries in AI/ML-Enabled Medical Devices
3.8. Clinical Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artificial Intelligence (AI) Coined at Dartmouth. Celebrate Our 250th 2018. Available online: https://250.dartmouth.edu/highlights/artificial-intelligence-ai-coined-dartmouth (accessed on 4 December 2022).
- Le Cun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131.e9. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.B.A.; Wang, S.; Marinsek, N.; Ranganath, R.; Foschini, L.; Ghassemi, M. Reproducibility in machine learning for health research: Still a ways to go. Sci. Transl. Med. 2021, 13, eabb1655. [Google Scholar] [CrossRef] [PubMed]
- Screening for Early Detection—CPR22. Cancer Progress Report n.d. Available online: https://cancerprogressreport.aacr.org/progress/cpr22-contents/cpr22-screening-for-early-detection/ (accessed on 4 December 2022).
- Zhu, S.; Gilbert, M.; Chetty, I.; Siddiqui, F. The 2021 landscape of FDA-approved artificial intelligence/machine learning-enabled medical devices: An analysis of the characteristics and intended use. Int. J. Med. Inform. 2022, 165, 104828. [Google Scholar] [CrossRef] [PubMed]
- Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. FDA, 2023. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 10 January 2024).
- De Novo Classification Request. FDA, 2023. Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/de-novo-classification-request (accessed on 10 January 2024).
- Premarket Approval (PMA). FDA, 2023. Available online: https://cacmap.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma (accessed on 23 October 2023).
- Premarket Notification 510(k). FDA, 2023. Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k (accessed on 23 October 2023).
- Software as a Medical Device (SaMD): Clinical Evaluation|International Medical Device Regulators Forum 2017. Available online: https://www.imdrf.org/documents/software-medical-device-samd-clinical-evaluation (accessed on 10 January 2024).
- Gerke, S.; Babic, B.; Evgeniou, T.; Cohen, I.G. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. NPJ Digit. Med. 2020, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Clusmann, J.; Kolbinger, F.R.; Muti, H.S.; Carrero, Z.I.; Eckardt, J.-N.; Laleh, N.G.; Löffler, C.M.L.; Schwarzkopf, S.-C.; Unger, M.; Veldhuizen, G.P.; et al. The future landscape of large language models in medicine. Commun. Med. 2023, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, S.M.; Graham, M.G.; Brereton, T.; Pencina, M.J.; Halamka, J.D.; Vidal, D.E.; Economou-Zavlanos, N.J. Implementing quality management systems to close the AI translation gap and facilitate safe, ethical, and effective health AI solutions. NPJ Digit. Med. 2023, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xing, L.; Zou, J.; Wu, J.C. Shifting machine learning for healthcare from development to deployment and from models to data. Nat. Biomed. Eng. 2022, 6, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- De Kok, J.W.T.M.; de la Hoz, M.Á.A.; de Jong, Y.; Brokke, V.; Elbers, P.W.G.; Thoral, P.; Castillejo, A.; Trenor, T.; Castellano, J.M.; Bronchalo, A.E.; et al. A guide to sharing open healthcare data under the General Data Protection Regulation. Sci. Data 2023, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, M.; Muto, K. Public attitudes toward cloud computing and willingness to share personal health records (PHRs) and genome data for health care research in Japan. Hum. Genome Var. 2023, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Perkel, J.M. Terra takes the pain out of ‘omics’ computing in the cloud. Nature 2022, 601, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chia, M.A.; Wagner, S.K.; Ayhan, M.S.; Williamson, D.J.; Struyven, R.R.; Liu, T.; Xu, M.; Lozano, M.G.; Woodward-Court, P.; et al. A foundation model for generalizable disease detection from retinal images. Nature 2023, 622, 156–163. [Google Scholar] [CrossRef] [PubMed]
- CDCBreastCancer. Breast Cancer Statistics. Centers for Disease Control and Prevention, 2022. Available online: https://www.cdc.gov/cancer/breast/statistics/index.htm (accessed on 10 January 2024).
- CDCBreastCancer. What CDC Is Doing about Breast Cancer. Centers for Disease Control and Prevention, 2023. Available online: https://www.cdc.gov/cancer/breast/what_cdc_is_doing/index.htm (accessed on 10 January 2024).
- Bains, A.; Osathanugrah, P.; Sanjiv, N.; Chiu, C.; Fiorello, M.G.; Siegel, N.H.; Peeler, C.E.; Distefano, A.G.; Lee, H.J.; Ness, S.; et al. Diverse Research Teams and Underrepresented Groups in Clinical Studies. JAMA Ophthalmol. 2023, 141, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations (accessed on 10 January 2024).
- Shandhi, M.M.H.; Dunn, J.P. AI in medicine: Where are we now and where are we going? CR Med. 2022, 3, 100861. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Badal, K.; Lee, C.M.; Esserman, L.J. Guiding principles for the responsible development of artificial intelligence tools for healthcare. Commun. Med. 2023, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Bienefeld, N.; Boss, J.M.; Lüthy, R.; Brodbeck, D.; Azzati, J.; Blaser, M.; Willms, J.; Keller, E. Solving the explainable AI conundrum by bridging clinicians’ needs and developers’ goals. NPJ Digit. Med. 2023, 6, 94. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, G.; Jain, A.; Araveeti, S.R.; Adhikari, S.; Garg, H.; Bhandari, M. FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics 2024, 13, 498. https://doi.org/10.3390/electronics13030498
Joshi G, Jain A, Araveeti SR, Adhikari S, Garg H, Bhandari M. FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics. 2024; 13(3):498. https://doi.org/10.3390/electronics13030498
Chicago/Turabian StyleJoshi, Geeta, Aditi Jain, Shalini Reddy Araveeti, Sabina Adhikari, Harshit Garg, and Mukund Bhandari. 2024. "FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape" Electronics 13, no. 3: 498. https://doi.org/10.3390/electronics13030498
APA StyleJoshi, G., Jain, A., Araveeti, S. R., Adhikari, S., Garg, H., & Bhandari, M. (2024). FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics, 13(3), 498. https://doi.org/10.3390/electronics13030498







