Evaluation of the Electronic Properties of Atomic Layer Deposition-Grown Ge-Doped Zinc Oxide Thin Films at Elevated Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Thin Films
2.1.1. Atomic Layer Deposition
2.1.2. Annealing of the Films
2.2. Film Characterization
2.2.1. Scanning Electron Microscopy (SEM)
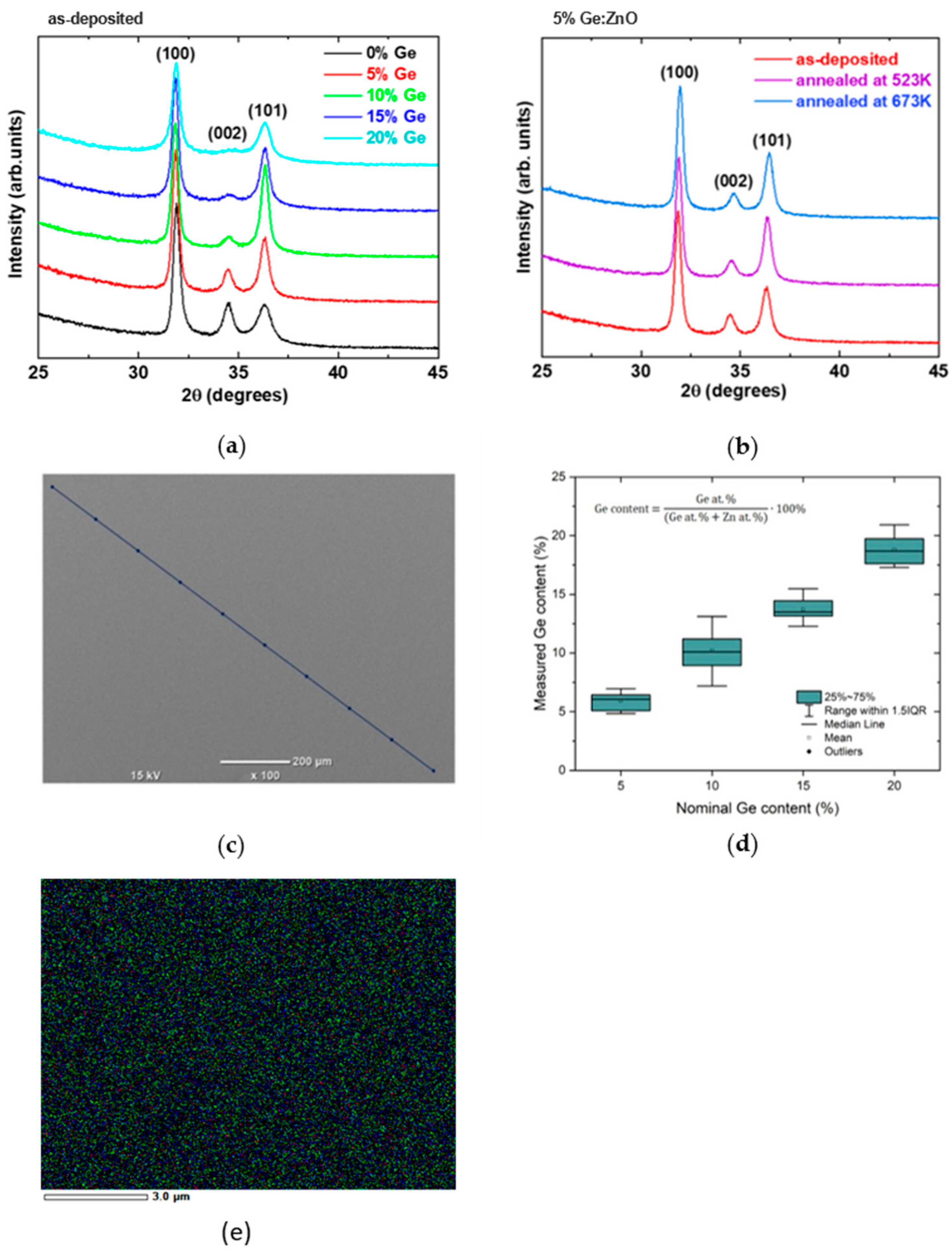
2.2.2. X-ray Diffraction
2.2.3. Laser Ellipsometry
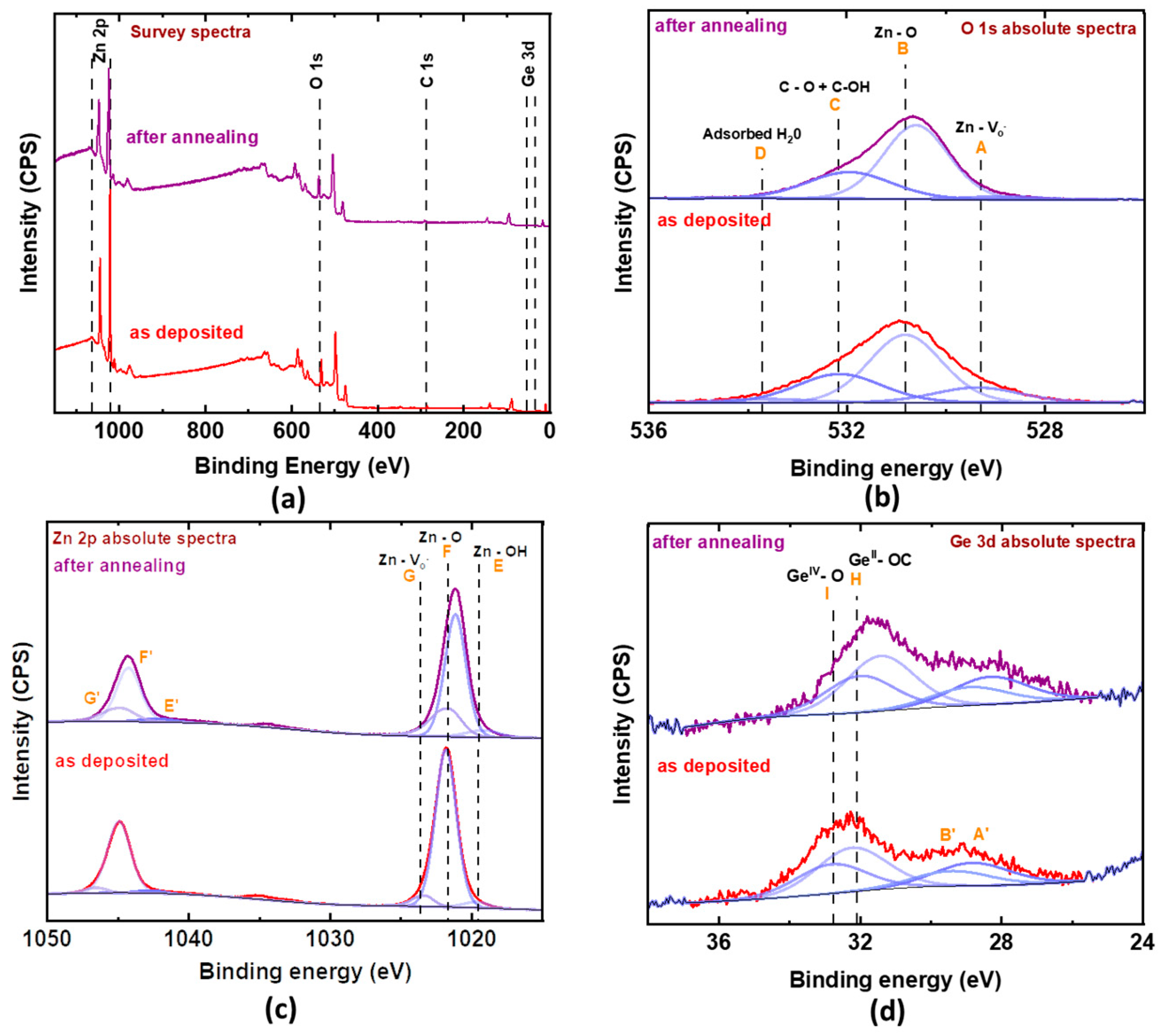
2.2.4. X-ray Photoelectron Spectroscopy (XPS)
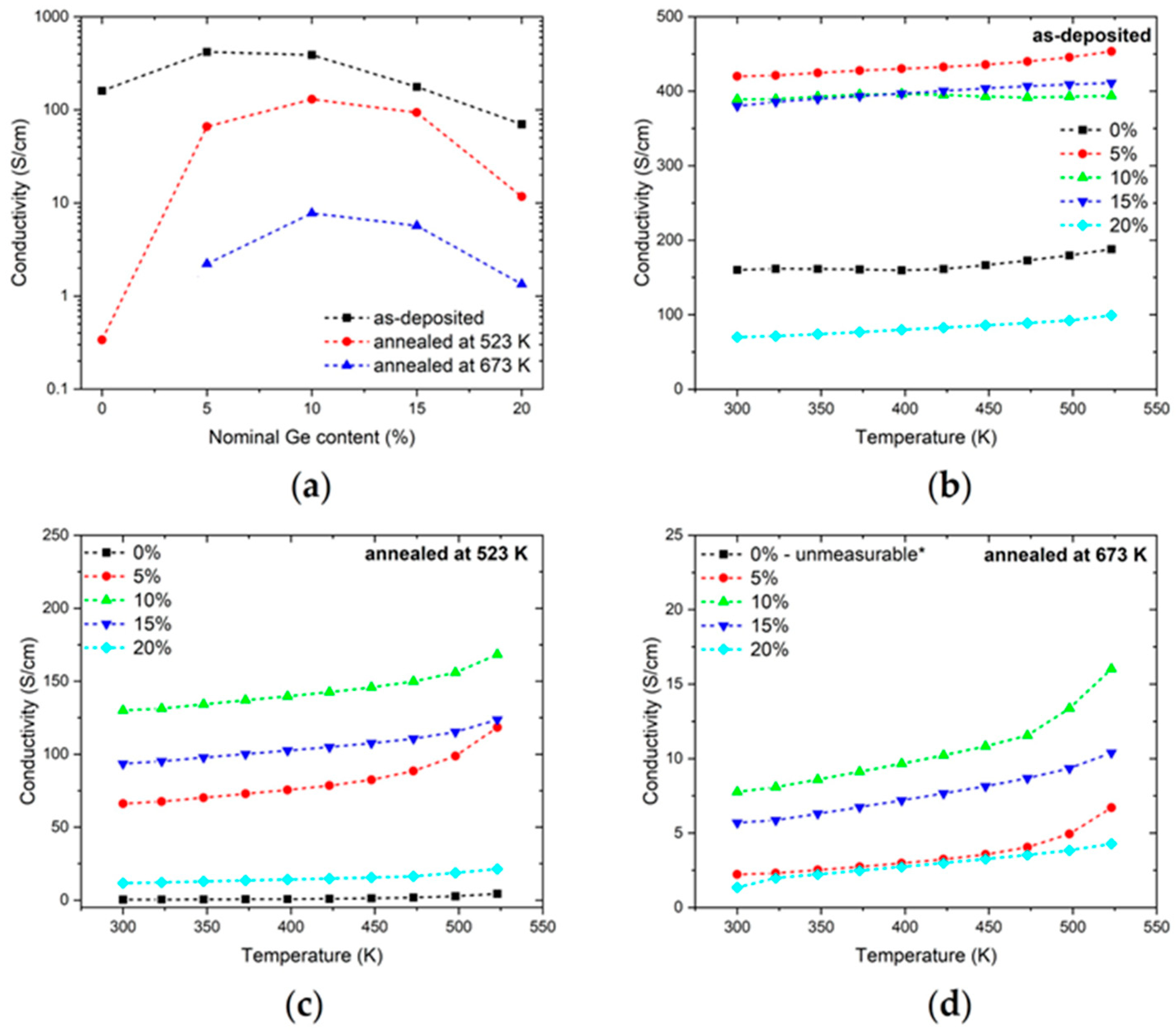
2.2.5. Electrical Properties
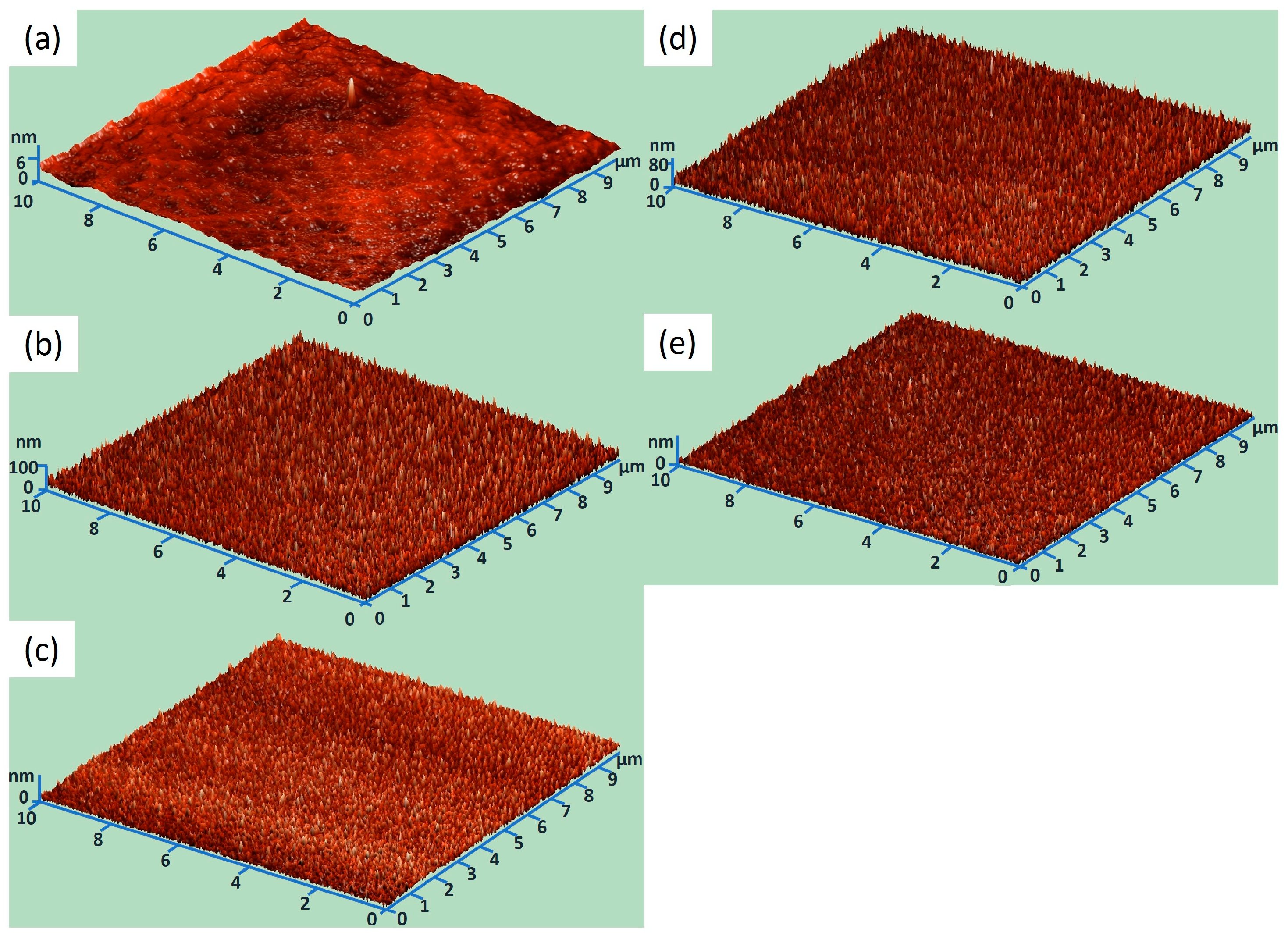
2.2.6. Atomic force microscopy (AFM) Analysis
3. Results and Discussion
3.1. Structural Characterization
3.2. Annealing Kinetics
3.3. Annealing in Air
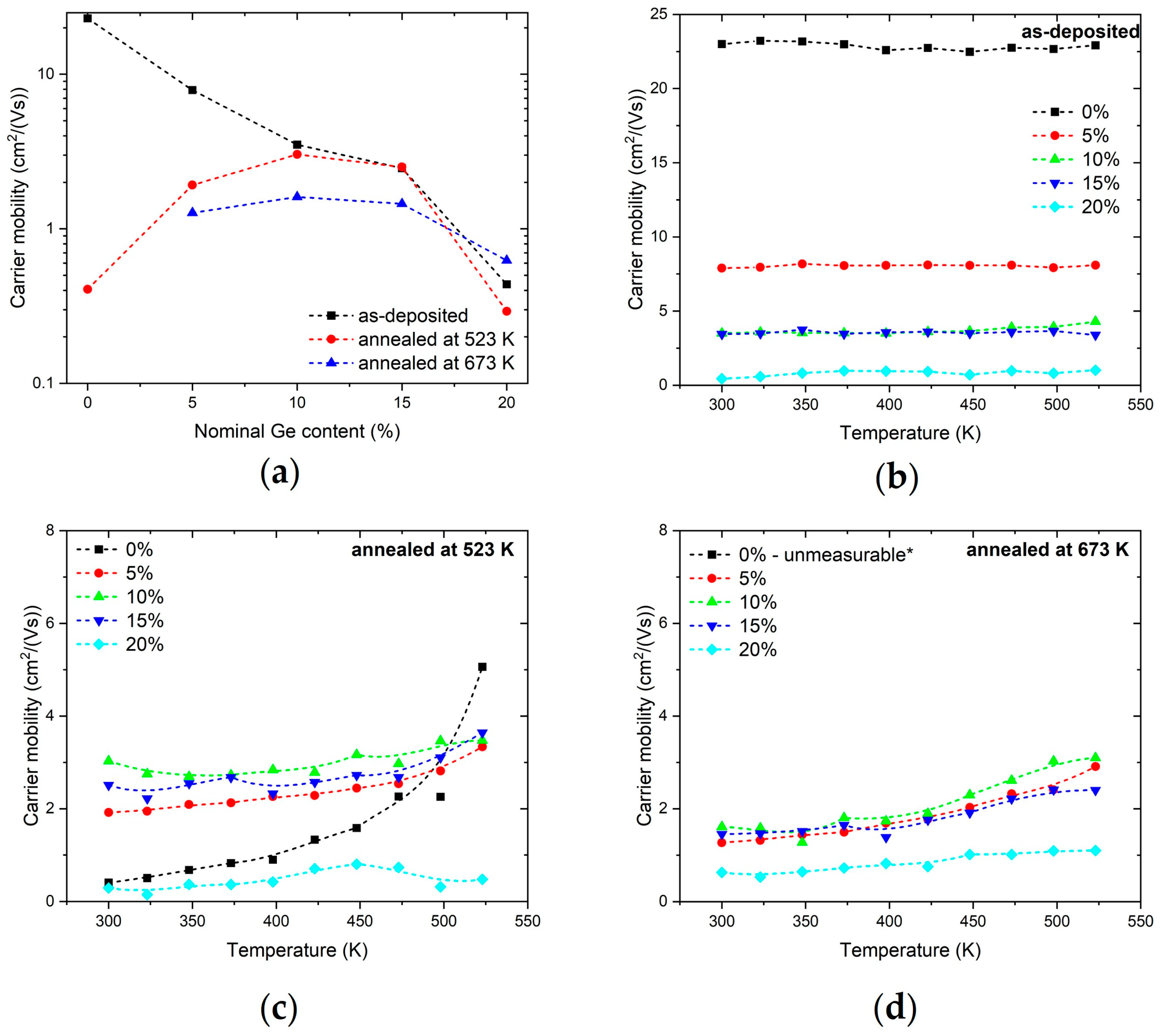
3.4. Discussion of Electronic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Li, M.Y.; Su, D.; Yu, M.; Kan, H.; Liu, H.; Wang, X.; Jiang, S. Broad-Band High-Sensitivity ZnO Colloidal Quantum Dots/Self-Assembled Au Nanoantennas Heterostructures Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 32516–32525. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yu, M.; Su, D.; Zhang, J.; Jiang, S.; Wu, J.; Wang, Q.; Liu, S. Ultrahigh Responsivity UV Photodetector Based on Cu Nanostructure/ZnO QD Hybrid Architectures. Small 2019, 15, 1901606. [Google Scholar] [CrossRef]
- Zheng, T.; Li, Z.; Chen, J.; Shen, K.; Sun, K. Transitions of Microstructure and Photoluminescence Properties of the Ge/ZnO Multilayer Films in Certain Annealing Temperature Region. Appl. Surf. Sci. 2006, 252, 8482–8486. [Google Scholar] [CrossRef]
- Suganthi, K.; Vinoth, E.; Sudha, L.; Bharathi, P.; Navaneethan, M. Manganese (Mn2+) Doped Hexagonal Prismatic Zinc Oxide (ZnO) Nanostructures for Chemiresistive NO2 Sensor. Sens. Actuators B Chem. 2023, 380, 133293. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Ovanesyan, R.A.; Filatova, E.A.; Elliott, S.D.; Hausmann, D.M.; Smith, D.C.; Agarwal, S. Atomic Layer Deposition of Silicon-Based Dielectrics for Semiconductor Manufacturing: Current Status and Future Outlook. J. Vac. Sci. Technol. A Vac. Surf. Film. 2019, 37, 060904. [Google Scholar] [CrossRef]
- Zaidi, S.J.A.; Basit, M.A.; Park, T.J. Advances in Atomic Layer Deposition of Metal Sulfides: From a Precursors Perspective. Chem. Mater. 2022, 34, 7106–7138. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Meng, X.; Elam, J.W.; Martinson, A.B.F. Atomic Layer Deposition of Metal Sulfide Materials. Acc. Chem. Res. 2015, 48, 341–348. [Google Scholar] [CrossRef]
- Witkowski, M.; Starowicz, Z.; Zięba, A.; Adamczyk-Cieślak, B.; Socha, R.P.; Szawcow, O.; Kołodziej, G.; Haras, M.; Ostapko, J. The Atomic Layer Deposition (ALD) Synthesis of Copper-Tin Sulfide Thin Films Using Low-Cost Precursors. Nanotechnology 2022, 33, 505603. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Liu, A.; Gao, L.; Zhang, C.; Wei, G.; Ma, T. Recent Progress in Metal Sulfide-Based Electron Transport Layers in Perovskite Solar Cells. Nanoscale 2021, 13, 17272–17289. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yin, Z.; Collins, F.; Pinna, N. Atomic Layer Deposition of Metal Oxides and Chalcogenides for High Performance Transistors. Adv. Sci. 2022, 9, 2104599. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic Layer Deposition of ZnO: A Review. Semicond. Sci. Technol. 2014, 29, 43001. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, J.; Zhao, Y.; Han, D.; Wang, Y.; Liu, B.; Dong, J. Investigation on Transparent, Conductive ZnO:Al Films Deposited by Atomic Layer Deposition Process. Nanomaterials 2022, 12, 172. [Google Scholar] [CrossRef]
- Fei, Y.; Li, Y.; Li, Y.; Xie, A.; Li, Y.; Sun, D. Properties Study of ZnO Films Prepared by ALD. J. Mol. Struct. 2022, 1269, 133804. [Google Scholar] [CrossRef]
- Kruszyńska, J.; Ostapko, J.; Ozkaya, V.; Surucu, B.; Szawcow, O.; Nikiforow, K.; Hołdyński, M.; Tavakoli, M.M.; Yadav, P.; Kot, M.; et al. Atomic Layer Engineering of Aluminum-Doped Zinc Oxide Films for Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2200575. [Google Scholar] [CrossRef]
- Starowicz, Z.; Zięba, A.; Ostapko, J.; Wlazło, M.; Kołodziej, G.; Jakub Szczerba, M.; Putynkowski, G.; Piotr Socha, R. Synthesis and Characterization of Al-Doped ZnO and Al/F Co-Doped ZnO Thin Films Prepared by Atomic Layer Deposition. Mater. Sci. Eng. B 2023, 292, 116405. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS Characterization of ALD Al2O3 Coated Porous Silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Myers, T.J.; Throckmorton, J.A.; Borrelli, R.A.; O’Sullivan, M.; Hatwar, T.; George, S.M. Smoothing Surface Roughness Using Al2O3 Atomic Layer Deposition. Appl. Surf. Sci. 2021, 569, 150878. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-Dimensional Anodic TiO2 Nanotubes Coated by Atomic Layer Deposition: Towards Advanced Applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Moehl, T.; Suh, J.; Sévery, L.; Wick-Joliat, R.; David Tilley, S. Investigation of (Leaky) ALD TiO2 Protection Layers for Water-Splitting Photoelectrodes. ACS Appl. Mater. Interfaces 2017, 9, 43614–43622. [Google Scholar] [CrossRef]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, Doping & Processing. Mater. Today 2004, 7, 34–40. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Yang, J.; Park, J.K.; Kim, S.; Choi, W.; Lee, S.; Kim, H. Atomic-Layer-Deposited ZnO Thin-Film Transistors with Various Gate Dielectrics. Phys. Status Solidi A 2012, 209, 2087–2090. [Google Scholar] [CrossRef]
- Yang, J.; Bahrami, A.; Ding, X.; Lehmann, S.; Kruse, N.; He, S.; Wang, B.; Hantusch, M.; Nielsch, K. Characteristics of ALD-ZnO Thin Film Transistor Using H2O and H2O2 as Oxygen Sources. Adv. Mater. Interfaces 2022, 9, 2101953. [Google Scholar] [CrossRef]
- Shih, Y.T.; Wu, M.K.; Li, W.C.; Kuan, H.; Yang, J.R.; Shiojiri, M.; Chen, M.J. Amplified Spontaneous Emission from ZnO in N-ZnO/ZnO Nanodots-SiO2 Composite/p-AlGaN Heterojunction Light-Emitting Diodes. Nanotechnology 2009, 20, 165201. [Google Scholar] [CrossRef]
- Chen, M.-J.; Yang, J.-R.; Shiojiri, M. ZnO-Based Ultra-Violet Light Emitting Diodes and Nanostructures Fabricated by Atomic Layer Deposition. Semicond. Sci. Technol. 2012, 27, 74005. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y. Surface Modifications of Li-Ion Battery Electrodes with Various Ultrathin Amphoteric Oxide Coatings for Enhanced Cycleability. J. Solid State Electrochem. 2013, 17, 1049–1058. [Google Scholar] [CrossRef]
- Szabó, Z.; Volk, J.; Horváth, Z.E.; Medveczky, Z.; Czigány, Z.; Vad, K.; Baji, Z. Atomic Layer Deposition and Annealing of Ga Doped ZnO Films. Mater. Sci. Semicond. Process 2019, 101, 95–102. [Google Scholar] [CrossRef]
- Nam, T.; Lee, C.W.; Kim, H.J.; Kim, H. Growth Characteristics and Properties of Ga-Doped ZnO (GZO) Thin Films Grown by Thermal and Plasma-Enhanced Atomic Layer Deposition. Appl. Surf. Sci. 2014, 295, 260–265. [Google Scholar] [CrossRef]
- Hong, C.; Kang, K.M.; Kim, M.; Wang, Y.; Kim, T.; Lee, C.; Park, H.H. Structural, Electrical, and Optical Properties of Si-Doped ZnO Thin Films Prepared via Supercycled Atomic Layer Deposition. Mater. Sci. Eng. B 2021, 273, 115401. [Google Scholar] [CrossRef]
- Geng, Y.; Xie, Z.Y.; Yang, W.; Xu, S.S.; Sun, Q.Q.; Ding, S.J.; Lu, H.L.; Zhang, D.W. Structural, Optical, and Electrical Properties of Hf-Doped ZnO Films Deposited by Atomic Layer Deposition. Surf. Coat. Technol. 2013, 232, 41–45. [Google Scholar] [CrossRef]
- Ellmer, K.; Bikowski, A. Intrinsic and Extrinsic Doping of ZnO and ZnO Alloys. J. Phys. D Appl. Phys. 2016, 49, 413002. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.A.; King, P.J.; Dawson, K.; Romani, S.; Williams, P.A.; Ridealgh, J.; Rosseinsky, M.J. Atomic Layer Deposition of Germanium-Doped Zinc Oxide Films with Tuneable Ultraviolet Emission. J. Mater. Chem. 2012, 22, 12824–12829. [Google Scholar] [CrossRef]
- Zhu, W.; Kammuri, T.; Kitamura, S.; Sturaro, M.; Martucci, A.; Pezzotti, G. Structure and Composition Evaluation of Heavily Ge-Doped ZnO Nanocrystal Films. J. Phys. D Appl. Phys. 2018, 51, 085302. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, Y.; Yang, Y.; Zhang, W.; Zou, M.; Nan, C.-W.; Lin, Y.-H. Enhanced Thermoelectric Performance of ZnO-Based Thin Films via Interface Engineering. Crystals 2022, 12, 1351. [Google Scholar] [CrossRef]
- Li, L.; Fang, L.; Zhou, X.J.; Liu, Z.Y.; Zhao, L.; Jiang, S. X-ray Photoelectron Spectroscopy Study and Thermoelectric Properties of Al-Doped ZnO Thin Films. J. Electron Spectrosc. Relat. Phenom. 2009, 173, 7–11. [Google Scholar] [CrossRef]
- Yu, Y.S.; Kim, G.Y.; Min, B.H.; Kim, S.C. Optical Characteristics of Ge Doped ZnO Compound. J. Eur. Ceram. Soc. 2004, 24, 1865–1868. [Google Scholar] [CrossRef]
- Sypniewska, M.; Szczesny, R.; Popielarski, P.; Strzalkowski, K.; Derkowska-Zielinska, B. Structural, Morphological and Photoluminescent Properties of Annealed ZnO Thin Layers Obtained by the Rapid Sol-Gel Spin-Coating Method. Opto-Electron. Rev. 2020, 28, 182–190. [Google Scholar] [CrossRef]
- Ghosh, H.; Sadeghimakki, B.; Sivoththaman, S. Enhancement of UV Emission and Optical Bandgap of ZnO Nanowires via Doping and Post-Growth Annealing. Mater. Res. Express 2020, 7, 035013. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Progress. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- ISO 15472:2010; Surface Chemical Analysis—X-ray Photoelectron Spectrometers—Calibration of Energy Scales. ISO: Geneva, Switzerland, 2010.
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- NIST. X-ray Photoelectron Spectroscopy Database. In NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000; p. 20899. [Google Scholar]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. 7—Characterization of Biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 97–115. ISBN 978-0-08-100961-1. [Google Scholar]
- Liu, L.; Mei, Z.; Tang, A.; Azarov, A.; Kuznetsov, A.; Xue, Q.-K.; Du, X. Oxygen Vacancies: The Origin of n-Type Conductivity in ZnO. Phys. Rev. B 2016, 93, 235305. [Google Scholar] [CrossRef]
- Ke, L.; Lai, S.C.; Ye, J.D.; Kaixin, V.L.; Chua, S.J. Point Defects Analysis of Zinc Oxide Thin Films Annealed at Different Temperatures with Photoluminescence, Hall Mobility, and Low Frequency Noise. J. Appl. Phys. 2010, 108, 084502. [Google Scholar] [CrossRef]


| Nominal Ge Content (%) | Chemical Composition (at. %) | Nominal Ratio Ge/(Zn + Ge) (%) | Thickness (nm) | ||
|---|---|---|---|---|---|
| O | Zn | Ge | |||
| 0 | 69.9 ± 0.8 | 30.1 ± 0.8 | - | - | 103 |
| 5 | 70.4 ± 0.8 | 27.8 ± 0.8 | 1.8 ± 0.2 | 6.1 | 97 |
| 10 | 69.4 ± 1.3 | 27.5 ± 1.5 | 3.1 ± 0.5 | 10.1 | 116 |
| 15 | 69.5 ± 1.3 | 26.3 ± 1.3 | 4.2 ± 0.2 | 13.8 | 114 |
| 20 | 70.8 ± 0.8 | 23.7 ± 0.6 | 5.5 ± 0.4 | 18.8 | 111 |
| Sample | Roughness Average (Sa) [nm] |
|---|---|
| Si wafer | 0.5 |
| 5% Ge:ZnO | 13.5 |
| 5% Ge:ZnO annealed | 13.6 |
| 15% Ge:ZnO | 10.6 |
| 15% Ge:ZnO annealed | 8.3 |
| 5% Ge:ZnO | Chemical Composition (at. %) | |||
|---|---|---|---|---|
| Zn | Ge | O | C | |
| As-deposited | 41.7 | 0.84 | 40.7 | 16.7 |
| Annealed at 523 K | 43.45 | 0.87 | 43.75 | 11.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knura, R.; Skibińska, K.; Sahayaraj, S.; Marciszko-Wiąckowska, M.; Gwizdak, J.; Wojnicki, M.; Żabiński, P.; Sapeta, G.; Iwanek, S.; Socha, R.P. Evaluation of the Electronic Properties of Atomic Layer Deposition-Grown Ge-Doped Zinc Oxide Thin Films at Elevated Temperatures. Electronics 2024, 13, 554. https://doi.org/10.3390/electronics13030554
Knura R, Skibińska K, Sahayaraj S, Marciszko-Wiąckowska M, Gwizdak J, Wojnicki M, Żabiński P, Sapeta G, Iwanek S, Socha RP. Evaluation of the Electronic Properties of Atomic Layer Deposition-Grown Ge-Doped Zinc Oxide Thin Films at Elevated Temperatures. Electronics. 2024; 13(3):554. https://doi.org/10.3390/electronics13030554
Chicago/Turabian StyleKnura, Rafał, Katarzyna Skibińska, Sylvester Sahayaraj, Marianna Marciszko-Wiąckowska, Jakub Gwizdak, Marek Wojnicki, Piotr Żabiński, Grzegorz Sapeta, Sylwester Iwanek, and Robert P. Socha. 2024. "Evaluation of the Electronic Properties of Atomic Layer Deposition-Grown Ge-Doped Zinc Oxide Thin Films at Elevated Temperatures" Electronics 13, no. 3: 554. https://doi.org/10.3390/electronics13030554






