Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients
Abstract
:1. Introduction
2. Related Works
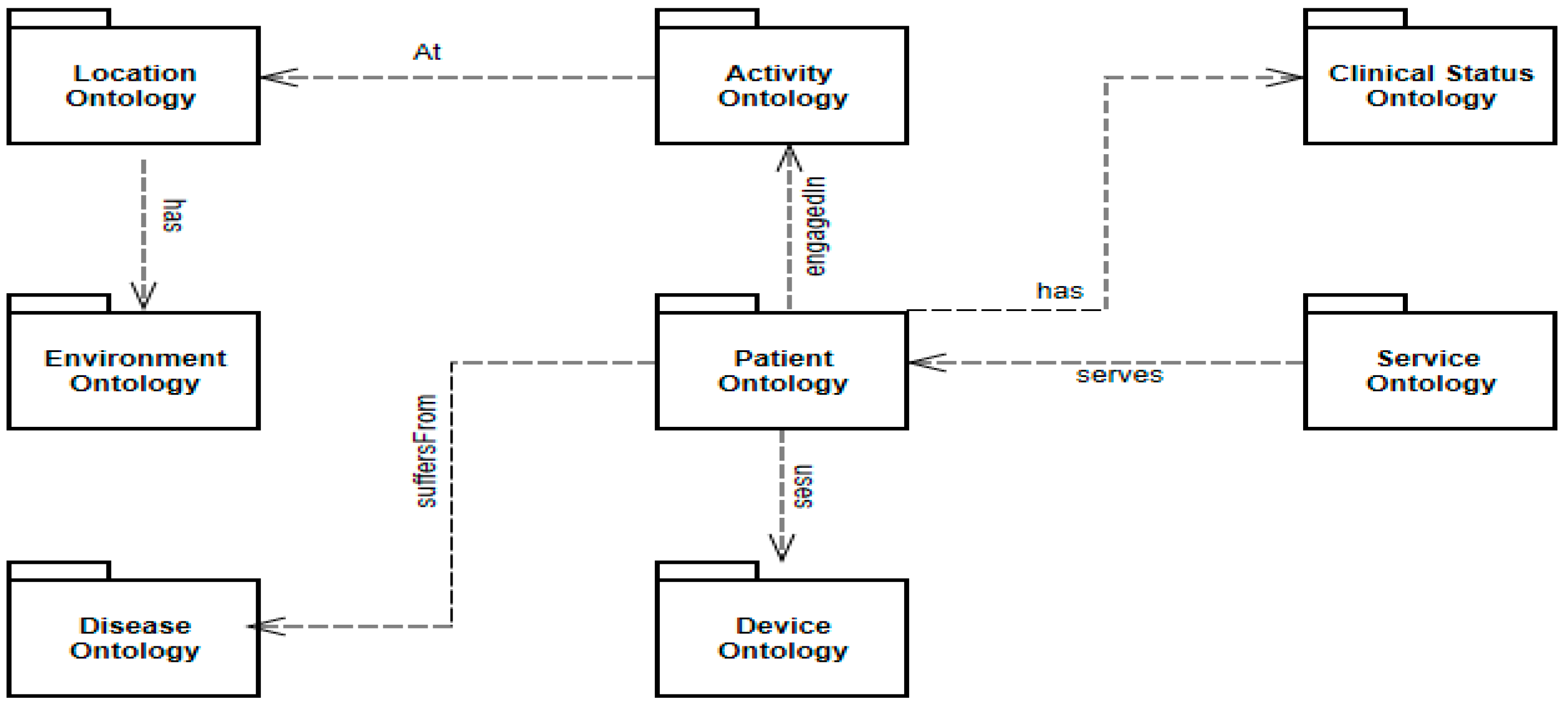
3. Proposed Model
3.1. Data Acquisition Layer
- i.
- Demographic factors:
- i.1
- Age: Aging is often associated with gradual weakness in bodily functions. A statistical analysis using clinical data from 28 countries refers that the prevalence of COPD is approaching 12% threshold with people over 40 years of age [7].
- i.2
- Sex: As we know, certain diseases are more common among men than among women or the inverse. Gender plays an important role in COPD, where the prevalence is doubled in men compared with women.
- i.3
- Race: Many diseases differ in prevalence by race and ethnicity. Recent research is uncovering evidence that ethnicity may influence the development of chronic COPD [14].
- i.4
- Country of residence: The World Health Organization sheet on COPD in the last year shows that most COPD deaths occur in low- and middle-income countries [14].
- ii.
- Physiological factors:
- iii.
- Psychological factors:
- iv.
- Environmental factors:
- iv.1
- Ambient air: Ambient air has been considered as a risk factor for COPD [32] as the concentration of air components (O2, CO2, He, etc.) must be kept in proportion with patient status.
- iv.2
- Weather: Weather conditions are also one of the factors that can trigger COPD symptoms. According to [32], extreme temperature and humidity, atmospheric pressure, precipitation and wind chill have a direct impact on the patient’s life.
- iv.3
- Air pollution: Short- and long-term exposure to indoor and outdoor air pollution have adverse effects and may induce the acute exacerbation of COPD. There is a long list of atmospheric pollutants, such as arsenic, carbon monoxide, nickel, chromium, etc. [33].
- v.
- Physical activity: Regular exercise is part of healthy living, where moderate exercise can improve COPD symptoms and help the organs better use oxygen. on the other hand, excessive exercise may harm COPD patient [34].
- vi.
- vii.
- Comorbidities: Comorbidities are other chronic problems that independently coexist with COPD. There is a set of comorbidities commonly associated with COPD, including coronary heart disease, diabetes mellitus, heart failure, lung cancer, osteoporosis and muscle weakness [35]. Comorbidities of COPD can adversely affect the stage of disease and might lead to early death
- viii.
- Food: Some foods and drinks can exacerbate COPD symptoms. Therefore, it is important to avoid foods which can potentially make this condition worse [34].
- ix.
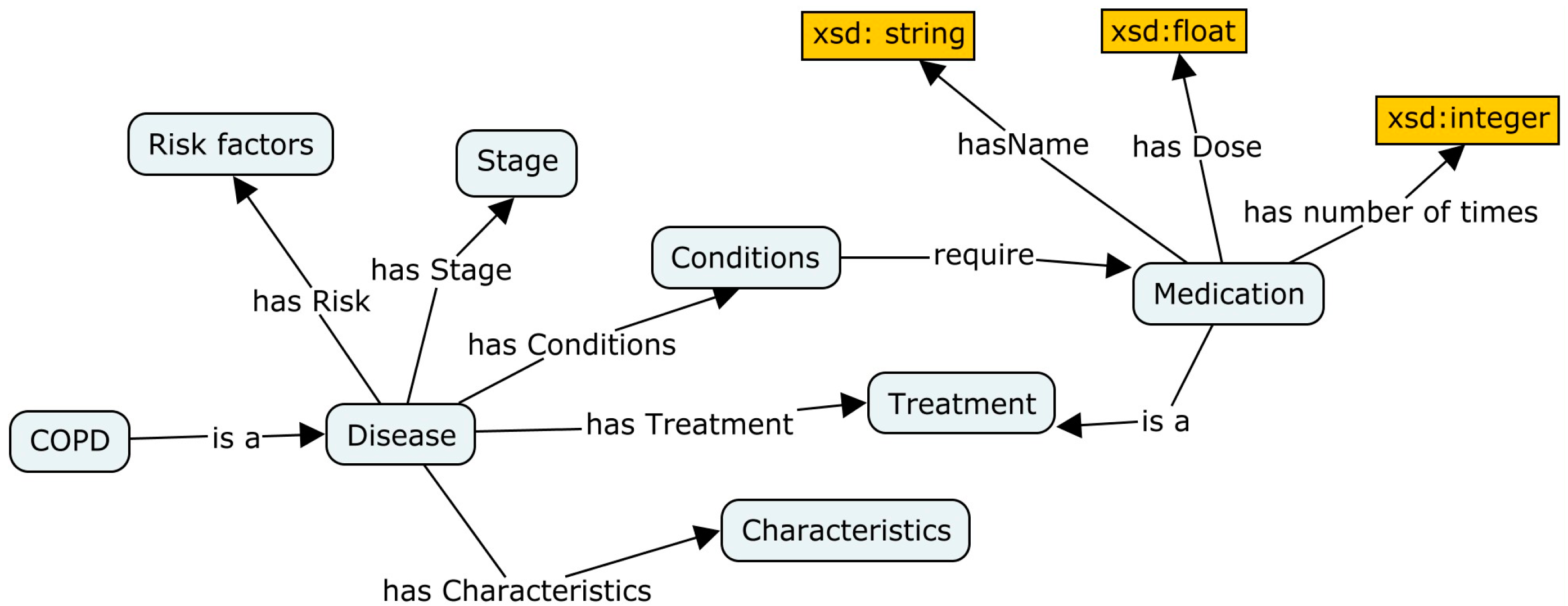
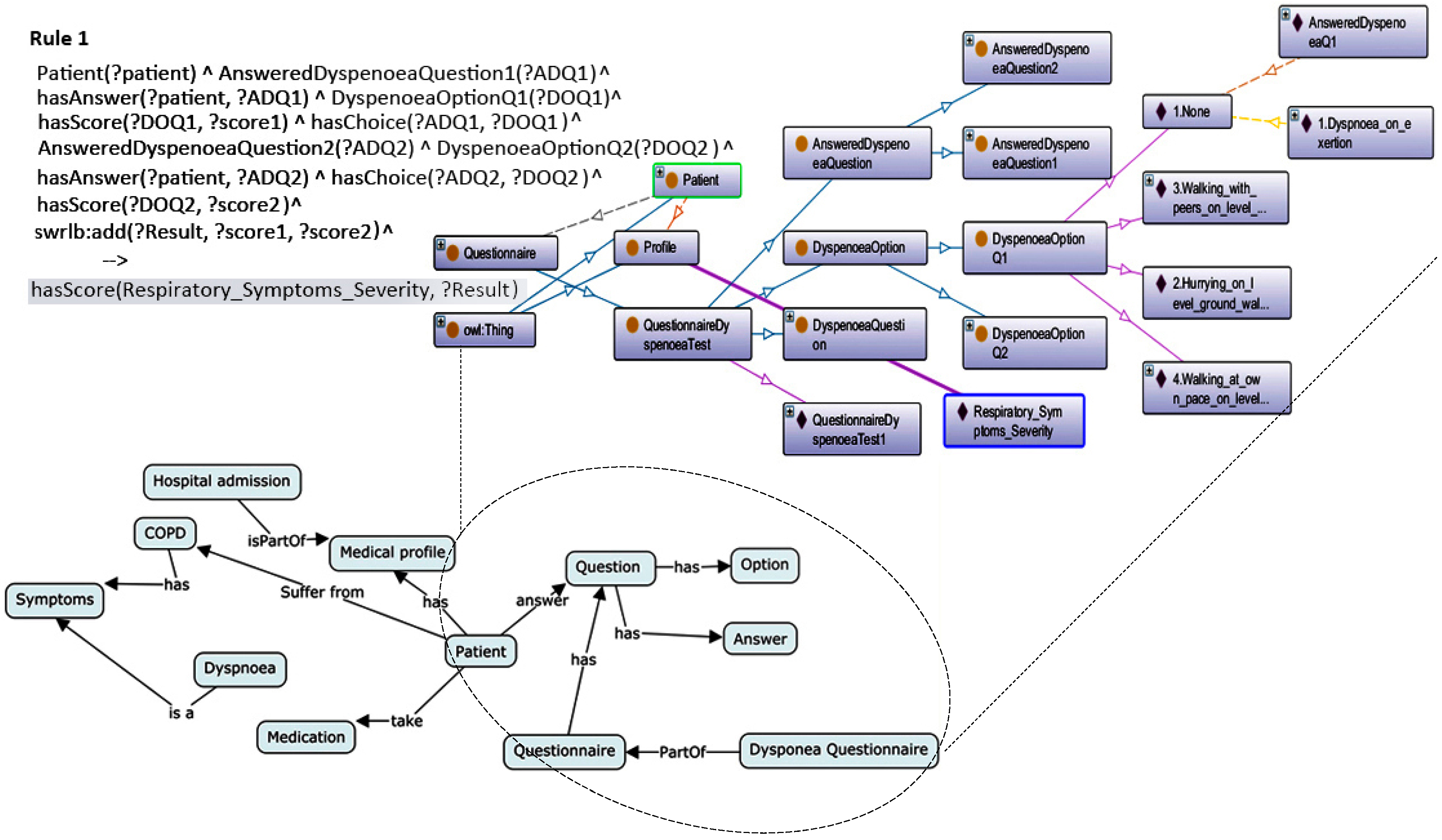
3.2. Semantic Layer (Ontology)
3.2.1. Classifications of Ontology
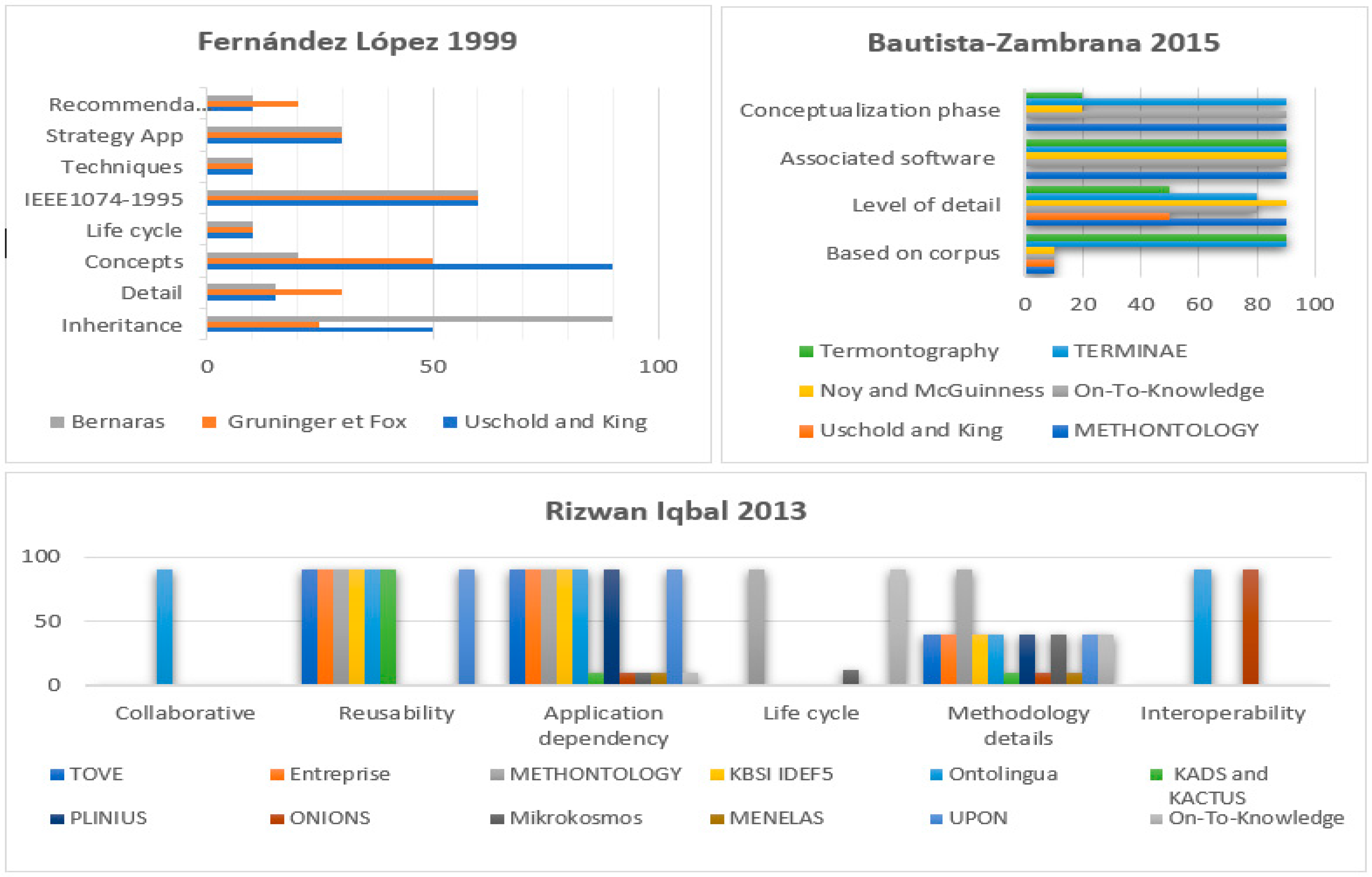
3.2.2. Methodologies for Building Ontologies
- ▪
- What is the domain that the ontology will cover?The chronic obstructive pulmonary disease is the domain of this ontology.
- ▪
- What is the purpose of this ontology?This ontology is designed for preventive management of COPD patients. The main purpose is to facilitate the systematic extraction of information from detailed observations. Our ontology is dedicated to support a personalized system for COPD patient. This ontology provides real-time monitoring and recommendations to help patients cease contact with risk factors and prevent progressive respiratory impairment and allows physicians to be kept informed of the patient’s condition.
- ▪
- Who will use the ontology?Potential users of this ontology are physicians and patients.
- ▪
- What types of questions should the information in the ontology provide answers for?The COPDology must provide answers to questions such as:
- What data should be collected to supervise the patient?
- How often should the patient take a measurement?
- Should the acquired data be transmitted to the healthcare site?
- How should the data be analyzed?
- Should an alarm be triggered according to the evaluation results?
- Which actions should be performed if an alarm is triggered?
- 1.
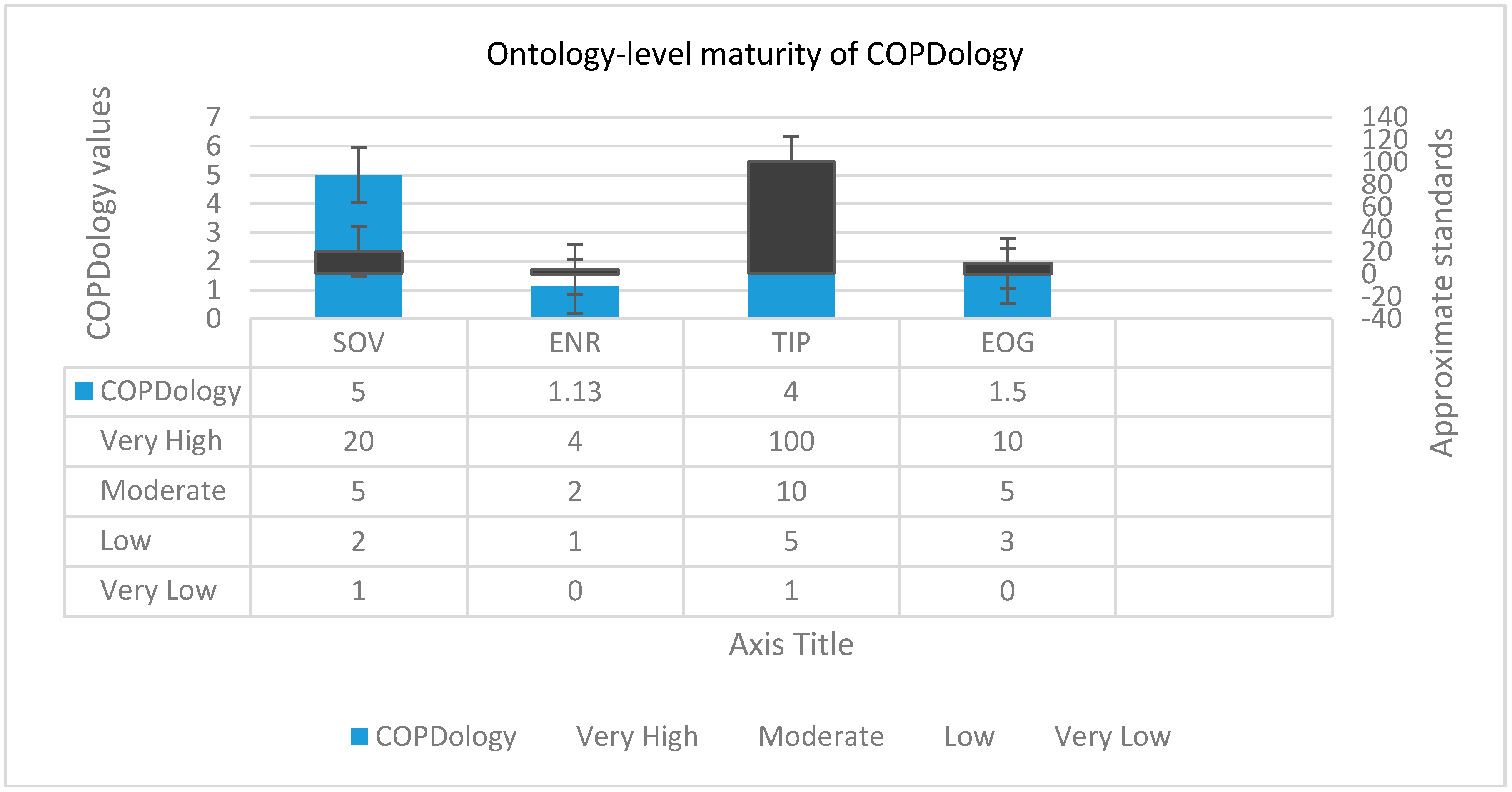
- Size of vocabulary (SOV): This metric includes the total number of created classes, instances and properties in the ontology; the SOV is defined as:where represents the number of named classes, while and are the number of properties and instances, respectively [79].
- 2.
- Edge node ratio (ENR): ENR represents the connectivity density which increases proportionally with the increment of the number of edges between nodes (classes and individuals). ENR is measured as follows:where the number of edges |E| is divided by the number of nodes .
- 3.
- Tree impurity (TIP): This indicator is mainly used to discover how far an ontology inheritance hierarchy digresses from a tree; the TIP is measured as in Equation (3):where represent the suite of relations and concepts in the inheritance hierarchy, respectively.
- 4.
- Entropy of ontology graph (EOG): This norm is an indicator of the graph complexity [80]. It is calculated directly by the application of the logarithm function to a probability distribution over the ontology graph:where p(i) represents the probability mass function for a concept to have i relations. Arithmetically, p(i) can be calculated for each vertex (concepts) in the ontology graph by dividing the degree of the vertex (i.e., properties) connected to that concept over the sum of all degrees of V vertices:
- 1.
- Number of classes (NOC): The NOC metric is simply a count of the defined classes in the ontology [85].
- 2.
- Number of instances (NOI): The NAO criterion is a census of the instances created in the ontology.
- 3.
- Number of properties (NOP): As its name implies, NOP is the number of properties found in an ontology [84].
- 4.
- Number of root classes (NORC): This metric corresponds to the number of non-rooted classes or the concepts that do not have super-classes in their upper layer. Let us consider C the classes in ontology:
- 5.
- Average Population (AP): This variable measures the mean distribution of instances across all classes. Theoretically, AP is defined as follow:According to the rules set [80], this metric has been proposed as an indication of whether there is sufficient information in the ontology.
- 6.
- Class Richness (CR): This value is the ratio between the number of non-empty classes that have instances and the total number of classes. CR percentage give us an idea of how many instances are related to classes defined in the graph.
- 7.
- Relationship Richness (RR): This metric represents the number of relationships divided by the sum of the number of subclasses and the number of relationships [80]:where |P| is considered the overall count of relationships and |SC| is the tally of subclasses or the number of inheritance relationships.
- 8.
- Inheritance richness (IR): The IR describes the distribution of knowledge overall levels of the ontology’s inheritance tree. The inheritance richness of the schema (IRs) is known as the average number of subclasses per class. Formally, this value is calculated from the equation:
4. Processing and Reasoning Layer
4.1. Patient Chart Label
4.2. Patient Location Detector
4.3. Patient Activity Detector
4.4. Risk Factors Detector
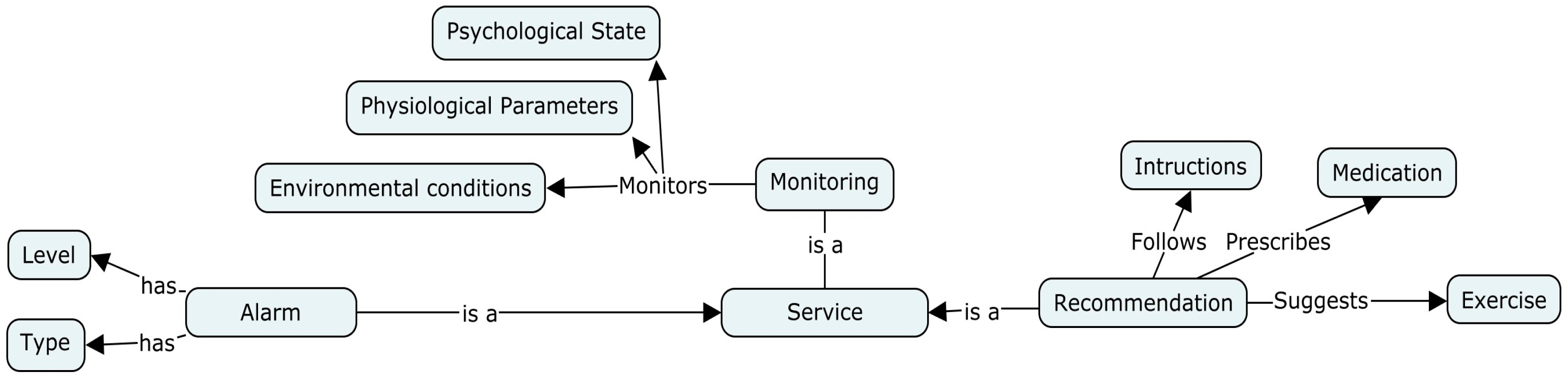
4.5. Medical Services
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GOLD. Available online: www.goldcopd.org (accessed on 15 July 2018).
- Global Strategy for Diagnosis, Management, and Prevention of COPD: 2016. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016 (accessed on 10 July 2018).
- Burden of COPD. Available online: www.who.int/respiratory/copd/burden (accessed on 15 July 2018).
- Chronic Respiratory Diseases. Available online: www.who.int/respiratory (accessed on 15 July 2018).
- Institute for Clinical Systems Improvement. Diagnosis and Management of Chronic Obstructive Pulmonary Disease (COPD), 10th ed. January 2016. Available online: https://www.icsi.org/guidelines__more/catalog_guidelines_and_more/catalog_guidelines/catalog_respiratory_guidelines/copd/ (accessed on 20 April 2018).
- Chronic Respiratory Diseases: Chronic Disease Epidemics. Available online: www.who.int/gard/publications/chronic_respiratory_diseases.pdf (accessed on 1 April 2018).
- Badr, H.; Federman, A.D.; Wolf, M.; Revenson, T.A.; Wisnivesky, J.P. Depression in individuals with chronic obstructive pulmonary disease and their informal caregivers. Aging Ment. Health 2017, 21, 975–982. [Google Scholar] [CrossRef]
- Rospocher, M.; Ghidini, C.; Serafini, L. An ontology for the Business Process Modelling Notation. Front. Artif. Intell. Appl. 2014, 267, 133–146. [Google Scholar]
- Blanc, P.D.; Iribarren, C.; Trupin, L.; Earnest, G.; Katz, P.P.; Balmes, J.; Sidney, S.; Eisner, M.D. Occupational exposures and the risk of COPD: Dusty trades revisited. Thorax 2009, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.; Franssen, F.M.; Wesseling, G.; Wouters, E.F. The prevalence of chronic obstructive pulmonary disease in Maastricht, The Netherlands. Respir. Med. 2012, 106, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Feshchenko, Y.; Iashyna, L.; Nugmanova, D.; Gyrina, O.; Polianska, M.; Markov, A.; Moibenko, M.; Makarova, J.; Tariq, L.; Pereira, M.H. Chronic obstructive pulmonary disease, bronchial asthma and allergic rhinitis in the adult population within the commonwealth of independent states: Rationale and design of the CORE study. BMC Pulm. Med. 2017, 17, 131. [Google Scholar]
- Janssen, D.J.; Spruit, M.A.; Leue, C.; Gijsen, C.; Hameleers, H.; Schols, J.M.; Wouters, E.F. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chronic Respir. Dis. 2010, 7, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Tanni, S.E.; Caram, L.M.; Corrêa, C.; Corrêa, C.R.; Godoy, I. Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir. Res. 2013, 14. [Google Scholar] [CrossRef]
- National Center for Injury Prevention and Control. Web-Based Injury Statistics Query and Reporting System (WISQARS). Leading Causes of Death Report. 2015. Available online: https://webappa.cdc.gov/sasweb/ncipc/leadcause.html (accessed on 10 October 2017).
- Sulaiman, I.; Cushen, B.; Greene, G.; Seheult, J.; Seow, D.; Rawat, F.; MacHale, E.; Mokoka, M.; Moran, C.N.; Sartini Bhreathnach, A. Objective Assessment of Adherence to Inhalers by COPD Patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1333–1343. [Google Scholar] [CrossRef]
- Martínez-Rivera, C.; Vennera Mdel, C.; Cañete, C.; Bardagí, S.; Picado, C. Psychological profile of patients with bronchial asthma and functional dyspnea: A comparison with a non-asthmatic population and impact on the disease. Arch. Bronconeumol. 2011, 47, 73–78. [Google Scholar]
- Wu, W.H.; Batalin, M.A.; Au, L.K.; Bui, A.A.; Kaiser, W.J. Context-aware sensing of physiological signals. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5271–5275. [Google Scholar]
- Bricon-Souf, N.; Newman, C.R. Context awareness in health care: A review. Int. J. Med. Inform. 2007, 76, 2–12. [Google Scholar] [CrossRef]
- Trappenburg, J.C.; Niesink, A.; de Weert-van Oene, G.H.; van der Zeijden, H.; van Snippenburg, R.; Peters, A.; Lammers, J.-W.J.; Schrijvers, A.J. Effects of telemonitoring in patients with chronic obstructive pulmonary disease. Telemed.J. e-Health 2008, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kuilboer, M.M.; Van Wijk, M.A.; Mosseveld, M.; Van Der Does, E.; De Jongste, J.C.; Overbeek, S.E.; Ponsioen, B.; Van der Lei, J. Computed critiquing integrated into daily clinical practice affects physicians’ behavior—A randomized clinical trial with AsthmaCritic. Methods Inf. Med. 2006, 45, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mohktar, M.S.; Basilakis, J.; Redmond, S.J.; Lovell, N.H. A guideline-based decision support system for generating referral recommendations from routinely recorded home telehealth measurement data. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6166–6169. [Google Scholar]
- Rosso, R.; Munaro, G.; Salvetti, O.; Colantonio, S.; Ciancitto, F. CHRONIOUS: An open, ubiquitous and adaptive chronic disease management platform for chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD) and renal insufficiency. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6850–6853. [Google Scholar]
- Song, B.; Wolf, K.-H.; Gietzelt, M.; Scharaa, O.A.; Tegtbur, U.; Haux, R.; Marschollek, M. Decision support for teletraining of COPD patients. In Proceedings of the 2009 3rd International Conference on Pervasive Computing Technologies for Healthcare, London, UK, 1–3 April 2009; pp. 1–6. [Google Scholar]
- Lasierra, N.; Alesanco, A.; Guillén, S.; García, J. A three stage ontology-driven solution to provide personalized care to chronic patients at home. J. Biomed. Inform. 2013, 46, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Lee, H.J.; Lee, J.W. Ontology-based context modeling and reasoning for u-healthcare. IEICE Trans. Inf. Syst. 2007, 90, 1262–1270. [Google Scholar] [CrossRef]
- Paganelli, F.; Giuli, D. Context Aware Information Services to Suppot Tourist Communities. Inf. Technol. Tourism 2018, 10, 313–327. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Quantifying Physical Activity in Daily Life with Questionnaires and Motion Sensors in COPD. Eur. Respire. J. 2006, 27, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- El-Sappagh, S.; Franda, F.; Ali, F.; Kwak, K.S. SNOMED CT standard ontology based on the ontology for general medical science. BMC Med. Inform. Decis. Mak. 2018, 18, 76. [Google Scholar] [CrossRef]
- De Meo, P.; Quattrone, G.; Ursino, D. Integration of the HL7 Standard in a Multiagent System to Support Personalized Access to e-Health Services. IEEE Trans. Knowl. Data Eng. 2011, 23, 1244–1260. [Google Scholar] [CrossRef]
- Bhatt, M.; Rahayu, W.; Soni, S.P.; Wouters, C. Ontology driven semantic profiling and retrieval in medical information systems. J. Web Semant. 2009, 7, 317–331. [Google Scholar] [CrossRef]
- Farfan, F.; Hristidis, V.; Ranganathan, A.; Weiner, M. XOntoRank: Ontology-Aware Search of Electronic Medical Records. In Proceedings of the 2009 IEEE 25th International Conference on Data Engineering, Shanghai, China, 29 March–2 April 2009; pp. 820–831. [Google Scholar]
- Carey, I.M.; Atkinson, R.W.; Kent, A.J.; van Staa, T.; Cook, D.G.; Anderson, H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 1226–1233. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Obstructive Pulmonary Disease (COPD). Available online: http://www.cdc.gov/copd/ (accessed on 30 August 2018).
- National Heart, Lung, and Blood Institute. COPD Learn More Breathe Better Campaign. Available online: http://www.nhlbi.nih.gov/health/public/lung/copd/index. htm (accessed on 30 December 2010).
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef]
- Brewster, C.; O’Hara, K.; Fuller, S.; Wilks, Y.; Franconi, E.; Musen, M.A.; Ellman, J.; Shum, S.B. Knowledge representation with ontologies: The present and future. IEEE Intell. Syst. 2004, 19, 72–81. [Google Scholar] [CrossRef]
- Gruber, T.R. A Translation Approach to Portable Ontologies. Knowl. Acquis. 1993, 5, 199–220. [Google Scholar] [CrossRef]
- Borst, W.N.; Akkermans, J.M. Engineering Ontologies. Int. J. Hum.-Comput. Stud. 1997, 46, 365–406. [Google Scholar] [CrossRef]
- Studer, R.; Benjamins, R.; Fensel, D. Knowledge engineering: Principles and methods. Data Knowl. Eng. 1998, 25, 161–198. [Google Scholar] [CrossRef]
- Maedche, A. Ontology Learning for the Semantic Web; Kluwer Academic Publishers: Boston, MA, USA, 2002. [Google Scholar]
- Falquet, G.; Métral, C.; Teller, J.; Tweed, C. Ontologies in Urban Development Projects; Springer: London, UK, 2011. [Google Scholar] [CrossRef]
- Mizoguchi, R.; Ikeda, M. Towards Ontological Engineering; Technical Report AI-TR-96-1; ISIR, Osaka University: Osaka, Japan, 1996. [Google Scholar]
- Uschold, M.; Gruninger, M. Ontologies: Principles, methods, and applications. Knowl. Eng. Rev. 1996, 11, 93–155. [Google Scholar] [CrossRef]
- Guarino, N. Understanding, building and using ontologies. Int. J. Hum.-Comput. Stud. 1997, 46, 293–310. [Google Scholar] [CrossRef] [Green Version]
- Van Heijst, G.; Schreiber, A.T.; Wielinga, B.J. Using Explicit Ontologies in KBS Development. Int. J. Hum.-Comput. Stud. 1997, 46, 183–292. [Google Scholar] [CrossRef]
- Jurisica, I.; Mylopoulos, J.; Yu, E. Using ontologies for knowledge management: An information systemsperspective. In Proceedings of the 62nd Annual Meeting of the American Society for Information Science (ASISI99), Washington, DC, USA, 31 October–4 November 1999; pp. 482–496. [Google Scholar]
- Sowa, J.F. Knowledge Representation: Logical, Philosophical, and Computational Foundations; Brooks/Cole Publishing Co.: Pacific Grove, CA, USA, 2000. [Google Scholar]
- Lassila, O.; McGuinness, D. The Role of Frame-Based Representation on the Semantic Web; Technical Report KSL-01-02; Knowledge Systems Laboratory, Stanford University: Stanford, CA, USA, 2001. [Google Scholar]
- Fensel, D.; van Harmelen, F. Towards the Semantic Web: Ontology-Driven Knowledge Management; Wiley: New York, NY, USA, 2003; pp. 4–5. [Google Scholar]
- Ruiz, F.; Hilera, J.R. Using Ontologies in Software Engineering and Technology Ontologies for Software Engineering and Software Technology; Springer: Berlin, Germany, 2006; pp. 49–102. [Google Scholar]
- Berdier, C. Urban renewal: How to make a comparison between different approachs: Ase studies: France, Italy and Spain. In Proceedings of the COST C21 Meeting, Belfast, Northern Ireland, 8–9 May 2006. [Google Scholar]
- Obrst, L. Ontological architectures. In TAO—Theory and Applications of Ontology; Poli, R., Healy, M., Kameas, A., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bullinger, A. Innovation and Ontologies: Structuring the Early Stages of Innovation Management; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Fernández-López, M. Overview of methodologies for building ontologies. In Proceedings of the Workshop Ontologies and Problem-Solving Methods: Lessons Learned and Future Trends de la Conferencia International Joint Conference for Artificial Intelligence (IJCAI’99), Stockholm, Sweden, 2 August 1999. [Google Scholar]
- Bautista-Zambrana, M.R. Methodologies to Build Ontologies for Terminological Purposes. Procedia Soc. Behav. Sci. 2015, 173, 264–269. [Google Scholar] [CrossRef]
- Iqbal, R.; Murad, M.A.; Mustapha, A.; Sharef, N.M. An Analysis of Ontology Engineering Methodologies: A Literature Review. Res. J. Appl. Sci. Eng. Technol. 2013, 6, 2993–3000. [Google Scholar] [CrossRef]
- Gruninger, M.; Fox, M.S. Methodology for the Design and Evaluation of Ontologies. In Proceedings of the Workshop on Basic Ontological Issues in Knowledge Sharing, IJCAI-95, Montreal, QC, Canada, 19–20 August 1995. [Google Scholar]
- Sánchez, D.; Batet, M. Semantic similarity estimation in the biomedical domain: An ontology-based information-theoretic perspective. J. Biomed. Inform. 2011, 44, 749–759. [Google Scholar] [CrossRef]
- Fernández-López, M.; Gómez-Pérez, A.; Juristo, N. Methontology: From Ontological Art towards Ontological Engineering. In Proceedings of the Spring Symposium on Ontological Engineering of AAAI, Stanford University, Stanford, CA, USA, 24–26 March 1997; pp. 33–40. [Google Scholar]
- Lenat, D.B.; Guha, R.V. Building Large Knowledge-Based Systems: Representation and Inference in the Cyc Project; Addison-Wesley: Boston, MA, USA, 1990. [Google Scholar]
- Mcheick, H.; Saleh, L.; Ajami, H.; Mili, H. Context Relevant Prediction Model for COPD Domain Using Bayesian Belief Network. Sensors 2017, 17, 1486. [Google Scholar] [CrossRef] [PubMed]
- Slimani, T. A Study Investigating Typical Concepts and Guidelines for Ontology Building. arXiv, 2015; arXiv:1509.05434. [Google Scholar]
- Turpin, B.J.; Weisel, C.P.; Morandi, M.; Colome, S.; Stock, T.; Eisenreich, S.; Buckley, B. Relationships of indoor, outdoor, and personal air (RIOPA): Part II. Analyses of concentrations of particulate matter species. Res. Rep. Health Eff. Inst. 2007, 130, 1–77. [Google Scholar]
- Ferrari, U.; Exner, T.; Wanka, E.R.; Bergemann, C.; Meyer-Arnek, J.; Hildenbrand, B.; Tufman, A.; Heumann, C.; Huber, R.M. Influence of air pressure, humidity, solar radiation, temperature, and wind speed on ambulatory visits due to chronic obstructive pulmonary disease in Bavaria, Germany. Int. J. Biometeorol. 2012, 56, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.G.; Bell, M.L. Weather-related mortality: How heat, cold, and heat waves affect mortality in the United States. Epidemiology 2009, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Zanobetti, A.; Schwartz, J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ. Health Perspect. 2002, 110, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Alatrish, E.S. Comparison Some of Ontology. J. Manag. Inf. Syst. 2013, 8, 18–24. [Google Scholar]
- Abburu, S. A Survey on Ontology Reasoners and Comparison. Int. J. Comput. Appl. 2012, 57, 975–8887. [Google Scholar]
- Yu, J. Requirements-Oriented Methodology for Evaluating Ontologies. Ph.D. Thesis, RMIT University, Melbourne, Australia, July 2008. [Google Scholar]
- Gómez-Pérez, A. Ontology Evaluation. In Handbook on Ontologies. International Handbooks on Information Systems; Staab, S., Studer, R., Eds.; Springer: Berlin, Heidelberg, 2004. [Google Scholar]
- Lovrencic, S.; Cubrilo, M. Ontology evaluation—Comprising verification and validation. In Proceedings of the Central European Conference on Information and Intelligent Systems (CECIIS 2008), Varazdin, Croatia, 24–26 September 2008. [Google Scholar]
- Parsia, B.; Matentzoglu, N.; Gonçalves, R.S.; Glimm, B.; Steigmiller, A. The OWL Reasoner Evaluation (ORE) 2015 Competition Report. J. Autom. Reason. 2017, 59, 455–482. [Google Scholar] [CrossRef] [Green Version]
- Grau, B.; Halaschek-Wiener, C.; Kazakov, Y. History matters: Incremental ontology reasoning using modules. In The Semantic Web; Springer: Berlin, Germany, 2007; pp. 183–196. [Google Scholar]
- Wang, T.D.; Parsia, B. Ontology Performance Profiling and Model Examination: First Steps. In The Semantic Web; Springer: Berlin, Germany, 2007. [Google Scholar]
- Zakaria, N.H.; Hassan, R.; Othman, R.M.; Asmuni, H. Maturity-Based Analysis of Lightweight Ontology from the Aspect of Extensibility, Reusability and Evolutionary. Int. J. Adv. Soft Comput. Appl. 2015, 7, 55–74. [Google Scholar]
- Brewster, C.; Alani, H.; Dasmahapatra, S.; Wilks, Y. Data driven ontology evaluation. In Proceedings of the International Conference on Language Resources and Evaluation, Lisbon, Portugal, 24–30 May 2004. [Google Scholar]
- Obrst, L.; Ceusters, W.; Mani, I.; Ray, S.; Smith, B. The evaluation of ontologies. In Semantic Web; Springer: Boston, MA, USA, 2007; pp. 139–158. [Google Scholar]
- Zhang, H.; Li, Yu.; Tan, H.B.K. Measuring design complexity of semantic web ontologies. J. Syst. Softw. 2010, 83, 803–814. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Ye, C. Evaluation metrics for ontology complexity and evolution analysis. In Proceedings of the 2006 IEEE International Conference on e-Business Engineering (ICEBE’06), Shanghai, China, 24–26 October 2006; pp. 162–170. [Google Scholar]
- Srinivasulu, S.; Sakthivel, P.; Balamurugan, E. Measuring the ontology level and class level complexity metrics in the semantic web. Int. J. Adv. Comput. Eng. Netw. 2014, 2, 68–74. [Google Scholar]
- Santana, M.A.S. Ontologie Pour la traçabilité des Manipulations D’images Médicales. Ph.D. Thesis, Université de Franche-Comté, Besançon, France, 2014. [Google Scholar]
- Brank, J.; Mladenic, D.; Grobelnik, M. Gold standard-based ontology evaluation using instance assignment. In Proceedings of the Workshop on Evaluation of Ontologies for the Web, EON 2006, Edinburgh, UK, 22 May 2006. [Google Scholar]
- Riaño, D.; Real, F.; López-Vallverdú, J.; Campana, F.; Ercolani, S.; Mecocci, P.; Annicchiarico, R.; Caltagirone, C. An ontology-based personalization of health-care knowledge to support clinical decisions for chronically ill patients. J. Biomed. Inform. 2012, 45, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, M.A.; Rodríguez, D.; García-Barriocanal, E.; Sánchez-Alonso, S. Empirical Findings on Ontology Metrics. Expert Syst. Appl. 2012, 39, 6706–6711. [Google Scholar] [CrossRef]
- Kazadi, Y.K.; Fonou-Dombeu, J.V. Analysis of Advanced Complexity Metrics of Biomedical Ontologies in the Bioportal Repository. Int. J. Biosci. Biochem. Bioinform. 2017, 7, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Baader, F.; Calvanese, D.; McGuinness, D.; Nardi, D.; Patel-Schneider, P. The Description Logic Handbook, Theory, Implementation and Applications; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Eiter, T.; Ianni, G.; Krennwallner, T.; Polleres, A. Rules and ontologies for the semantic web. In Reasoning Web; Springer: Berlin, Germany, 2008; pp. 1–53. [Google Scholar]
- Pan, J.Z.; Stoilos, G.; Stamou, G.; Tzouvaras, V.; Horrocks, I. f-SWRL: A fuzzy extension of SWRL. In Journal on Data Semantics VI: Special Issue on Emergent Semantics; Springer: Berlin, Germany, 2006. [Google Scholar]
- Calero, J.M.A.; Ortega, A.M.; Perez, G.M.; Blaya, J.A.B.; Skarmeta, A.F.G. A non-monotonic expressiveness extension on the semantic web rule language. J. Web Eng. 2011, 11, 93–118. Available online: http://dl.acm.org/citation.cfm?id=2230896. 2230897 (accessed on 1 August 2018).
- Miravitlles, M.; Anzueto, A.; Legnani, D.; Forstmeier, L.; Fargel, M. Patient’s perception of exacerbations of COPD-the PERCEIVE study. Respir. Med. 2007, 101, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Golightly, Y.M.; Allen, K.D.; Caine, D.J. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys. Sportsmed. 2012, 40, 52–65. [Google Scholar] [CrossRef]
- Greenwood, J.L.; Joy, E.A.; Stanford, J.B. The Physical Activity Vital Sign: A primary care tool to guide counseling for obesity. J. Phys. Act. Health 2010, 7, 571–576. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, H.; Zhuang, J.; Zhang, J.; Ransdell, L.; Zhu, Z.; Wang, S. Metabolic equivalents of selected sedentary and physical activities in chinese youth. J. Phys. Act. Health 2016, 13, S48–S52. [Google Scholar] [CrossRef]



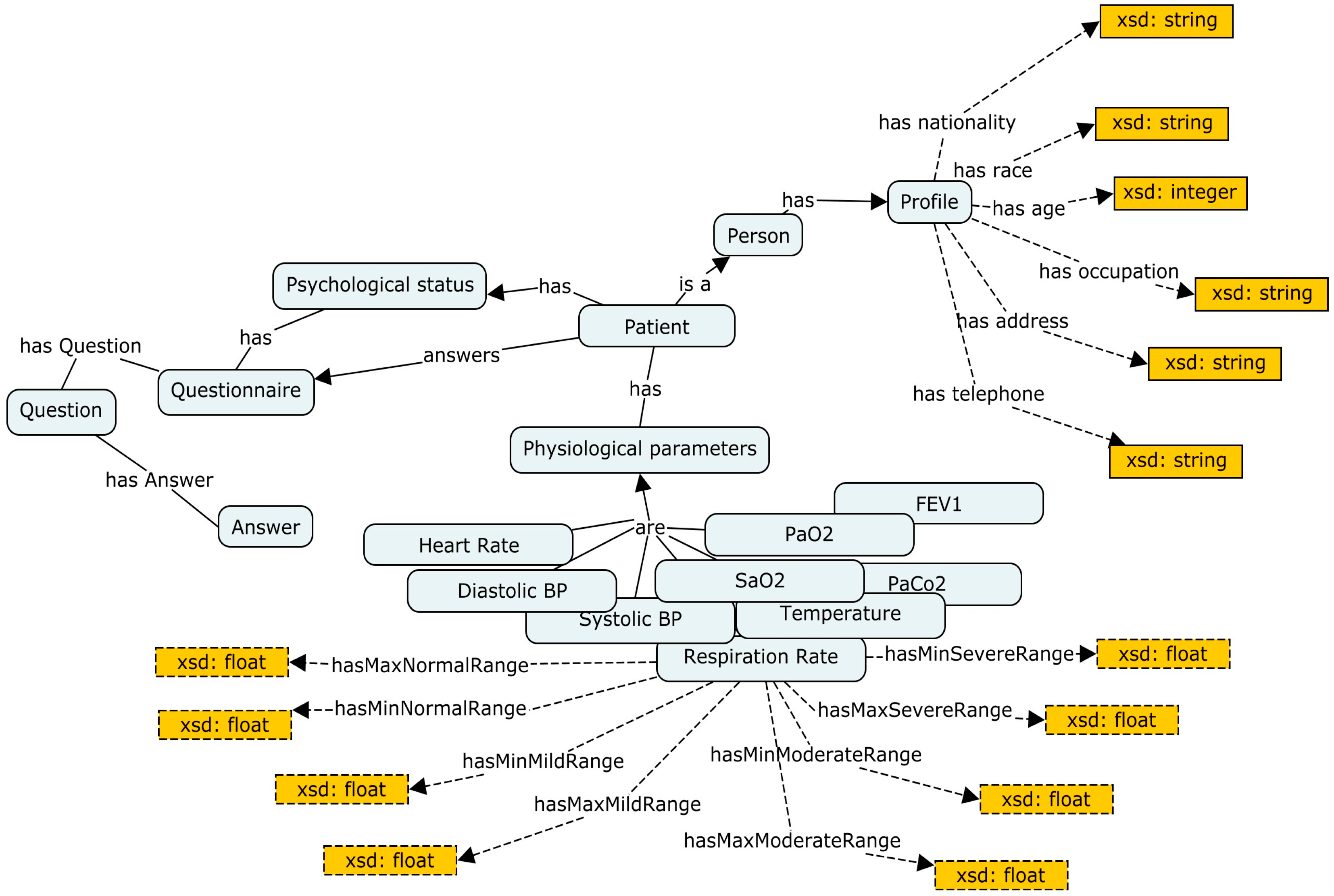









| Albumin | Creatinine | Glomerular Filtration Rate |
| Oxygen Consumption | Hematocrit | PH Level |
| Systolic pressure | Oxygen saturation | Glucose |
| Sodium level | Diastolic pressure | PaO2 |
| Blood Urea Nitrogen | Respiration rate | FEV1 |
| Temperature | Heartrate | PaCo2 |
| Author | Metrics of Classification | Types |
|---|---|---|
| Mizoguchi (1995) | Typology | Content (task, domain and general ontologies), Communication, Indexing, and Meta-ontologies |
| Uschold (1996) | Formality | Highly informal, Structured informal, Semi-formal, Rigorously formal ontologies |
| Purpose | Communication among humans, Inter-operability among systems, System engineering benefits | |
| Subject Matter | Domain, Task/Method/Problem solving, Representation/Meta ontologies | |
| Heijst (1997) | Type of structure of the conceptualization | Terminological, Information, and Knowledge modeling ontologies |
| Subject of the conceptualization | Representation, Generic, Domain, and Application ontologies | |
| Guarino (1998) | level of dependence on a particular task | Top-level, Domain, Task, and Application ontologies |
| Jurisica (1999) | Nature of issue | Static, Dynamic, Intentional ontologies, and Social ontologies |
| Pérez (1999) | Content | Task, Domain and Representation ontologies |
| Issue of the conceptualization | Application, Domain, Generic, and Representation Ontologies | |
| Sowa (2000) | Level of axiomatization | Terminological and Formal ontologies |
| Lassila (2001) | Richness of the internal structure | Controlled vocabulary, Glossary, Thesauri, Term hierarchies, Strict subclass hierarchies, Frames, Ontology with value restrictions, Ontology with logical constraints |
| Fensel (2003) | Level of generality | Generic, Representational, Domain, Method and Task ontologies. |
| Ruiz (2006) | Software engineering | Ontologies of Domain and Ontologies as software artifacts |
| Berdier (2007) | Formalization | Highly informal, Semi-informal, Semi-formal and Rigorously formal |
| Expressiveness | Heavyweight and Lightweight ontologies | |
| Purpose | Application and Reference ontologies | |
| Specificity | Generic, Core and Domain ontologies | |
| Obrst (2010) | Level of generality | Upper, Mid-level, and Domain ontologies |
| Roussey (2011) | Expressivity and formality | Information, Terminological, Software, and Formal ontologies |
| Scope of the objects | Foundational, General, Core reference, Domain, Task, and Local or Application ontologies |
| Class | SNOMED-CT | Class | SNOMED-CT |
|---|---|---|---|
| Patient | 116,154,003 | Systolic pressure | 271,649,006 |
| Profile | 263,878,001 | Glomerular Filtration Rate | 802,740,01 |
| Psychological status | 704,488,001 | Hematocrit | 365,616,005 |
| Oxygen saturation | 449,171,008 | PH Level | 945,600,6 |
| Temperature | 703,421,000 | Total lung capacity (TLC) | 575,660,09 |
| Pulmonary circulation | 177,850,05 | Forced expiratory flow | 251,930,006 |
| Forced vital capacity (FVC) | 508,340,05 | Blood Urea Nitrogen | 723,410,03 |
| Diffusion capacity | 547,150,06 | Diastolic Pressure | 271,650,006 |
| Object Property | Domain | Range | Datatype Property | Domain | Range |
|---|---|---|---|---|---|
| hasTemprature | Physiological | Temperature | hasFname | Profile | String |
| hasHeartRate | Physiological | heartRate | hasLname | Profile | String |
| hasFEV1 | Physiological | FEV1 | hasGender | Profile | String |
| hasHematocrit | Physiological | Hematocrit | hasTelephone | Profile | String |
| hasOxSaturation | Physiological | Oxygen saturation | hasHabits | Profile | String |
| hasRespRate | Physiological | Respiration rate | hasMinNormalRange | vital signs | Float |
| hasBodyWeight | Physiological | Weight | hasaxNormalRange | vital signs | Float |
| hasBodyHeight | Physiological | Height | hasMinSevereRange | vital signs | Float |
| hasGlucose | Physiological | Glucose | hasMaxSevereRange | vital signs | Float |
| Metric | NOC | NOI | NOP | NORC | AP | CR | RR | IR |
|---|---|---|---|---|---|---|---|---|
| COPDology | 180 | 4K | 285 | 12 | 6.52 | 0.80 | 0.389 | 2.210 |
| Degree | Example | Service | Recommendation |
|---|---|---|---|
| Low | Room temperature is one degree lower than the ideal indoor temperature | Notification message | The ambient temperature is out of range |
| Mild | Patient intends to make a mountain trip to a high altitude (6000 feet above sea level) | Warning message | You need an oxygen mask and winter clothing. |
| Moderate | Air quality index rises above 151 | Alert doctor | - |
| Severe | Fever; increase in wheezes, an increase in coughing, increase in heart rate ≥ 20% | Call the emergency services | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajami, H.; Mcheick, H. Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients. Electronics 2018, 7, 371. https://doi.org/10.3390/electronics7120371
Ajami H, Mcheick H. Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients. Electronics. 2018; 7(12):371. https://doi.org/10.3390/electronics7120371
Chicago/Turabian StyleAjami, Hicham, and Hamid Mcheick. 2018. "Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients" Electronics 7, no. 12: 371. https://doi.org/10.3390/electronics7120371
APA StyleAjami, H., & Mcheick, H. (2018). Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients. Electronics, 7(12), 371. https://doi.org/10.3390/electronics7120371






