Monitoring System for Laboratory Mice Transportation: A Novel Concept for the Measurement of Physiological and Environmental Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Legislation about Laboratory Animals Transport
- EU Directive on the protection of animals during transport;
- European Convention ETS 193 for the protection of animals during international transport;
- International Air Transportation Association (IATA) Live Animal Regulations;
- Guidelines for the Humane Transportation of Animals Research.
- Adequate ventilation to avoid or minimize the entry of bacteria and viruses.
- Provide appropriate gripping systems that do not compromise the animal (keeping the box horizontal, for example) or the personnel handling the container.
- The material of which the container is made must be hard, rigid, and resistant, especially to moisture. For this, specially coated cardboard, fiberglass, aluminum, or more commonly, plastics may be employed. The interior surfaces should be durable and smooth, so that the animal cannot damage or gnaw them.
- The space specified for each animal must be broad enough to allow the animal a normal posture and to move freely, avoiding claustrophobic feelings that can cause stress, but not so large as to allow the animal to hit the walls hard in any case or crush with other animals.
- It should also allow sufficient space between the upper part of the body and the top of the container, so that air can circulate freely.
- The publication “Guidance on the transport of laboratory animals” [32] includes information on the minimum dimensions for different species of rodent, depending on their weight.
- Temperature: Suitable temperature for the transport of rodents may vary between 4 °C and 34 °C but should ideally be kept between 20 °C and 26 °C at all times.
- Humidity: The acceptable range is between 40% and 70%. Very low humidity can cause respiratory problems and higher humidity favors the development of bacteria and microorganisms.
- Amount of light: For common animals, the amount of light should be kept as small as possible, always less than 60 lux. Ideally, a 12 h ON and 12 h OFF pattern should be used, including dimmed simulation of sunrise and sunset.
- Sound: Another major source of stress is the sound level, which must be kept as low as possible. Of course, this is often difficult during transport. Mice have the ability to make sounds between 23 Hz and 85 kHz, while the range for rats is between 250 Hz and 70 kHz. If they suffer stress, rats typically emit sounds at a frequency close to 22 kHz [33].
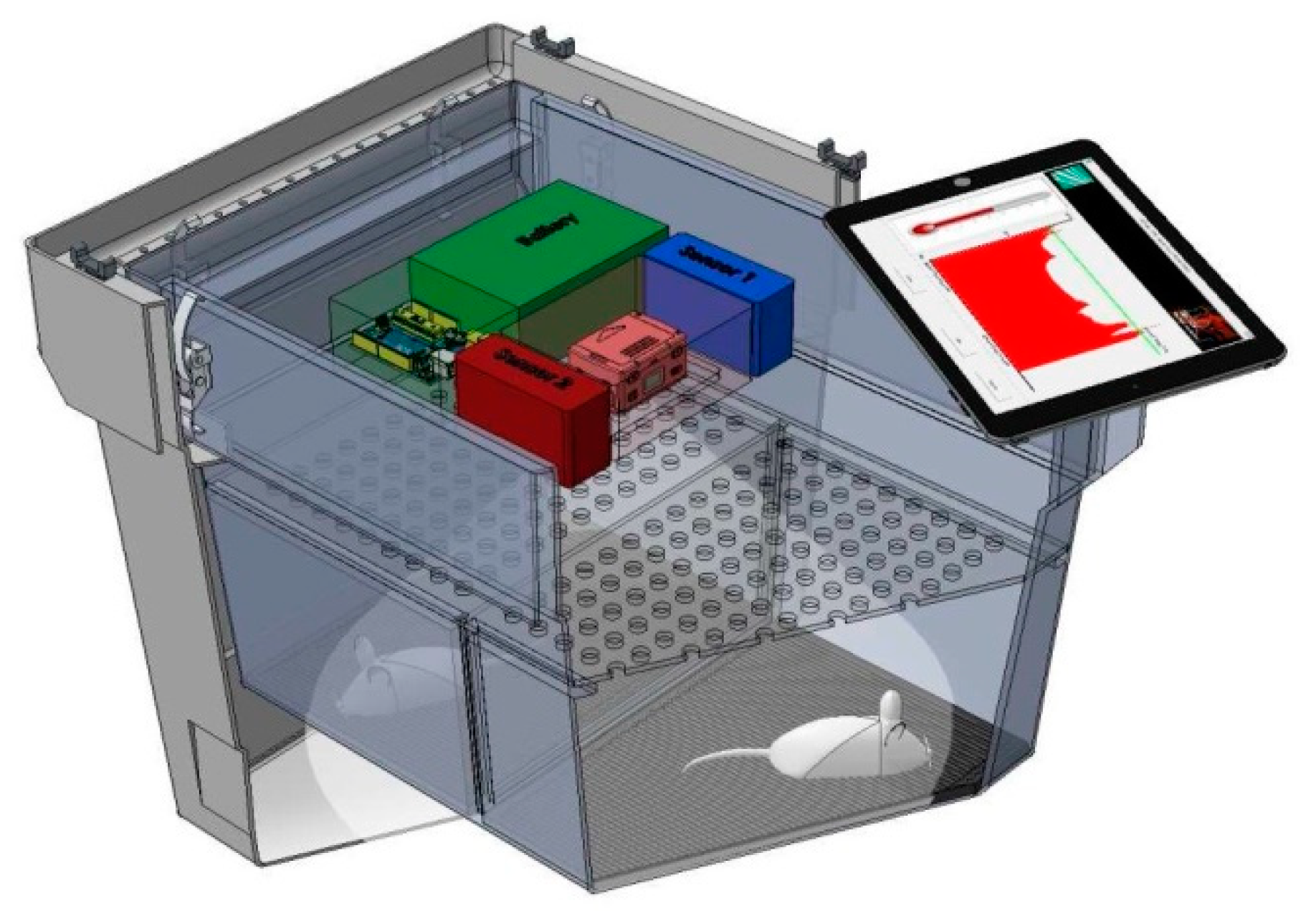
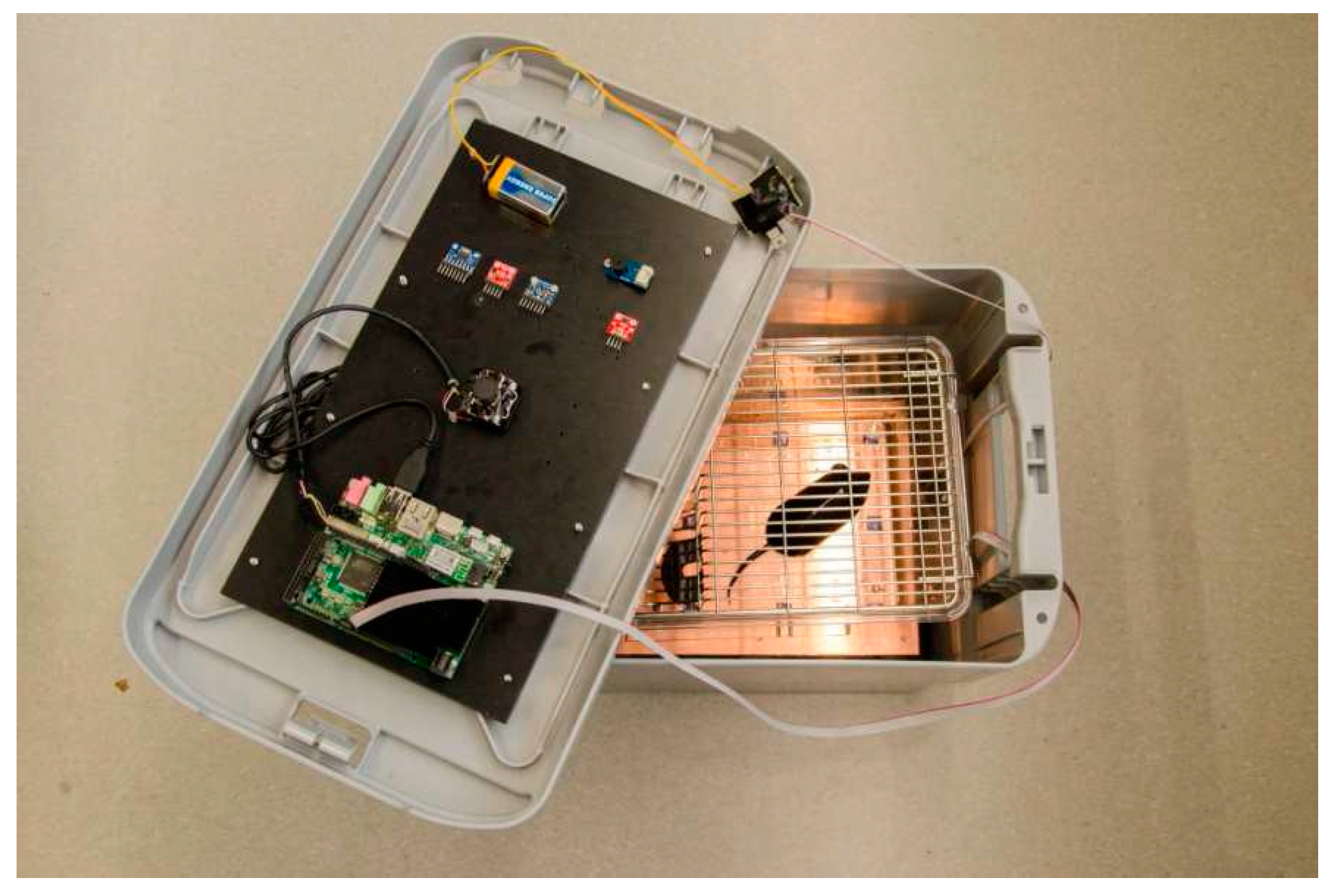
2.2. Design of Monitoring System and Transport Box
- Use of a rechargeable battery due to the portability of the system.
- Capture, storage, and alarm management for the following environmental parameters: Temperature, humidity, movement, light intensity inside the box, and atmospheric pressure. A camera must be included to record the interior of the transport box for visual information about the state of the mice. This could also be used to calculate the position and movement of the mice and assess their level of activity.
- Detection of the stress level of the mice using the matrix of EPIC sensors including power management as well as signal capture, filtering, and analysis.
- Acoustical or optical alert for values outside the acceptable range.
- Wireless access to information: The monitoring system must be able to exchange all data, preferably using WiFi, cellular networks, or RFID. This exchange could take place during transportation or once the transport box has already arrived at the destination, downloading the data to a tablet or similar device.
2.3. Matrix of EPIC Sensors for Capturing Physiological Signals in Mice
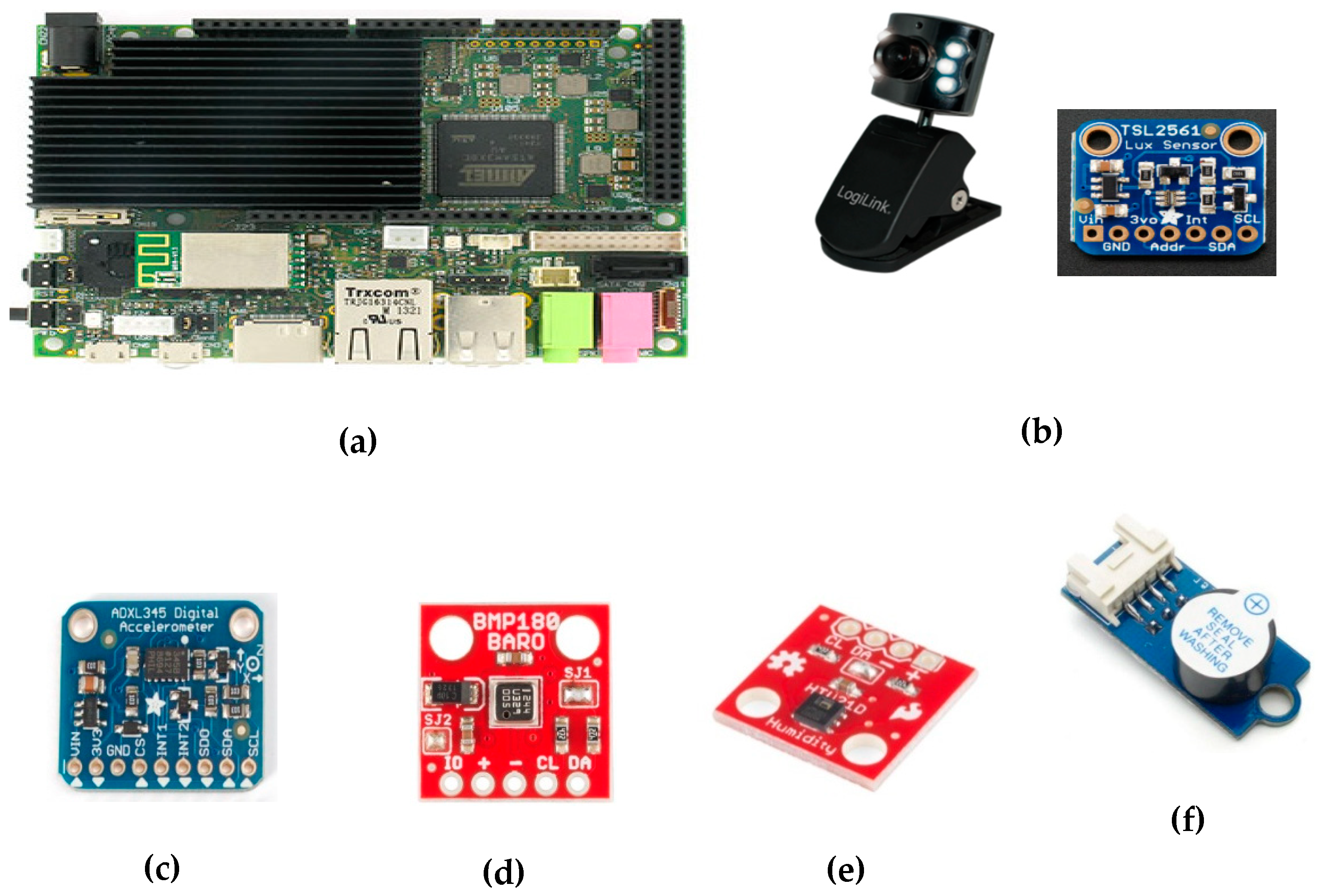
2.4. Selection of Commercial Components and Modules
- Webcam—Logilink UA0072: For capturing images and video inside the transport box, we included a webcam featuring: 0.3 Megapixels, CMOS sensor (640 × 480), F14mm lenses, 54° angle, automatic exposure and brightness adjustments, USB 2.0 connection, refresh rate of 30 frames per second, and 24-bit color depth. In order to use infrared light, which does not interfere with mice since they are not sensitive to it, we removed the IR camera filter and replaced the integrated white power LEDs with 10 infrared ones.
- Visible light and infrared sensor—TAOS TSL2561: This sensor is able to convert a light intensity to a digital output of up to 16 bits of precision. It counts with two photodiodes, one for visible radiation and other for infrared radiation. It also has automatic ripple filtering at 50 or 60 Hz and supports I2C communication.
- Digital accelerometer—Analog Devices ADXL345: This small, very low power consumption accelerometer (0.1 μA in standby mode and 40 μA during measurement) is able to measure acceleration in three axes with at least 10 and up to 13 bits of precision. The measurement range is user adjustable and varies from ± 2 to ± 16 g. It allows to use serial peripheral interface (SPI) and I2C communication protocol.
- Barometric pressure sensor—Bosch BMP180: Based on piezoresistive technology, this sensor offers high accuracy, linearity, and long-term stability. It includes a temperature sensor to correct the pressure value, having a resolution of up to 0.01 hPa for pressure and up to 0.1 °C for temperature. This sensor is capable of providing up to 128 measurements per second.
- Humidity sensor—TE Connectivity HTU21: This low-cost sensor is able to measure temperature in the −40 to 125 °C range and the relative humidity on a scale from 0% to 100%. Its accuracy is 0.04% for relative humidity (12 bits) and 0.01 °C (14 bits) for temperature.
- Buzzer: To implement sound alarms, a small piezoelectric buzzer was included. Its resonant frequency is 3 kHz, but using pulse width modulation (PWM), the pitch can be changed.
3. Results
- A lower platform was fixed to the bottom of the transport box and served as support for the cage where animals were housed during transport. It also contained the 4 × 4 matrix of EPIC sensors.
- An upper platform was fixed at the bottom of the box cover. It included the CPU board and the environmental sensors.
3.1. Measurements with Matrix of EPIC Sensors
3.2. Software for Monitoring and Control of the Transport Box
- “Items” section: It includes type, name, and appearance of the parameters displayed in the user interface.
- “Sitemaps” section: In this section, the graphical user interface is generated, defining the type of menus and submenus. A screenshot of the “main menu” is shown in Figure 11.
- “Rules” section: It handles the automation, allowing the creation of event-based rules (changes of parameters, timers, etc.).
- “Persist” section: It allows us to add different methods of creating and maintaining databases to store the parameters obtained.
- Address: This parameter was only 1 bit set to 0 or 1 depending on the direction of communication (0 for openHAB → Arduino and 1 in the opposite direction).
- Type: The second parameter corresponded to a byte, which identified the type of data being processed (for example, 00000000 indicates temperature, 00000001 indicates humidity, and so on). This data type was not limited to sensor data identification and could also be used to modify alarms or switches.
- Data: The third parameter indicated the value of the requested sensor.
4. Discussion
- (1)
- Development of an algorithm for the stress detection of mice based on EPIC sensor signals: Although the ability of these sensors to capture physiological signals in mice has been proved, and the function for the activation and selection of different sensors has been implemented, the development of an adequate algorithm for the automated sensor selection and stress level classification is necessary.
- (2)
- Signal analysis and integration with the openHAB platform: An algorithm for the capture and automatic filtering of the signal (mainly focused around 50 Hz electrical noises) has been developed using MATLAB. This software has to be conveniently ported to Arduino or another programming language supported by the CPU board. The integration of the acquired signal on the openHAB platform is another important task to be performed.
- (3)
- Power management: The current version of the prototype is designed to be powered using lithium polymer batteries or similar. This adds weight to the transport box, which is far from ideal for such an application. It is necessary to optimize the power consumption of the system.
- (4)
- Secure wireless access to information: A sufficient level of encryption must be provided to prevent third parties from obtaining data captured or stored in the transport box.
- (5)
- Tests under realistic conditions: The number of measurements performed on laboratory mice under realistic conditions has not been as high as we would have liked due to a variety of limitations. Further tests should be carried out in a controlled environment, closer to the reality of transporting laboratory mice.
Author Contributions
Funding
Conflicts of Interest
References
- European Directive 2010/63/EU on “The Protection of Animals Used for Scientific Purposes”. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 5 November 2018).
- Commission Decision of 24 January 2017 Establishing the Commission Expert Group ‘Platform on Animal Welfare’. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1485860312484&uri= CELEX:32017D0131%2801%29 (accessed on 5 November 2018).
- Public Health Service Policy on Humane Care and Use of Laboratory Animals; U.S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2015. Available online: https://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf (accessed on 5 November 2018).
- The Eighth Edition of the Guide for the Care and Use of Laboratory Animals. American Association for Accreditation of Laboratory Animal Care (AAALAC). National Research Council 2011. Available online: http://www.aaalac.org/resources/theguide.cfm (accessed on 5 November 2018).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Seventh Report from the Commission to the Council and the European Parliament on the Statistics on the Number of Animals Used for Experimental and Other Scientific Purposes in the Member States of the European Union. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52013DC0859 (accessed on 5 November 2018).
- Castelhano-Carlos, M.J.; Baumans, V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 2009, 43, 311–327. [Google Scholar] [CrossRef] [PubMed]
- The US Institute for Laboratory Animal Research (ILAR) NRC. Guidelines for the Humane Transportation of Research Animals; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Obernier, J.A.; Baldwin, R.L. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 2006, 47, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Swallow, J.; Anderson, D.; Buckwell, A.C.; Harris, T.; Hawkins, P.; Kirkwood, J.; Lomas, M.; Meacham, S.; Peters, A.; Prescott, M.; et al. Guidance on the transport of laboratory animals. Lab. Anim. 2005, 39, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.W.; Kramer, K.C.; Arndt, S.S.C.; Ohl, F. The impact of transportation on physiological and behavioral parameters in Wistar rats: Implications for acclimatization periods. ILAR J. 2012, 53, E82–E98. [Google Scholar] [CrossRef]
- Arts, J.W.; Oosterhuis, N.R.; Kramer, K.; Ohl, F. Effects of transfer from breeding to research facility on the welfare of rats. Animals 2014, 4, 712–728. [Google Scholar] [CrossRef]
- Capdevila, S.; Giral, M.; Ruiz de La Torre, J.L.; Russell, R.J.; Kramer, K. Acclimatization of rats after ground transportation to a new animal facility. Lab. Anim. 2007, 41, 255–261. [Google Scholar] [CrossRef]
- Van Ruiven, R.; Meijer, G.W.; Wiersma, A.; Baumans, V.; Van Zutphen, L.F.M.; Ritskes-Hoitinga, J. The influence of transportation stress on selected nutritional parameters to establish the necessary minimum period for adaptation in rat feeding studies. Lab. Anim. 1998, 32, 446–456. [Google Scholar] [CrossRef]
- Claassen, V. Neglected factors in pharmacology and neuroscience research. In Techniques in the Behavioral and Neural Sciences; Huston, J.P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1994; Volume 12, pp. 422–459. [Google Scholar]
- Van Ruiven, R.; Meijer, G.W.; Van Zutphen, L.F.M.; Ritskes-Hoitinga, J. Adaptation period of laboratory animals after transport: A review. Scand. J. Lab. Anim. Sci. 1996, 4, 185–190. [Google Scholar]
- Landi, M.S.; Kreider, J.W.; Lang, C.M.; Bullock, L.P. Effects of shipping on the immune function in mice. Am. J. Vet. Res. 1982, 43, 1654–1657. [Google Scholar]
- Drozdowicz, C.K.; Bowman, T.A.; Webb, M.L.; Lang, C.M. Effect of in-house transport on murine plasma corticosterone concentration and blood lymphocyte populations. Am. J. Vet. Res. 1990, 51, 1841–1846. [Google Scholar]
- Tuli, J.; Smith, J.; Morton, D. Stress measurements in mice after transportation. Lab. Anim. 1995, 29, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Harland, C.; Clark, T.; Prance, R. High resolution ambulatory electrocardiographic monitoring using wrist-mounted electric potential sensors. Meas. Sci. Technol. 2003, 14, 923–928. [Google Scholar] [CrossRef]
- Sato, S.; Yamada, K.; Inagaki, N. System for simultaneously monitoring heart and breathing rate in mice using a piezoelectric transducer. Med. Biol. Eng. Comput. 2006, 44, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.; Otero, J.M.; Lopez, O.; Morgan, J.P.; Amende, I.; Hampton, T.G. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001, 1, 6. [Google Scholar] [CrossRef]
- Van Vliet, B.N.; Chafe, L.L.; Antic, V.; Schnyder-Candrian, S.; Montani, J.P. Direct and indirect methods used to study arterial blood pressure. J. Pharmacol. Toxicol. Methods 2000, 44, 361–373. [Google Scholar] [CrossRef]
- Arcelus, A.; Sardar, M.; Mihailidis, A. Design of a capacitive ECG sensor for unobtrusive heart rate measurements. In Proceedings of the IEEE International Conference in Instrumentation and Measurement Technology Conference, The Depot, MN, USA, 6–9 May 2013; pp. 407–410. [Google Scholar]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A smart health monitoring chair for nonintrusive measurement of biological signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef]
- Lee, H.J.; Hwang, S.H.; Yoon, H.N.; Lee, W.K.; Park, K.S. Heart Rate Variability Monitoring during Sleep Based on Capacitively Coupled Textile Electrodes on a Bed. Sensors 2015, 15, 11295–11311. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A Wearable Capacitive Sensor for Monitoring Human Respiratory Rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05-1–04CL05-7. [Google Scholar] [CrossRef]
- Oum, J.H.; Lee, S.E.; Kim, D.W.; Hong, S. Non-contact heartbeat and respiration detector using capacitive sensor with Colpitts oscillator. Electron. Lett. 2008, 44, 233–245. [Google Scholar] [CrossRef]
- González-Sánchez, C.; Fraile, J.-C.; Pérez-Turiel, J.; Damm, E.; Schneider, J.G.; Zimmermann, H.; Schmitt, D.; Ihmig, F.R. Capacitive Sensing for Non-Invasive Breathing and Heart Monitoring in Non-Restrained, Non-Sedated Laboratory Mice. Sensors 2016, 16, 1052. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Animal Science Association (LASA). Guidance on the transport of laboratory animals. Report of the Transport Working Group established by LASA. Lab. Anim. 2005, 39, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, A.; Rose, M. Guidelines for the housing of mice in scientific institutions. Animal Welfare Unit, NSW Department of Primary Industries, West Pennant Hills. Anim. Res. Rev. Panel 2012, 1, 1–143. [Google Scholar]
- Stryjek, R. A transportation device for rats. Lab Anim. 2010, 39, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Tada, T.; Fujikura, E.; Matsumoto, K.; Tanaka, Y.; Hongo, K. A container for transporting small laboratory animals for magnetic resonance imaging. J. Neurosci. Methods 2005, 144, 143–146. [Google Scholar] [CrossRef] [PubMed]










| (1,1) | (1,2) | (1,3) | (1,4) |
| 1 | 2 | 4 | 8 |
| (2,1) | (2,2) | (2,3) | (2,4) |
| 16 | 32 | 64 | 128 |
| (3,1) | (3,2) | (3,3) | (3,4) |
| 256 | 512 | 1024 | 2048 |
| (4,1) | (4,2) | (4,3) | (4,4) |
| 4096 | 8192 | 16384 | 32768 |
| (1,1) | (1,2) | (1,3) | (1,4) |
| 0–0000 | 1–0001 | 2–0010 | 3–0011 |
| (2,1) | (2,2) | (2,3) | (2,4) |
| 4–0100 | 5–0101 | 6–0110 | 7–0111 |
| (3,1) | (3,2) | (3,3) | (3,4) |
| 8–1000 | 9–1001 | 10–1010 | 11–1011 |
| (4,1) | (4,2) | (4,3) | (4,4) |
| 12–1100 | 13–1101 | 14–1110 | 15–1111 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Sánchez, C.; Fraile, J.-C.; Pérez-Turiel, J.; Damm, E.; Schneider, J.G.; Schmitt, D.; Ihmig, F.R. Monitoring System for Laboratory Mice Transportation: A Novel Concept for the Measurement of Physiological and Environmental Parameters. Electronics 2019, 8, 34. https://doi.org/10.3390/electronics8010034
González-Sánchez C, Fraile J-C, Pérez-Turiel J, Damm E, Schneider JG, Schmitt D, Ihmig FR. Monitoring System for Laboratory Mice Transportation: A Novel Concept for the Measurement of Physiological and Environmental Parameters. Electronics. 2019; 8(1):34. https://doi.org/10.3390/electronics8010034
Chicago/Turabian StyleGonzález-Sánchez, Carlos, Juan-Carlos Fraile, Javier Pérez-Turiel, Ellen Damm, Jochen G. Schneider, Daniel Schmitt, and Frank R. Ihmig. 2019. "Monitoring System for Laboratory Mice Transportation: A Novel Concept for the Measurement of Physiological and Environmental Parameters" Electronics 8, no. 1: 34. https://doi.org/10.3390/electronics8010034
APA StyleGonzález-Sánchez, C., Fraile, J.-C., Pérez-Turiel, J., Damm, E., Schneider, J. G., Schmitt, D., & Ihmig, F. R. (2019). Monitoring System for Laboratory Mice Transportation: A Novel Concept for the Measurement of Physiological and Environmental Parameters. Electronics, 8(1), 34. https://doi.org/10.3390/electronics8010034





