3.1. Analysis of Dispersion Characteristics of Glass Tube Waveguide
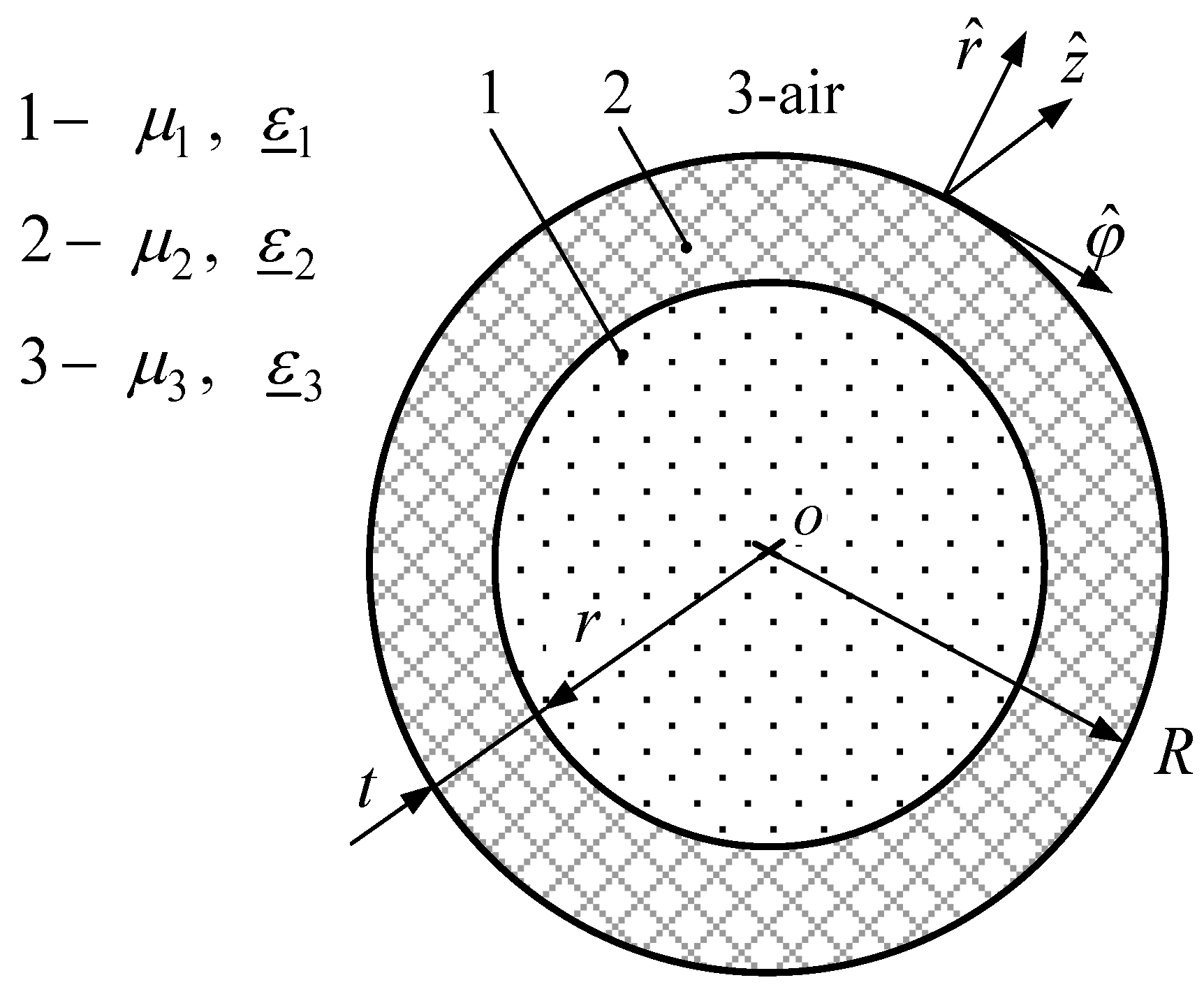
Dispersion characteristics of the glass tube waveguide express the phase constant h′ and attenuation constant h′′ as functions of frequency f, channel radius is r = 1 mm, whereas the external radius of the glass tube waveguide is R = 5 mm.
In
Figure 3 and
Figure 4 the normalized phase constant h′/k and the attenuation constant h′′ dependences of the waveguides in the frequency range from 1 GHz to 100 GHz are given, where k is the propagation constant in free space. These figures display the frequency characteristics of the fundamental mode for three cases: empty glass tube waveguide (black line), waveguide filled with blood plasma (red line), and waveguide filled with blood cells (green line). In
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9, the inner waveguide radius (corresponding to the channel radius) is r = 1 mm, whereas the external radius of the glass tube waveguide is R = 5 mm.
We have discovered two kinds of different modes in the frequency range from 1 GHz to 100 GHz. These modes can propagate in the waveguides, which are filled with blood plasma and blood cells. This phenomenon might be explained as follows. In order to understand the processes taking place in the layered “air-glass-blood” waveguide, we can mentally imagine that the one is dissected into two almost independent waveguides, i.e., a glass tube waveguide and cylindrical core waveguide, which is made of very dissipative liquid blood components. The implied cylindrical core waveguide boundary matches with the central channel boundary of the tube waveguide. The cylindrical core waveguide is surrounded by lossless and low-permittivity materials such as glass and air.
These waveguides as the glass tube and the biomaterial core of the waveguide assume the existence of two independent groups of modes.
This is the reason why modes of several kinds may propagate in the layered waveguide when it is filled with blood components. The two kinds of the propagating hybrid modes investigated in the present study feature remarkably dissimilar dispersion curves as well as quite different distributions of the EM field.
In the case of the first kind of the modes, the EM field energy concentrates mostly in the glass tube. For this reason, the modes of the waveguide filled with blood components have dispersion characteristics similar to those of the empty glass tube waveguide and the modes have relatively low losses. We shall call these the “tube” modes.
The EM field energy of the second kind of the hybrid modes is concentrated in the channel of the waveguide filled with the absorbent substance, such as blood plasma and blood cells. Losses of those modes are higher, and we shall call them the “core” modes.
This paper presents dispersion characteristics of the fundamental and the first higher “tube” modes (
Figure 3) and the ЕМ field distributions of the fundamental “tube” mode (
Figure 7 and
Figure 8). Dispersion characteristics of the fundamental HE
11 and the first higher EH
11 modes are required for calculating the broad-bandwidth (or the operating frequency range) of the waveguide.
Since the higher mode types (the second, the third, etc.) are seldom used when developing microwave devices, their analysis was not included in the current study. The results of our calculations of dispersion characteristics using the commercial program CST MWS are marked with squares for the empty waveguide and with triangles when filled with blood cells (
Figure 3). For the “core” modes, the EM field energy is concentrated in the waveguide’s central channel, which is filled with an absorbent substance. Dispersion characteristics of the “core” modes are shown in
Figure 4. The “core” mode is accompanied by higher losses (
Figure 4b and
Figure 9b).
Let us examine alternately the properties of the EM “tube” and “core” modes. The cutoff frequency of the “tube” mode present in the waveguide filled with air, blood plasma, and blood cells is 7.10, 6.61, and 19.27 GHz, respectively. Meanwhile, for the first higher mode, the cutoff frequency is 21.70, 19.69, and 20.86 GHz, respectively (
Figure 3).
As seen in
Figure 3a, the phase coefficient of the fundamental mode for the waveguide filled with blood plasma has an anomalous dispersion in the frequency band of 40‒52 GHz. In the frequency band of 47‒49 GHz, such an anomaly is observed for the first higher “tube” mode as well.
The anomalous dispersion is revealed by the abnormal changes of phase coefficient h’, which is equivalent to the wavelength λ that decreases with frequency in a certain frequency range. One can also observe anomalous dispersion at the frequency range of 48‒54 GHz but only for the fundamental “tube” modes present in a waveguide filled with blood cells.
Above 70 GHz, the frequency characteristic of the phase constant for the fundamental mode in a waveguide filled with liquid is virtually identical to that of an empty waveguide (
Figure 3a).
This is due to the fundamental mode propagating almost inside the glass layer in the high-frequency part of the considered range where the EM parameters of a biological liquid have almost no effect on the propagation of the modes (see
Figure 3a).
At high frequencies, the attenuation coefficient of the fundamental mode approaches zero since the mode’s EM energy is completely drawn into the lossless glass medium with ε’’ = 0. This way the mode propagates along the glass tube with very small attenuation, as seen in
Figure 3b. We would like to note that the losses in the glass were not taken into account in order to observe the impact on the waves of absorbent materials.
A completely different situation occurs for the first higher mode present in the waveguide filled with blood plasma. Its phase coefficient starts decreasing slightly at frequencies above 65 GHz (
Figure 3a). Meanwhile, the attenuation coefficient (i.e., the waveguide loss) for this mode increases substantially (
Figure 3b).
As a consequence, a larger part of the EM energy of the first higher mode propagates in blood plasma when f > 65 GHz. However, the mode spreading along the waveguide disappears fast due to substantial losses.
Note that in the waveguide filled with blood plasma, the fundamental mode propagates with very small loss at frequencies above 65 GHz and the first higher mode is strongly attenuated. Due to this, one can improve the broad-bandwidth of the waveguide.
At frequencies above 65 GHz, the attenuation for the first higher “tube” mode present in the waveguide filled with air and blood cells is relatively low and decreases further with frequency.
In the frequency range of approximately 7 GHz to 60 GHz, the attenuation coefficient curves are quite complex. This is due to redeployment of some of the EM energy between the glass tube and the channel of the waveguide filled with blood components. The attenuation becomes higher when the EM energy of a mode penetrates deeper into the channel, which is filled by blood plasma with the permittivity ε
1’’ that can reach 39 and blood cells with ε
1’’ that can reach up to about 10 at the 7–60 GHz frequency range (
Figure 3b).
Figure 4a,b show how the phase and attenuation constants depend on frequency for the fundamental “core” mode only. As seen in
Figure 4a, with increasing value of
the mode wavelength decreases while the attenuation increases. Therefore, for the waveguide filled with blood plasma, the attenuation coefficient of the fundamental “core” mode is large and can be in the range of 1000–2000 m
−1 (
Figure 4b).
When the waveguide is empty, the cutoff frequency of the “core” mode is 7.10 GHz. For the waveguide, which is filled with blood plasma and blood cells, it is equal to 4.95 GHz and 6.67 GHz respectively. The fundamental “core” mode is of the hybrid magnetic type HE11. We have implemented this classification by calculating all the EM field components of this mode. It has turned out that longitudinal components relate Hz > Ez. Waveguides with the empty channel as well as those filled with blood cells have low attenuation (h’’ < 20 m−1) in the frequency range of 5–15 GHz.
Figure 5 displays the waveguide’s broad-bandwidth for the “tube” modes (see
Figure 3) as a function of the inner radius r of the glass tube when its external radius is R = 5 mm. As the inner radius r varies from 0.1 mm to 4.5 mm, thickness of the glass tube’s wall decreases from 4.9 mm to 0.5 mm.
The broad-bandwidth of the waveguide is calculated using the following expression:
where Δf = f
cut2–f
cut1, f
cut1 is the cutoff frequency of the fundamental mode, f
cut2 is the cutoff frequency of the first higher mode, and f
c = ( f
cut1+f
cut2)/2 is the center frequency.
The relationship between the waveguide’s broad-bandwidth and the radius r is presented in
Figure 5 for the cases of an empty glass tube waveguide (black line), and for the waveguide filled with blood plasma (red line) or blood cells (green line).
The broad-bandwidth of an empty waveguide gradually decreases with an increase in the channel’s inner radius. However, the broad-bandwidth drops when the radius exceeds 3.5 mm. This is a result of the waveguide walls becoming very thin and part of the EM mode power leaves the boundaries of the waveguide. In turn, this leads to a significant increase of non-linearity in the frequency dependence of the phase constant.
For a waveguide filled with liquid, the mode critical frequencies become very sensitive to changes in the waveguide’s geometry.
Figure 5 shows minima and maxima of the broad-bandwidth at certain values of r. They are highly influenced by the frequency-dependent complex relative dielectric permittivity of biologic liquids.
Hence, one can choose a suitable value for the broad-bandwidth of the “tube” modes by selecting the thickness of the wall of the glass tube waveguide.
3.2. Analysis of Electric Field Distributions in the Glass Tube Waveguide
Analysis of the distributions of magnetic and electric field components is an important part of the solution for the EM problem. These distributions allow us to determine the type of hybrid mode as well as its location. A waveguide can support propagation of the modes of magnetic and/or electric types.
Dispersion characteristics for the “tube” (
Figure 3) and “core” (
Figure 4) modes were used as the basis for calculating the EM field components (E
r, E
φ, E
z) in various points of the transverse and longitudinal cross-sections. We performed a simulation of the EM field components in 40,400 points of the transverse waveguide’s cross-section (
Figure 6а,
Figure 7a,
Figure 8a,
Figure 9а) and in 29,400 points of the longitudinal waveguide’s cross-section (
Figure 6b,
Figure 7b,
Figure 8b,
Figure 9b).
Only the distributions of the electric field for the fundamental “tube” and “core” modes are presented here. Wavelength λ of the modes is given at the top of
Figure 6b,
Figure 7b,
Figure 8b,
Figure 9b.
We used a method that allowed exploring the electric and magnetic field distributions of the higher modes as well [
15].
The electric field is represented in two ways, by the electrical field lines and by color intensity. In the first case, the electric field is visualized at every point of both the cross-sections with a collection of arrows each having a certain length and direction. The tip of an arrow indicates direction of the electric field lines at the point considered, whereas the arrow’s length is proportional to the strength of the electric field vector at the point.
For a better display, color coding is also used in
Figure 6,
Figure 7,
Figure 8 and
Figure 9 for the electric field densities. A scale placed on the right side of the corresponding picture shows the normalized intensity of the EM field. The greatest intensity of an electric field corresponds to the unit, i.e., to the dark red color.
Figure 6 shows the electric field’s distribution of the fundamental mode for the glass tube waveguide filled with air, i.e., the one with an empty channel. The radii of this waveguide are r = 1 mm and R = 5 mm. Calculations were performed at the frequency of 50 GHz.
The electric field’s vector is
, where
are the field projections, and
are the unit vectors (see
Figure 1). The electric field’s vector at an arbitrary point of the transverse cross-section was calculated as a sum of two vectors
and
Meanwhile, the electric field’s vector at an arbitrary point of the longitudinal cross-section was calculated as a sum of two vectors
and
These plots clearly demonstrate the electric field’s structure at each layer and outside of the waveguide. Moreover, they identify the location of the highest concentration of the electric field. For the case of an empty glass waveguide, the electric field concentrates within the glass material (
Figure 6).
The “tube” mode is not strongly attenuated along the waveguide, as we chose zero value for the imaginary part of the glass permittivity in our calculations. As can be seen, the electric field extends only about 3–4 mm beyond the waveguide’s external radius. In the inner channel of the waveguide, the electric field is weaker than that within the glass layer by a factor of 2.5. Such a structure of the field’s fundamental mode is typical for dielectric hollow cylindrical waveguides made of other materials as well [
15].
The structure of the electric field’s fundamental mode changes substantially when the inner channel of the waveguide is filled with blood plasma (
Figure 7) or blood cells (
Figure 8). Similar to the case of an empty waveguide, the largest portion of the EM mode’s power does not leave the boundaries of the waveguide. The electric field is most intense within glass at the boundary between the inner r and the outer R of the waveguide. As can be seen from
Figure 7 and
Figure 8, the structure of the field is very similar in both cases.
We can see that the electric field concentrates around the channel with the (repeating) form of the field maxima.
Figure 7 and
Figure 8 also demonstrate a small phase shift between the propagating modes due to the different phase constants (h’ = 2π/λ) of the modes at 50 GHz (
Figure 3a). In the inner channel of the waveguide filled with blood cells, the electric field is stronger than that found in the waveguide filled with blood plasma. This is due to the fact that the parameter
of blood cells is lower than that of blood plasma. For the same reason, the losses of the fundamental mode are also lower in the waveguide filled with blood cells (
Figure 3b).
In the waveguide filled with blood cells, the “core” mode can propagate as well, with its power focused in the inner channel (
Figure 9). According to the electric field’s structure, this mode also corresponds to the hybrid magnetic HE
11 mode. The mode propagates within the inner channel of the waveguide filled with blood cells, as if it were a single-layer waveguide wrapped with a glass layer from the outside. Since blood cells have high absorption of EM modes’ radiation, the strength of the electric field of the mode that propagates in the waveguide diminishes by a factor of four per one wavelength
λ (
Figure 9b).