A Study of the Sulfidation Behavior on Palladium-Coated Copper Wire with a Flash-Gold Layer (PCA) after Wire Bonding
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Morphologies and Cross Sections of the Wires
3.2. Mechanical and Electrical Properties of the Wires
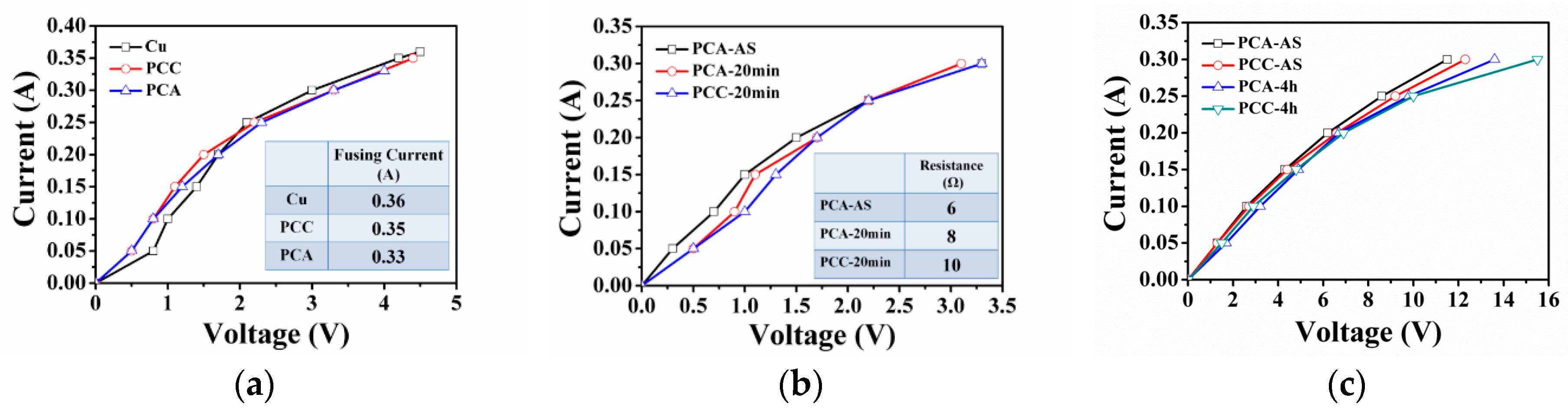
3.3. Bonding Strength and Electrical Properties
3.4. Limitations and Suggestion of this Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harman, G.G. Wire Bonding in Microelectronics Materials, Processes, Reliability, and Yield, 2nd ed.; McGraw-Hill: New York, NY, USA, 1997; pp. 1–21. [Google Scholar]
- Nguyen, L.; McDonald, D.; Danker, A.; Ng, P. Optimization of copper wire bonding on Al-Cu metallization. IEEE Trans. Components Packag. Manuf. Technol. Part A 1995, 18, 423–429. [Google Scholar] [CrossRef]
- So, H.; Senesky, D.G. ZnO nanorod arrays and direct wire bonding on GaN surfaces for rapid fabrication of antireflective, high-temperature ultraviolet sensors. Appl. Surf. Sci. 2016, 387, 280–284. [Google Scholar] [CrossRef]
- Lum, I.; Mayer, M.; Zhou, Y. Footprint study of ultrasonic wedge-bonding with aluminum wire on copper substrate. J. Electron. Mater. 2006, 35, 433–442. [Google Scholar] [CrossRef] [Green Version]
- So, H.; Senesky, D.G. Rapid fabrication and packaging of AlGaN/GaN high-temperature ultraviolet photodetectors using direct wire bonding. J. Phys. D Appl. Phys. 2016, 49, 285109. [Google Scholar] [CrossRef]
- Kaimori, S.; Mizoguchi, A.; Nonaka, T. The Development of Cu Bonding Wire with Oxidation-Resistant Metal Coating. IEEE Trans. Adv. Packag. 2006, 29, 227–231. [Google Scholar] [CrossRef]
- Saraswati; Theint, E.P.P.; Stephan, D.; Goh, H.M.; Pasamanero, E.; Calpito, D.R.M.; Wulff, F.W.; Breach, C.D. High temperature storage (HTS) performance of copper ball bonding wires. In Proceedings of the 2005 7th Electronic Packaging Technology Conference, Singapore, 7–9 December 2005; pp. 602–607. [Google Scholar]
- Lin, Y.W.; Wang, R.Y.; Ke, W.B.; Wang, I.S.; Chiu, Y.T.; Lu, K.C.; Lin, K.L.; Lai, Y.S. The Pd distribution and Cu flow pattern of the Pd-plated Cu wire bond and their effect on the nanoindentation. Mater. Sci. Eng. A 2012, 543, 152–157. [Google Scholar] [CrossRef]
- Leong, G.C.; Uda, H. Comparative Reliability Studies and Analysis of Au, Pd-Coated Cu and Pd-Doped Cu Wire in Microelectronics Packaging. PLoS ONE 2013, 8, 78705. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.; Albers, J. The Ultrasonic Welding Mechanism as Applied to Aluminum-and Gold-Wire Bonding in Microelectronics. IEEE Trans. Parts Hybrids Packag. 1977, 13, 406–412. [Google Scholar] [CrossRef]
- Uno, T. Enhancing bondability with coated copper bonding wire. Microelectron. Reliab. 2011, 51, 88–96. [Google Scholar] [CrossRef]
- Romano-Rodríguez, A.; Hernández-Ramírez, F. Dual-beam focused ion beam (FIB): A prototyping tool for micro and nanofabrication. Microelectron. Eng. 2007, 84, 789–792. [Google Scholar] [CrossRef]
- Chang, C.C. Auger Electron Spectroscopy. Surf. Sci. 1971, 25, 53–79. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hung, F.Y.; Lui, T.S. Mechanical and Electrical Properties of Palladium-Coated Copper Wires with Flash Gold. J. Electron. Mater. 2017, 18, 4384–4391. [Google Scholar] [CrossRef]
- Van Ooij, W.J. The role of XPS in the study and understanding of rubber-to-metal bonding. Surf. Sci. 1977, 68, 1–9. [Google Scholar] [CrossRef]
- Ozawa, K.; Kakubo, T.; Shimizu, K.; Amino, N.; Mase, K.; Komatsu, T. High-resolution photoelectron spectroscopy analysis of sulfidation of brass at the rubber/brass interface. Appl. Surf. Sci. 2013, 264, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. Effect of the direct current on microstructure, tensile property and bonding strength of pure silver wires. Microelectron. Reliab. 2013, 53, 1159–1163. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, C.; Yao, Z.; Zhao, Q.; Xie, Y. Large-Scale Synthesis of Single-Crystal Double-Fold Snowflake Cu2S Dendrites. Cryst. Growth Des. 2006, 6, 1717–1719. [Google Scholar] [CrossRef]
- Shu, Q.W.; Lan, J.; Gao, M.X.; Wang, J.; Huang, C.Z. Controlled synthesis of CuS caved superstructures and their application to the catalysis of organic dye degradation in the absence of light. CrystEngComm 2015, 17, 1374–1380. [Google Scholar] [CrossRef]
- Kar, P.; Farsinezhad, S.; Zhang, X.; Shankar, K. Anodic Cu2S and CuS nanorod and nanowall arrays: Preparation, properties and application CO2 photoreduction. Nanoscale 2016, 6, 14305–14318. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xu, K. Formation of well-defined Cu2S dendrites on TEM grids from sulfur/CS2 under ambient conditions. Mater. Lett. 2012, 85, 4–6. [Google Scholar] [CrossRef]
- Han, Q.; Sun, S.; Li, J.; Wang, X. Growth of copper sulfide dendrites and nanowires from elemental sulfur on TEM Cu grids under ambient conditions. Nanotechnology 2011, 22, 155607. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-J.; Hung, F.-Y.; Chang, C.-Y. A Study of the Sulfidation Behavior on Palladium-Coated Copper Wire with a Flash-Gold Layer (PCA) after Wire Bonding. Electronics 2019, 8, 792. https://doi.org/10.3390/electronics8070792
Chen K-J, Hung F-Y, Chang C-Y. A Study of the Sulfidation Behavior on Palladium-Coated Copper Wire with a Flash-Gold Layer (PCA) after Wire Bonding. Electronics. 2019; 8(7):792. https://doi.org/10.3390/electronics8070792
Chicago/Turabian StyleChen, Kuan-Jen, Fei-Yi Hung, and Chia-Yun Chang. 2019. "A Study of the Sulfidation Behavior on Palladium-Coated Copper Wire with a Flash-Gold Layer (PCA) after Wire Bonding" Electronics 8, no. 7: 792. https://doi.org/10.3390/electronics8070792




