New Application of an Instantaneous Frequency Parameter for Assessing Far Infrared Fabric Effects in Aged Subjects
Abstract
:1. Introduction
2. Study Design, System Design, and Finger Blood Flow Assessment
2.1. Study Population and Study Protocol
2.1.1. Study Population and Grouping
2.1.2. Study Protocol
2.2. Photoplethysmographic Electrical Device (PED) for Data Analysis
2.2.1. Parameters for Finger Blood Flow Assessment
- Stiffness Index (SI)
- Crest Time (CT) and Crest Time Ratio (CTR)
- Finger Perfusion Index (FPI)
- Instantaneous Frequency Difference, ΔfEmax
2.2.2. Procedures of Examinations
2.2.3. Hardware of PED
- a PPG sensor: one pair of infrared transmitter and receiver with a 940 nm wavelength;
- analog filters: a 2nd order band pass filter, with cut-off frequencies of 0.48–10 Hz;
- an analog amplification circuit: digital volume pulses (DVPs) with 1–10 mV;
- a USB-6008 DAQ: a sampling frequency of 1000 Hz and 12-bit ADC with USB (DVPs stored in a computer for later computation);
- a notebook computer for real data analysis.
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Testing Subjects
3.2. Failure of CT, CTR, SI, and FPI Change during SY2 Attachment for a Group 1 Subject
3.3. Comparison of Computational Parameters for Finger Blood Flow Assessment
3.3.1. Impact of Far-Infrared Fabric for Five Parameters in the Same Group
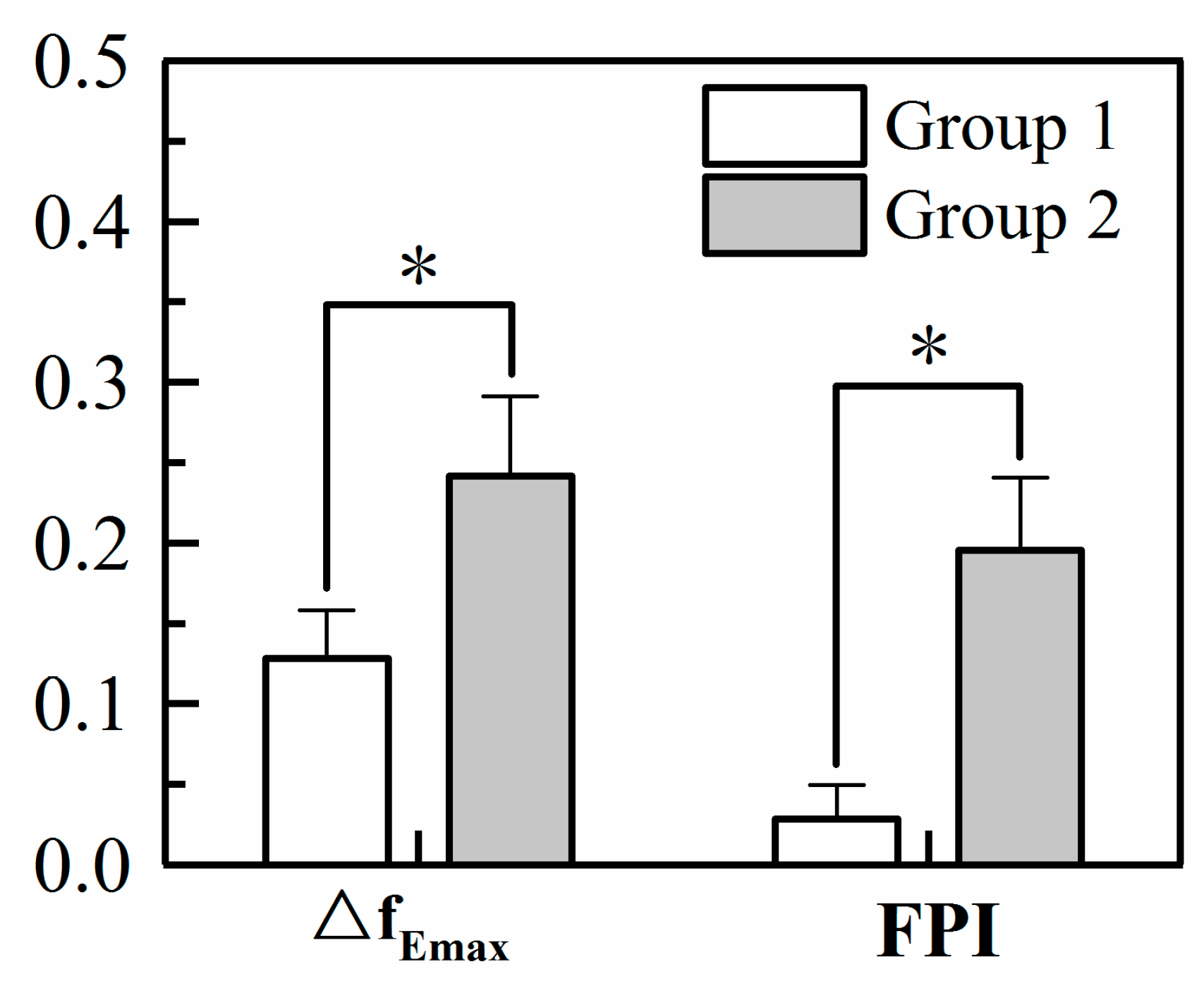
3.3.2. Comparison of FPI and ΔfEmax for the Two Groups
3.4. Multivariate Analysis for ΔfEmax
3.4.1. Correlations between ΔfEmax and Demographic and Anthropometric Parameters
3.4.2. Multivariate Regression Analysis for ΔfEmax
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CT | Crest Time |
| CTR | Crest Time Ratio |
| DVP | Digital Volume Pulse |
| ECG | Electrocardiography |
| EMD | Ensemble Empirical Mode Decomposition |
| fEmax | instantaneous frequency of maximal energy |
| FIR | Far-Infrared Radiation |
| FP | Finger Perfusion |
| FPI | Finger Perfusion Index |
| HHT | Hilbert–Huang transformation |
| IMF5 | the 5th decomposed Intrinsic Mode Function |
| LabVIEW | Laboratory Virtual Instrumentation Engineering Workbench |
| Matlab | MATrix LABoratory |
| PC | Personal Computer |
| PED | Photoplethysmographic Electrical Device |
| PPG | Photoplethysmography |
| PWV | Pulse Wave Velocity |
| RRI | R-R Interval of ECG |
| SI | Stiffness Index |
| SPSS | Statistical Package for the Social Sciences |
| SY2 | a far infrared fabric |
| SD | Standard Deviation |
| ΔfEmax | instantaneous frequency difference |
References
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photonics Lasers Med. 2012, 4, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.; Kabaya, M. Biological activities caused by far infrared radiation. Int. J. Biometeorol. 1989, 33, 145–150. [Google Scholar] [CrossRef]
- Pang, X.-F. Vibrational energy-spectra of protein molecules and non-thermally biological effect of infrared light. Int. J. Infrared Millim. Waves 2001, 22, 291–307. [Google Scholar] [CrossRef]
- Akasaki, Y.; Miyata, M.; Eto, H.; Shirasawa, T.; Hamada, N.; Ikeda, Y.; Biro, S.; Otsuji, Y.; Tei, C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ. J. 2006, 70, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Chang, C.F.; Lai, M.Y.; Chen, T.W.; Lee, P.C.; Yang, W.C. Far-infrared therapy: A novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. J. Am. Soc. Nephrol. 2007, 18, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.Y.; Chiu, J.H.; Yang, S.D.; Hsu, Y.C.; Liu, W.Y.; Wu, C.W. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol. Photoimmunol. Photomed. 2006, 22, 78–86. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, G.M.; Wang, J.H.; Chu, H.C.; Wu, H.T.; Chen, J.J.; Sun, C.K. Effects of combined far-infrared radiation and acupuncture at ST36 on peripheral blood perfusion and autonomic activities. Evidence-Based Complement. Altern. Med. 2017, 2017, 1947315. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.H.; Park, C.M.; Oh, T.J.; Han, S.H.; Kang, H.H.; Chang, I.S. Investigation of jewelry powders radiating far-infrared rays and the biological effects on human skin. J. Cosmet. Sci. 2002, 53, 175–184. [Google Scholar]
- Meng, J.; Jin, W.; Liang, J.; Ding, Y.; Gan, K.; Yuan, Y. Effects of particle size on far infrared emission properties of tourmaline superfine powders. J. Nanosci. Nanotechnol. 2010, 10, 2083–2087. [Google Scholar] [CrossRef]
- Chen, S.Z.; Li, J.; Li, X.Y.; Xu, L.S. Effects of vacuum-assisted closure on wound microcirculation: An experimental study. Asian J. Surg. 2005, 28, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Baran, U.; Shi, L.; Wang, R.K. Capillary blood flow imaging within human finger cuticle using optical microangiography. J. Biophotonics 2015, 8, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, K.-I.; Fukutake, T.; Hattori, T. Fingertip photoplethysmography and migraine. J. Neurol. Sci. 2003, 216, 17–21. [Google Scholar] [CrossRef]
- Korhonen, I.; Yli-Hankala, A. Photoplethysmography and nociception. Acta Anaesthesiol. Scand. 2009, 53, 975–985. [Google Scholar] [CrossRef]
- Hsu, P.C.; Wu, H.T.; Sun, C.K. Assessment of subtle changes in diabetes-associated arteriosclerosis using photoplethysmographic pulse wave from index finger. J. Med. Syst. 2018, 42, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Lin, B.Y.; Yang, C.C.; Ou, Y.N.; Sun, C.K. Assessment of vascular health with photoplethysmographic waveforms from the fingertip. IEEE J. Biomed. Health Inform. 2017, 21, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Wu, H.T.; Chuang, W.Y.; Liu, T.C.; Tsai, I.T.; Chen, J.J.; Sun, C.K. Application of short time MSE in assessing the impact of acupuncture on peripheral blood flow and autonomic activities in normal and overweight subjects. J. Med. Biol. Eng. 2016, 36, 386–395. [Google Scholar] [CrossRef]
- Wei, H.C.; Xiao, M.X.; Chen, H.Y.; Li, Y.Q.; Wu, H.T.; Sun, C.K. Instantaneous frequency from Hilbert-Huang transformation of digital volume pulse as indicator of diabetes and arterial stiffness in upper-middle-aged subjects. Sci. Rep. 2018, 8, 15771. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Fryzowicz, A.; Czepulis, N.; Kaluźniak-Szymanowska, A.; Dworak, L.B.; Wieczorowska-Tobis, K. The impact of the age range of young healthy reference population on the cut-off points for low muscle mass necessary for the diagnosis of sarcopenia. Eur. Rev. Med. Pharm. Sci. 2019, 23, 4321–4332. [Google Scholar] [CrossRef]
- Millasseau, S.; Kelly, R.; Ritter, J.; Chowienczyk, P. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. 2002, 103, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Alty, S.R.; Angarita-Jaimes, N.; Millasseau, S.C.; Chowienczyk, P.J. Predicting arterial stiffness from the digital volume pulse waveform. IEEE Trans. Biomed. Eng. 2007, 54, 2268–2275. [Google Scholar] [CrossRef]
- Wu, H.T.; Liu, C.C.; Lin, P.H.; Chung, H.M.; Liu, M.C.; Yip, H.K.; Liu, A.B.; Sun, C.K. Novel application of parameters in waveform contour analysis for assessing arterial stiffness in aged and atherosclerotic subjects. Atherosclerosis 2010, 213, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Hsu, P.C.; Lin, C.F.; Wang, H.J.; Sun, C.K.; Liu, A.B.; Lo, M.T.; Tang, C.J. Multiscale entropy analysis of pulse wave velocity for assessing atherosclerosis in the aged and diabetic. IEEE Trans. Biomed. Eng. 2011, 58, 2978–2981. [Google Scholar] [PubMed]
- Wu, H.T.; Lee, K.W.; Pan, W.Y.; Liu, A.B.; Sun, C.K. Difference in bilateral digital volume pulse as a novel noninvasive approach to assessing arteriosclerosis in aged and diabetic subjects: A preliminary study. Diabetesvasc. Dis. Res. 2017, 14, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Klarhofer, M.; Csapo, B.; Balassy, C.; Szeles, J.C.; Moser, E. High-resolution blood flow velocity measurementsin the human finger. Magn. Reson. Med. 2001, 45, 716–719. [Google Scholar] [CrossRef]
- Zhang, T.; Xia, L.H.; Bian, Y.Y.; Feng, B.; Wang, C.; Meng, F.X.; Zhang, Y.H.; Chen, M. Blood flow of the acral finger arterioles in patients with type 2 diabetes by quality doppler profiles. Cell Biochem. Biophys. 2013, 67, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Wang, Q.Y.; Yi, J.B.; Song, D.; Xiang, X. A numerical model of blood flow velocity measurement based on finger ring. J. Healthc. Eng. 2018, 2018, 3916481. [Google Scholar]
- Li, L.; Mac-Mary, S.; Sainthillier, J.-M.; Nouveau, S.; de Lacharriere, O.; Humbert, P. Age-related changes of the cutaneous microcirculation in vivo. Gerontology 2006, 52, 142–153. [Google Scholar] [CrossRef]
- Hile, C.; Veves, A. Diabetic neuropathy and microcirculation. Curr. Diab. Rep. 2003, 3, 446–451. [Google Scholar] [CrossRef]
- Selim, M.; Jones, R.; Novak, P.; Zhao, P.; Novak, V. The effects of body mass index on cerebral blood flow velocity. Clin Auton Res. 2008, 18, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Scherrer, U.; Randin, D.; Tappy, L.; Vollenweider, P.; Jequier, E.; Nicod, P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 1994, 89, 2634–2640. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]



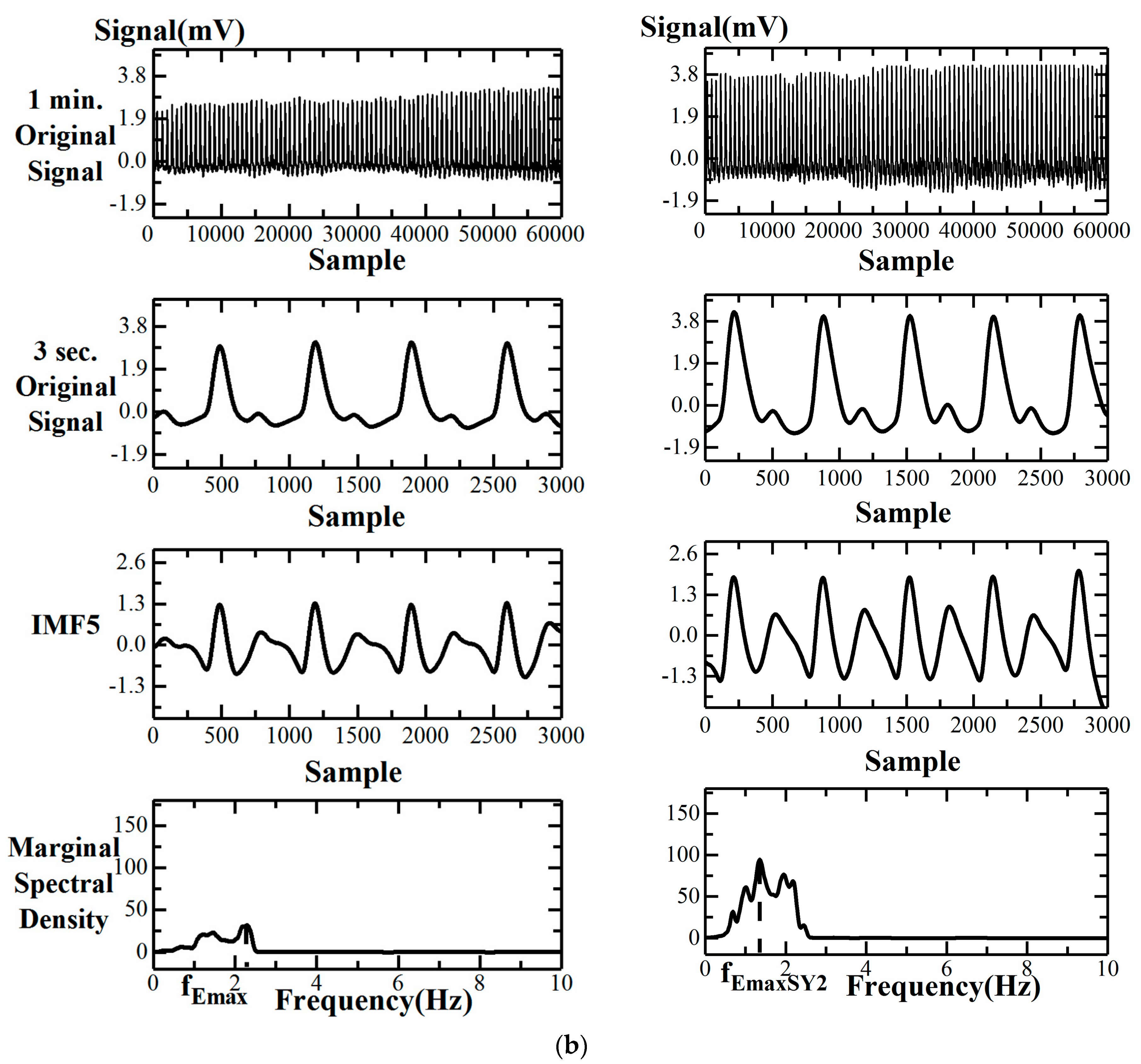
| Parameters | Group 1 Male/Female: 44/22 | Group 2 Male/Female: 17/16 | P Value |
|---|---|---|---|
| Age (years) | 21.26 ± 2.41 | 55.97 ± 11.88 ** | 0.000 |
| Body height (cm) | 169.23 ± 8.23 | 167.15 ± 8.26 | 0.240 |
| Body weight (kg) | 62.27 ± 12.01 | 67.11 ± 10.81 | 0.054 |
| BMI (kg/m2) | 21.61 ± 3.11 | 23.93 ± 2.87 * | 0.001 |
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| Pre-SY2 | Post-SY2 | Pre-SY2 | Post-SY2 | |
| CT (s) | 0.16 ± 0.03 | 0.17 ± 0.03 | 0.24 ± 0.05 | 0.23 ± 0.05 |
| CTR | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.16 ± 0.03 | 0.15 ± 0.03 |
| FP (mV*s) | 4407.72 ± 1504.80 | 4458.50 ± 1482.61 | 5819.01 ± 2956.27 | 6905.21 ± 3920.59 |
| SI (m/s) | 5.42 ± 0.66 | 5.34 ± 0.67 | 5.41 ± 0.92 | 5.17 ± 0.91 |
| fEmax (Hz) | 2.28 ± 0.35 | 2.15 ± 0.38 * | 1.99 ± 0.30 | 1.75 ± 0.31 ** |
| Group 1 Male/Female: 44/22 | Group 2 Male/Female: 17/16 | |
|---|---|---|
| Age (years) | r = −0.009 p = 0.945 | r = −0.014 p = 0.937 |
| Body height (cm) | r = −0.041 p = 0.744 | r = 0.103 p = 0.567 |
| Body weight (kg) | r = −0.073 p = 0.561 | r = 0.401 p = 0.021 * |
| BMI (kg/m2) | r = −0.054 p = 0.668 | r = 0.477 p = 0.005 ** |
| Advantages | Disadvantages | |
|---|---|---|
| microcirculation microscope (DMX 980) |
|
|
| Photoplethysmographic Electrical Device (PED) |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.-C.; Li, Y.-Q.; Wu, G.-S.; Xiao, M.-X.; Tang, X.-J.; Chen, J.-J.; Wu, H.-T. New Application of an Instantaneous Frequency Parameter for Assessing Far Infrared Fabric Effects in Aged Subjects. Electronics 2020, 9, 138. https://doi.org/10.3390/electronics9010138
Wei H-C, Li Y-Q, Wu G-S, Xiao M-X, Tang X-J, Chen J-J, Wu H-T. New Application of an Instantaneous Frequency Parameter for Assessing Far Infrared Fabric Effects in Aged Subjects. Electronics. 2020; 9(1):138. https://doi.org/10.3390/electronics9010138
Chicago/Turabian StyleWei, Hai-Cheng, Yun-Qin Li, Guan-Sheng Wu, Ming-Xia Xiao, Xiao-Jing Tang, Jian-Jung Chen, and Hsien-Tsai Wu. 2020. "New Application of an Instantaneous Frequency Parameter for Assessing Far Infrared Fabric Effects in Aged Subjects" Electronics 9, no. 1: 138. https://doi.org/10.3390/electronics9010138






