Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Description
2.2. Motor Imagery Task Analysis
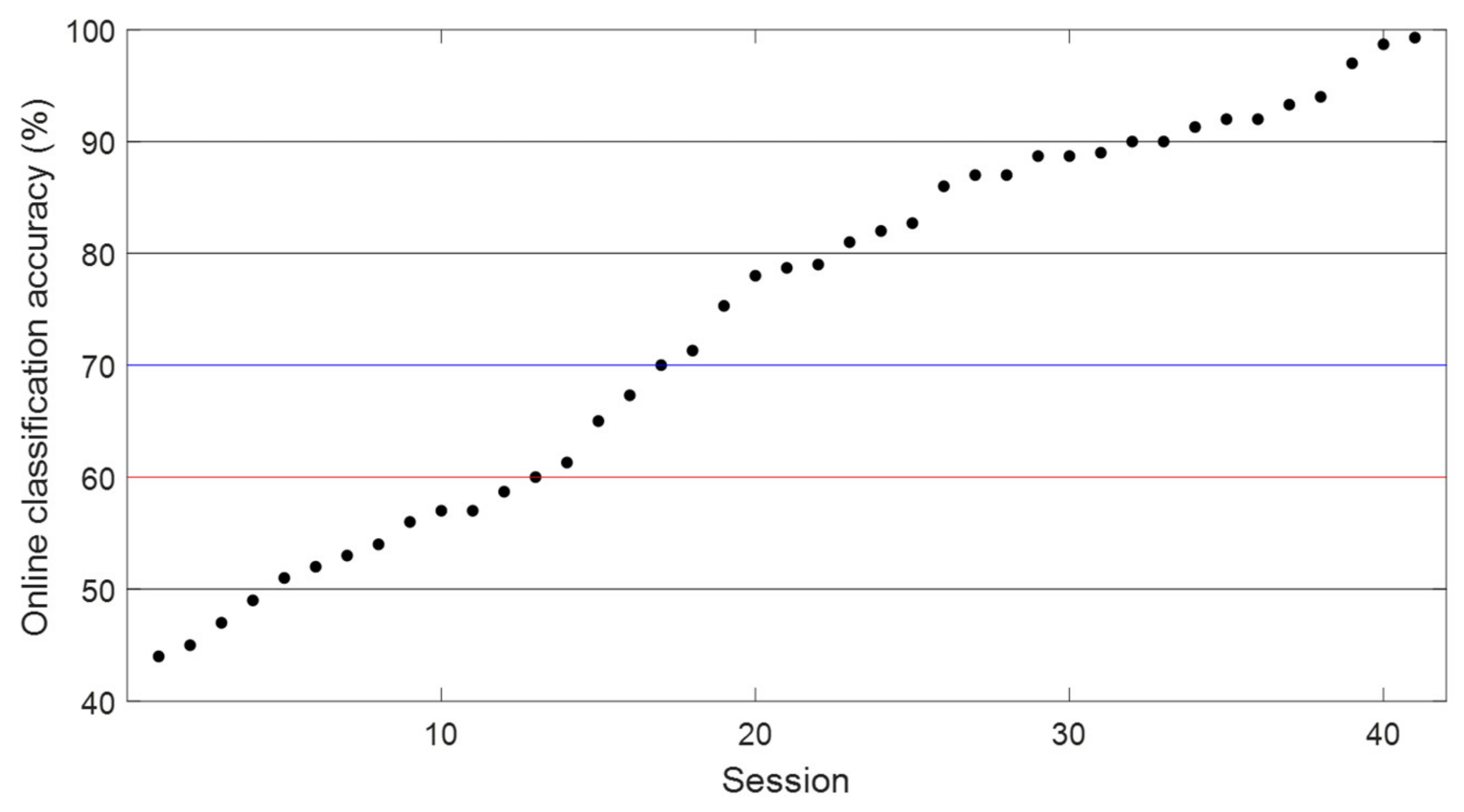
- High-performance group (classification accuracy ≥ 70%)
- Middle-performance group (60% ≤ classification accuracy < 70%)
- Low-performance group (classification accuracy < 60%).
2.3. Resting State Analysis
3. Results
3.1. Online BCI Performance
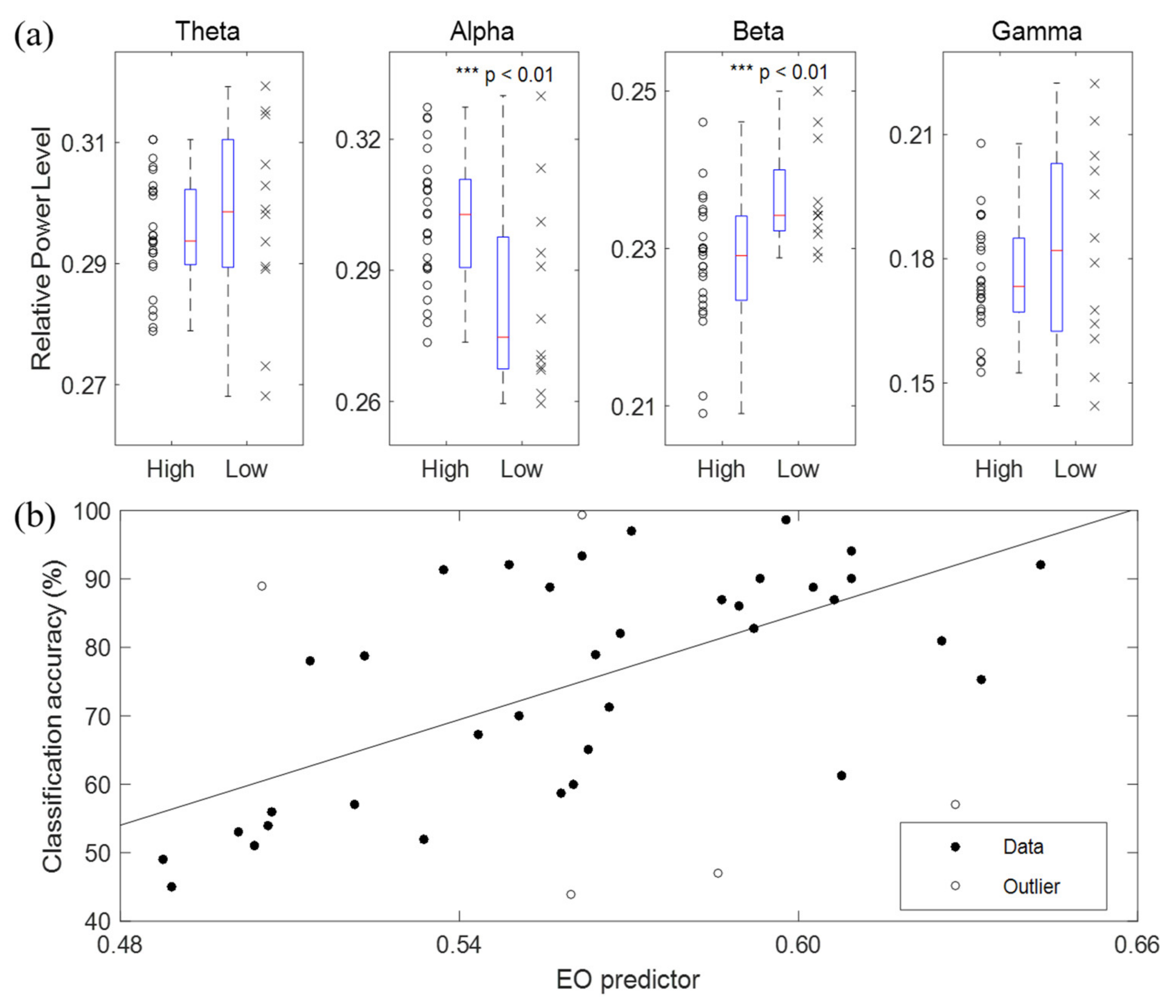
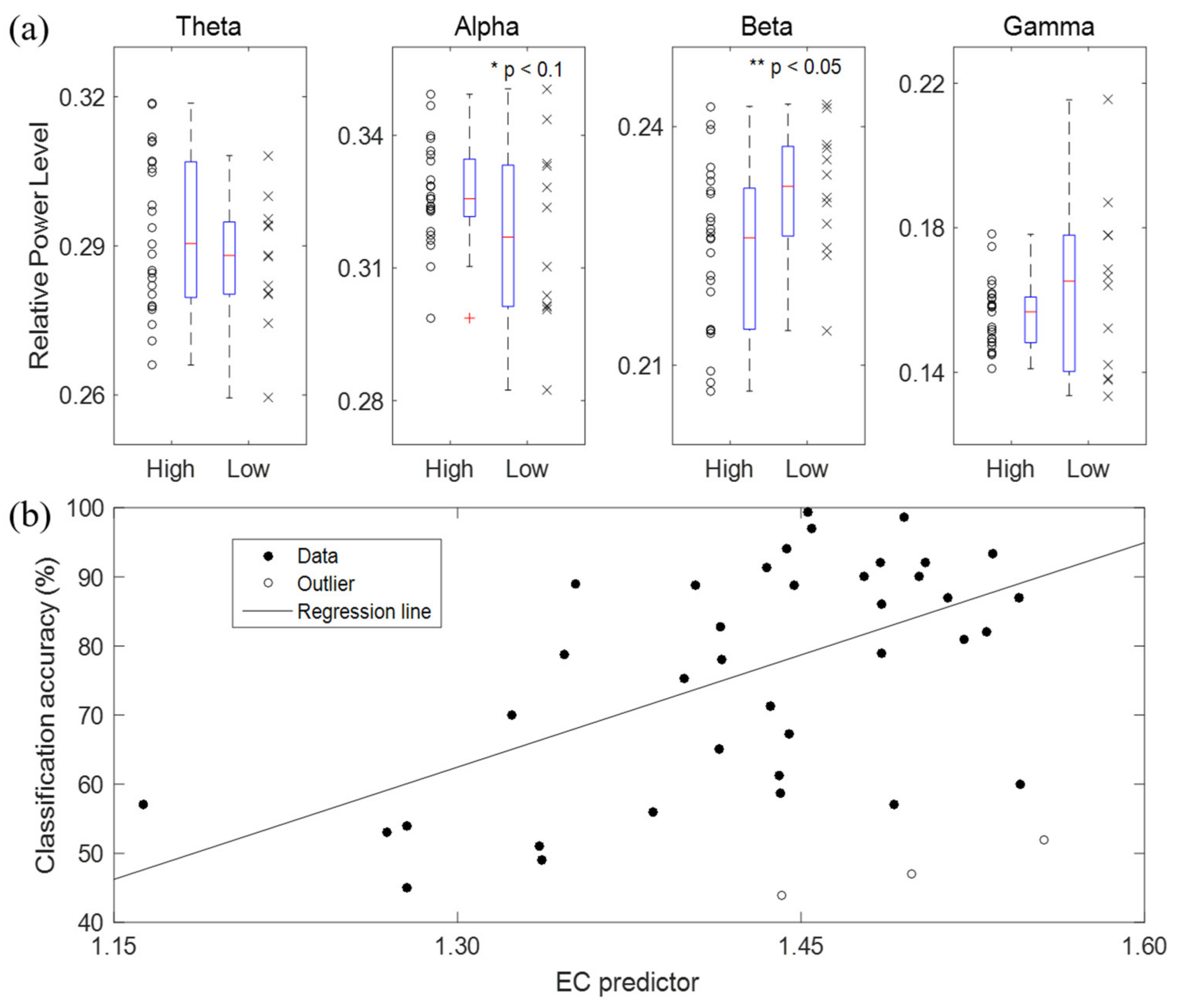
3.2. BCI Performance Predictors Using Eyes-Open or Eyes-Closed Resting State Alone
- The high-performance group’s alpha power was significantly higher than that of the low-performance group (p < 0.01).
- The high-performance group’s beta power was significantly lower than that of the low-performance group (p < 0.01).
- The theta powers did not differ significantly between the two groups (p > 0.1). However, except for two data points, the theta powers in the high-performance group had a significantly lower distribution than did those in the low-performance group (p < 0.05).
- The gamma powers did not differ significantly between the two groups (p > 0.1). However, the median of the gamma powers in the high-performance group was slightly lower than that in the low-performance group.
- Combination of two significant spectral bands’ (alpha and beta) powers
- Combination of three spectral bands’ (theta, alpha, and beta) powers
- Combination of all four spectral bands’ (theta, alpha, beta, and gamma) powers
- The high-performance group’s alpha power was higher than that of the low-performance group, but was only mildly significant (p < 0.1).
- The high-performance group’s beta power was significantly lower than that of the low-performance group (p < 0.05).
- There was no significant difference in the theta and gamma powers between the low- and high-performance groups (p > 0.1). However, theta’s median in the high-performance group was slightly higher than that in the low-performance group, while gamma’s median was slightly lower.
- The combination of two significant or moderately significant spectral bands’ (alpha and beta) powers
- The combination of three spectral bands’ (theta, alpha and beta) powers
- The combination of all four spectral bands’ (theta, alpha, beta and gamma) powers
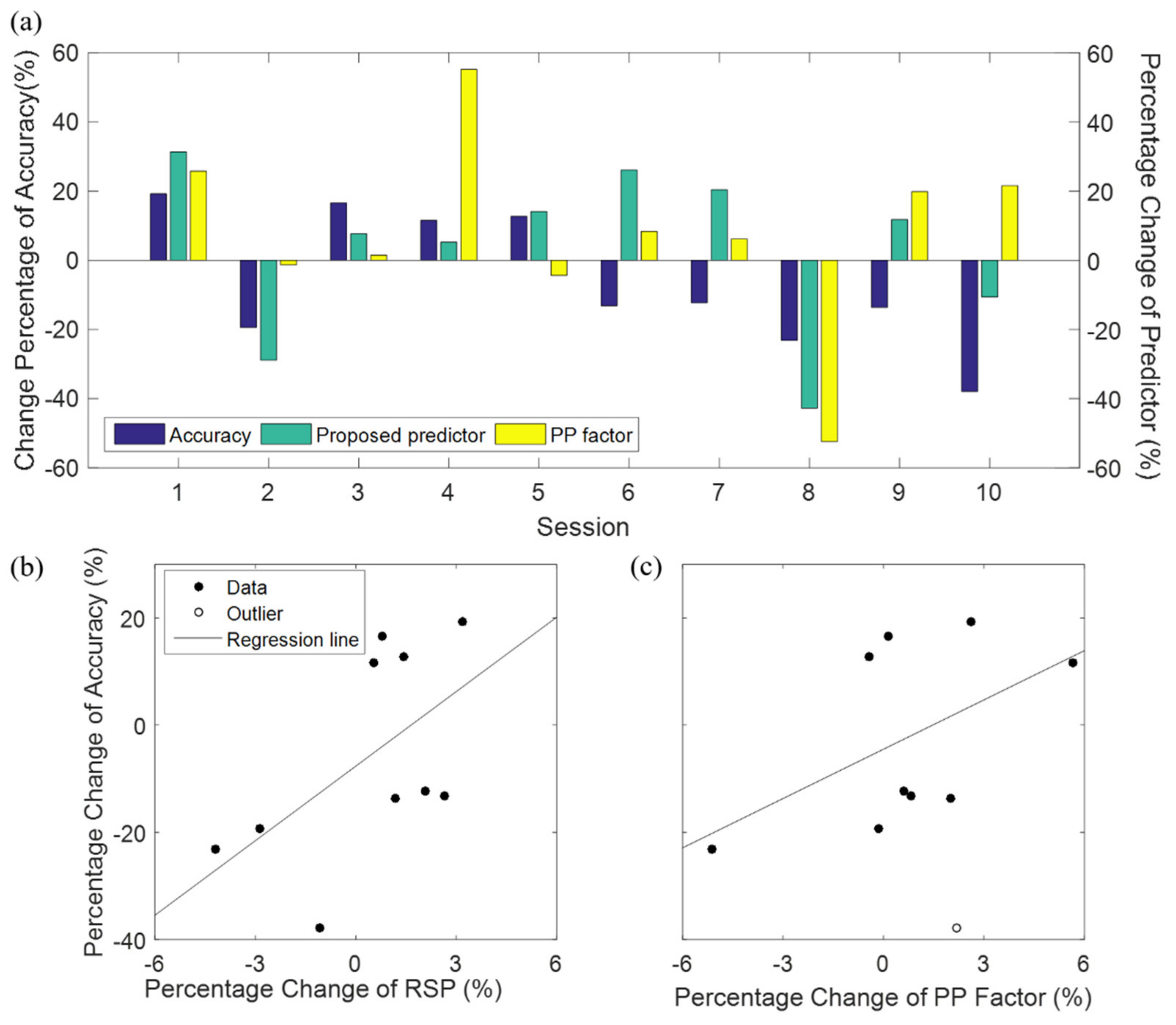
3.3. BCI Predictor Using both Eyes-Open and Eyes-Closed Resting States
4. Discussion
4.1. Relation between Spectral Powers and MI-BCI Performance
4.2. Using Beta Oscillations to Predict BCI
4.3. Comparisons with Previous Works
4.4. Inter-Session (Intra-Subject) Variability
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Kübler, A.; Kotchoubey, B.; Kaiser, J.; Wolpaw, J.; Birbaumer, N. Brain–computer communication: Unlocking the locked in. Psychol. Bull. 2001, 127, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Guger, C.; Edlinger, G.; Harkam, W.; Niedermayer, I.; Pfurtscheller, G. How many people are able to operate an EEG-based brain-computer interface (BCI)? IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Blankertz, B.; Sannelli, C.; Halder, S.; Hammer, E.M.; Kübler, A.; Müller, K.-R.; Curio, G.; Dickhaus, T. Neurophysiological predictor of SMR-based BCI performance. NeuroImage 2010, 51, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C. Critiquing the concept of BCI illiteracy. Sci. Eng. Ethics 2019, 25, 1217–1233. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kwon, K.; Im, C.-H. Neurofeedback-based motor imagery training for brain–computer interface (BCI). J. Neurosci. Methods 2009, 179, 150–156. [Google Scholar] [CrossRef]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef]
- Jeunet, C.; Glize, B.; McGonigal, A.; Batail, J.M.; Micoulaud-Franchi, J.A. Using EEG-based brain computer interface and neurofeedback targeting sensorimotor rhythms to improve motor skills: Theoretical background, applications and prospects. Neurophysiol. Clin. 2019, 49, 125–136. [Google Scholar] [CrossRef]
- Yao, L.; Meng, J.; Sheng, X.; Zhang, D.; Zhu, X. A novel calibration and task guidance framework for motor imagery BCI via a tendon vibration induced sensation with kinesthesia illusion. J. Neural Eng. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Shu, X.; Yao, L.; Sheng, X.; Zhang, D.; Zhu, X. Enhanced Motor Imagery-Based BCI Performance via Tactile Stimulation on Unilateral Hand. Front. Hum. Neurosci. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Aranibar, A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr. Clin. Neurophysiol. 1977, 42, 817–826. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Halder, S.; Varkuti, B.M.; Kübler, A.; Rosenstiel, W.; Sitaram, R.; Birbaumer, N. Prediction of brain-computer interface aptitude from individual brain structure. Front. Hum. Neurosci. 2013, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Aquorastos, D.; Veit, R.; Hammer, E.M.; Lee, S.; Varkuti, B.; Bogdan, M.; Rosenstiel, W.; Birbaumer, N.; Kübler, A. Neural mechanisms of brain-computer interface control. NeuroImage 2011, 55, 1779–1790. [Google Scholar] [CrossRef]
- Randolph, A.B.; Jackson, M.M.; Karmakar, S. Individual characteristics and their effect on predicting mu rhythm modulation. Int. J. Hum. Comput. Interact. 2010, 27, 24–37. [Google Scholar] [CrossRef]
- Nijboer, F.; Furdea, A.; Gunst, I.; Mellinger, J.; McFarland, D.J.; Birbaumer, N.; Kübler, A. An auditory brain-computer interface (BCI). J. Neurosci. Method 2008, 167, 43–50. [Google Scholar] [CrossRef]
- Cassady, K.; You, A.; Doud, A.; He, B. The impact of mind-body awareness training on the early learning of a brain-computer interface. Technol. (Singap. World Sci.) 2014, 2, 254–260. [Google Scholar] [CrossRef]
- Vuckovic, A.; Osuagwu, B.A. Using a motor imagery questionnaire to estimate the performance of a Brain-Computer Interface based on object oriented motor imagery. Clin. Neurophysiol. 2013, 124, 1586–1595. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, H.; Ahn, S.; Jun, S.C. User’s self-prediction of performance in motor imagery brain–computer interface. Front. Hum. Neurosci. 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Suk, H.I.; Fazli, S.; Mehnert, J.; Müller, K.R.; Lee, S.W. Predicting BCI subject performance using probabilistic spatio-temporal filters. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Botrel, L.; Kübler, A. Week-long visuomotor coordination and relaxation trainings do not increase sensorimotor rhythms (SMR) based brain–computer interface performance. Behav. Brain Res. 2019, 372, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, P.; Chen, R.; Li, F.; Guo, L.; Li, P.; Zhang, T.; Yao, D. Predicting inter-session performance of SMR-based Brain-coputer interface using the spectral entropy of resting-state EEG. Brain Topogr. 2015, 28, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Cho, H.; Ahn, S.; Jun, S.C. High theta and low alpha powers may be indicative of BCI-Illiteracy in motor imagery. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yang, B.; Huang, L. Feature Extraction of EEG Signals Using Power Spectral Entropy. 2008 Int. Conf. Biomed. Eng. Inform. 2008, 435–439. [Google Scholar] [CrossRef]
- Blanco, S.; Garay, A.; Coulombie, D. Comparison of frequency bands using spectral entropy for epileptic seizure prediction. Isrn Neurol. 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ahn, M.; Kim, K.; Jun, S.C. Increasing session-to-session transfer in a brain–computer interface with on-site background noise acquisition. J. Neural Eng. 2015, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ahn, M.; Ahn, S.; Jun, S.C. Invariant common spatio-spectral patterns. Proc. Tobi 3rd Workshop 2012, 31–32. [Google Scholar] [CrossRef]
- Lotte, F.; Congedo, M.; Lécuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain–computer interfaces. J. Neural Eng. 2007, 4, R1–R13. [Google Scholar] [CrossRef]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-based brain-computer interfaces using motor-imagery: Techniques and challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef]
- Venables, L.; Fairclough, S. The influence of performance feedback on goal-setting and mental effort regulation. Motiv. Emot. 2009, 33, 63–74. [Google Scholar] [CrossRef]
- Cruikshank, L.C.; Singhal, A.; Hueppelsheuser, M.; Caplan, J.B. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. J. Neurophysiol. 2012, 107, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.; Weiss, T.; Krause, W.; Beyer, L.; Rost, R.; Gutberlet, I.; Gertz, H.J. Power of theta waves in the EEG of human subjects increases during recall of haptic information. Neurosci. Lett. 1999, 260, 189–192. [Google Scholar] [CrossRef]
- Hinterberger, T.; Schmidt, S.; Kamei, T.; Walach, H. Decreased electrophysiological activity represents the conscious state of emptiness in meditation. Front. Psychol. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- De Lange, F.P.; Jensen, O.; Bauer, M.; Toni, I. Interactions between posterior gamma and frontal alpha/beta oscillations during imagined actions. Front. Hum. Neurosci. 2008, 2, 1–12. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trend Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Cattaneo, L.; Fabbri-Destro, M.; Rozzi, S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. Rev. 2014, 94, 655–706. [Google Scholar] [CrossRef]
- Naro, A.; Calabrò, R.S.; Leo, A.; Russo, M.; Milardi, D.; Cannavò, A.; Manuli, A.; Buda, A.; Casella, C.; Bramanti, P.; et al. Bridging the gap towards awareness detection in disorders of consciousness: An experimental study on the mirror neuron system. Brain Topogr. 2018, 31, 623–639. [Google Scholar] [CrossRef]
- EEG Motor Movement/Imagery Dataset. Available online: https://physionet.org/content/eegmmidb/1.0.0/ (accessed on 24 March 2020).


| Subject | Session | Class | Online Accuracy | Subject | Session | Class | Online Accuracy |
|---|---|---|---|---|---|---|---|
| S1 | 1 | RF | 47.0 | S7 | 1 | LF | 57.0 |
| 2 | RF | 57.0 | 2 | RF | 53.0 | ||
| S2 | 1 | LF | 79.0 | S8 | 1 | LR | 56.0 |
| 2 | LR | 82.0 | 2 | RF | 52.0 | ||
| 3 | LR | 65.0 | S9 | 1 | LF | 90.0 | |
| 4 | LR | 93.3 | 2 | LF | 71.3 | ||
| 5 | LR | 88.7 | S10 | 1 | RF | 70.0 | |
| S3 | 1 | RF | 81.0 | S11 | 1 | RF | 67.3 |
| 2 | LF | 87.0 | 2 | RF | 58.7 | ||
| 3 | RF | 87.0 | S12 | 1 | LF | 75.3 | |
| 4 | RF | 86.0 | 2 | LF | 82.7 | ||
| 5 | LF | 92.0 | S13 | 1 | LF | 88.7 | |
| S4 | 1 | RF | 89.0 | 2 | LF | 94.0 | |
| 2 | RF | 78.0 | S14 | 1 | LF | 90.0 | |
| 3 | RF | 51.0 | 2 | LF | 61.3 | ||
| 4 | LF | 78.7 | S15 | 1 | RF | 91.3 | |
| 5 | LF | 54.0 | 2 | RF | 44.0 | ||
| S5 | 1 | LF | 49.0 | ||||
| 2 | RF | 45.0 | |||||
| S6 | 1 | RF | 60.0 | ||||
| 2 | LF | 92.0 | |||||
| 3 | LF | 97.0 | |||||
| 4 | LF | 98.7 | |||||
| 5 | LF | 99.3 | |||||
| Predictor | |||||
|---|---|---|---|---|---|
| RSP | PP Factor | ||||
| Correlation, p-Value | Outlier | Correlation, p-Value | Outlier | ||
| Confidence Level | 90% | r = 0.71, p < | 4 | r = 0.48, p < | 3 |
| 95% | r = 0.66, p < | 3 | r = 0.31, p < | 1 | |
| 99% | r = 0.50, p < | 0 | r = 0.20, p > | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Cho, H.; Won, K.; Ahn, M.; Jun, S.C. Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance. Electronics 2020, 9, 690. https://doi.org/10.3390/electronics9040690
Kwon M, Cho H, Won K, Ahn M, Jun SC. Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance. Electronics. 2020; 9(4):690. https://doi.org/10.3390/electronics9040690
Chicago/Turabian StyleKwon, Moonyoung, Hohyun Cho, Kyungho Won, Minkyu Ahn, and Sung Chan Jun. 2020. "Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance" Electronics 9, no. 4: 690. https://doi.org/10.3390/electronics9040690
APA StyleKwon, M., Cho, H., Won, K., Ahn, M., & Jun, S. C. (2020). Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance. Electronics, 9(4), 690. https://doi.org/10.3390/electronics9040690






