Multimorbidity, Treatment, and Determinants among Chronic Patients Attending Primary Health Facilities in Tshwane, South Africa
Abstract
:1. Background
2. Methods
2.1. Study Design and Setting
2.2. Sample Size, Sampling, and Participants
2.3. Data Collection and Tools
2.4. Data Analysis
2.5. Ethics Committee
3. Results
3.1. Characteristics of Patients
3.2. Characteristics of Comorbidity
3.3. Factors Associated with Medication Adherence in Patients with Morbidity
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navickas, R.; Petric, V.K.; Feigl, A.B.; Seychell, M. Multimorbidity: What do we know? What should we do? J. Comorbidity 2016, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Academy of Medical Sciences. Multimorbidity: A Priority for Global Health Research; Academy of Medical Sciences: London, UK, 2018. [Google Scholar]
- Chowdhury, S.R.; Chandra Das, D.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: A systematic review and meta-analysis. E-Clin. Med. 2023, 57, 101860. [Google Scholar] [CrossRef]
- Ejeta, A.; Abdosh, T.; Hawulte, B.; Lamessa, A.; Belete Fite, M.; Fekadu, G. Diabetes Concordant Comorbidities and Associated Factors Among Adult Diabetic Out-Patients at Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2281–2289. [Google Scholar] [CrossRef]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef] [PubMed]
- Gouda, H.N.; Charlson, F.; Sorsdahl, K.; Ahmadzada, S.; Ferrari, A.J.; Erskine, H.; Leung, J.; Santamauro, D.; Lund, C.; Aminde, L.N.; et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Global Health. 2019, 7, e1375–e1387. [Google Scholar] [CrossRef] [PubMed]
- Kengne, A.P.; June-Rose McHiza, Z.; Amoah, A.G.; Mbanya, J.C. Cardiovascular diseases and diabetes as economic and developmental challenges in Africa. Prog. Cardiovasc. Dis. 2013, 56, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Chauke, G.D.; Nakwafila, O.; Chibi, B.; Sartorius, B.; Mashamba-Thompson, T. Factors influencing poor medication adherence amongst patients with chronic disease in low-and-middle-income countries: A systematic scoping review. Heliyon 2022, 8, e09716. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Agh, T.; Malmenäs, M.; Raval, A.D.; Bennett, B.M.; Borah, B.J.; Hutchins, D.S.; Manias, E.; Williams, A.F.; Hiligsmann, M. Methods for measuring multiple medication adherence: A systematic review–report of the ISPOR medication adherence and persistence special interest group. Value Health 2019, 22, 139–156. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- De-Graft Aikins, A.; Unwin, N.; Agyemang, C.; Allotey, P.; Campbell, C.; Arhinful, D. Tackling Africa’s chronic disease burden: From the local to the global. Glob. Health 2010, 6, 5. [Google Scholar] [CrossRef]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. BioMed Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef]
- Wong, E.B.; Olivier, S.; Gunda, R.; Koole, O.; Surujdeen, A.; Gareta, D.; Munatsi, D.; Modise, T.H.; Dreyer, J.; Nxumalo, S.; et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: A cross-sectional, population-based multimorbidity study. Lancet Glob. Health 2021, 9, e967–e976. [Google Scholar] [CrossRef] [PubMed]
- Oni, T.; Youngblood, E.; Boulle, A.; McGrath, N.; Wilkinson, R.J.; Levitt, N.S. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect. Dis. 2015, 15, 20. [Google Scholar] [CrossRef]
- While, A. Medication adherence: Understanding the issues and finding solutions. Br. J. Community Nurs. 2020, 25, 474–479. [Google Scholar] [CrossRef]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Makovski, T.T.; Schmitz, S.; Zeegers, M.P.; Stranges, S.; van den Akker, M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res. Rev. 2019, 53, 100903. [Google Scholar] [CrossRef]
- McPhail, S.M. Multimorbidity in chronic disease: Impact on health care resources and costs. Risk Manag. Healthc. Policy 2016, 9, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.P.; Flores, T.R.; Mielke, G.I.; Thumé, E.; Facchini, L.A. Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2016, 67, 130–138. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Wu, C.; Odden, M.C.; Kim, D.H. Multimorbidity Patterns, Frailty, and Survival in Community-Dwelling Older Adults. J. Gerontol. Ser. A 2018, 74, 1265–1270. [Google Scholar] [CrossRef]
- Aggarwal, P.; Woolford, S.J.; Patel, H.P. Multi-Morbidity and Polypharmacy in Older People: Challenges and Opportunities for Clinical Practice. Geriatrics 2020, 5, 85. [Google Scholar] [CrossRef]
- Kengne, A.P.; Mayosi, B.M. Readiness of the primary care system for non-communicable diseases in sub-Saharan Africa. Lancet Glob. Health 2014, 2, e247–e248. [Google Scholar] [CrossRef]
- Modjadji, P. Communicable and non-communicable diseases coexisting in South Africa. Lancet Glob. Health 2021, 9, e889–e890. [Google Scholar] [CrossRef]
- Peer, N. The converging burdens of infectious and non-communicable diseases in rural-to-urban migrant Sub-Saharan African populations: A focus on HIV/AIDS, tuberculosis and cardio-metabolic diseases. Trop. Dis. Travel Med. Vaccines 2015, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Flisher, A.J.; Lalloo, U.G.; Sitas, F.; Tollman, S.M.; Bradshaw, D. The burden of non-communicable diseases in South Africa. Lancet 2009, 374, 934–947. [Google Scholar] [CrossRef]
- Modjadji, P.; Mokgalaboni, K.; Nonterah, E.A.; Lebelo, S.L.; Mchiza, Z.J.-R.; Madiba, S.; Kengne, A.P. A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy. Viruses 2023, 15, 1441. [Google Scholar]
- Rifqah Abeeda, R.; Brian van, W.; Eunice Bolanle, T.; Victoria Pillay-van, W. Multimorbidity in South Africa: A systematic review of prevalence studies. BMJ Open 2021, 11, e048676. [Google Scholar]
- Roomaney, R.A.; van Wyk, B.; Cois, A.; Pillay van-Wyk, V. Multimorbidity patterns in South Africa: A latent class analysis. Front. Public Health 2023, 10, 1082587. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health. South Africa Demographic and Health Survey 2003; Department of Health: Pretoria, South Africa, 2007.
- Peltzer, K. Patient experiences and health system responsiveness in South Africa. BMC Health Serv. Res. 2009, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Inauen, J.; Bierbauer, W.; Lüscher, J.; König, C.; Tobias, R.; Ihle, A.; Zimmerli, L.; Holzer, B.M.; Battegay, E.; Siebenhüner, K.; et al. Assessing adherence to multiple medications and in daily life among patients with multimorbidity. Psychol. Health 2017, 32, 1233–1248. [Google Scholar] [CrossRef]
- González-Bueno, J.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Molist-Brunet, N.; Codina-Jané, C.; Espaulella-Panicot, J. Factors Associated with Medication Non-Adherence among Patients with Multimorbidity and Polypharmacy Admitted to an Intermediate Care Center. Int. J. Environ. Res. Public Health 2021, 18, 9606. [Google Scholar]
- UNAIDS. Country Factsheets; UNAIDS: Geneva, Switzerland, 2018. [Google Scholar]
- Kazooba, P.; Kasamba, I.; Mayanja, B.N.; Lutaakome, J.; Namakoola, I.; Salome, T.; Kaleebu, P.; Munderi, P. Cardiometabolic risk among HIV-POSITIVE Ugandan adults: Prevalence, predictors and effect of long-term antiretroviral therapy. Pan Afr. Med. J. 2017, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.G.; Weiner, B.J.; Sherr, K.H.; Kupfer, L.E.; Cherutich, P.K.; Wilson, D.; Geng, E.H.; Wasserheit, J.N. Implementation science for integration of HIV and non-communicable disease services in sub-Saharan Africa: A systematic review. AIDS 2018, 32, S93–S105. [Google Scholar]
- Gordon, T.; Booysen, F.; Mbonigaba, J. Socio-economic inequalities in the multiple dimensions of access to healthcare: The case of South Africa. BMC Public Health 2020, 20, 289. [Google Scholar] [CrossRef]
- Forslund, T.; Carlsson, A.C.; Ljunggren, G.; Ärnlöv, J.; Wachtler, C. Patterns of multimorbidity and pharmacotherapy: A total population cross-sectional study. Fam. Pract. 2021, 38, 132–140. [Google Scholar] [PubMed]
- National Department of Health South Africa. National User Guide on the Prevention and Treatment of Hypertension in Adults at the PHC. 2023. Available online: https://knowledgehubhealthgovza/system/files/elibdownloads/2023-04/HYPERTENSION%2520USER%2520GUIDE%2520FINAL%2520COPYpdf (accessed on 29 April 2023).
- Bruno, R.d.C.; Myriam, C.; Douglas, G.A.; Anne, W.S.R.; Matthias, E. Uses and misuses of the STROBE statement: Bibliographic study. BMJ Open 2011, 1, e000048. [Google Scholar]
- Oni T, McGrath N, BeLue R, Roderick P, Colagiuri S, May CR, Levitt NS: Chronic diseases and multi-morbidity—A conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health 2014, 14, 575.
- Sabaté, E.; Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Peh, K.Q.E.; Kwan, Y.H.; Goh, H.; Ramchandani, H.; Phang, J.K.; Lim, Z.Y.; Loh, D.H.F.; Østbye, T.; Blalock, D.V.; Yoon, S.; et al. An Adaptable Framework for Factors Contributing to Medication Adherence: Results from a Systematic Review of 102 Conceptual Frameworks. J. Gen. Intern. Med. 2021, 36, 2784–2795. [Google Scholar] [PubMed]
- Tshwane, Co. Primary Healthcare Clinic Services. 2023. Available online: https://wwwtshwanegovza/?page_id=649 (accessed on 12 January 2023).
- Wikipedia. City of Tshwane Metropolitan Municipality. 2023. Available online: https://enwikipediaorg/wiki/City_of_Tshwane_Metropolitan_Municipality#cite_note-adrianfrith-8 (accessed on 12 April 2023).
- Statistic South Africa. Data Reworked by Adrian Frith Tshwane—Census. 2011. Available online: https://census2011adrianfrithcom/place/799 (accessed on 11 March 2020).
- RAOSOFT.COM. Raosoft Sample Size Calculator. 2004. Available online: http://wwwraosoftcom/samplesizehtml (accessed on 10 March 2020).
- Elfil, M.; Negida, A. Sampling methods in Clinical Research; an Educational Review. Emergency 2017, 5, e52. [Google Scholar]
- Allaham, K.K.; Feyasa, M.B.; Govender, R.D.; Musa, A.M.A.; AlKaabi, A.J.; ElBarazi, I.; AlSheryani, S.D.; Al Falasi, R.J.; Khan, M.A.B. Medication Adherence Among Patients with Multimorbidity in the United Arab Emirates. Patient Prefer. Adherence 2022, 16, 1187–1200. [Google Scholar] [CrossRef]
- Diederichs, C.; Berger, K.; Bartels, D.B. The measurement of multiple chronic diseases—A systematic review on existing multimorbidity indices. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 301–311. [Google Scholar] [CrossRef]
- Maimela, E.; Alberts, M.; Modjadji, S.E.P.; Choma, S.S.R.; Dikotope, S.A.; Ntuli, T.S.; Van Geertruyden, J.-P. The Prevalence and Determinants of Chronic Non-Communicable Disease Risk Factors amongst Adults in the Dikgale Health Demographic and Surveillance System (HDSS) Site, Limpopo Province of South Africa. PLoS ONE 2016, 11, e0147926. [Google Scholar] [CrossRef]
- Zwane, J.; Modjadji, P.; Madiba, S.; Moropeng, L.; Mokgalaboni, K.; Mphekgwana, P.M.; Kengne, A.P.; Mchiza, Z.J.-R. Self-Management of Diabetes and Associated Factors among Patients Seeking Chronic Care in Tshwane, South Africa: A Facility-Based Study. Int. J. Environ. Res. Public Health 2023, 20, 5887. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Gómez-Olivé, F.X.; Payne, C.; Rohr, J.K.; Manne-Goehler, J.; Wade, A.N.; Wagner, R.G.; Montana, L.; Tollman, S.; Salomon, J.A. Chronic multimorbidity among older adults in rural South Africa. BMJ Glob. Health 2019, 4, e001386. [Google Scholar] [CrossRef]
- Kwan, Y.H.; Weng, S.D.; Loh, D.H.F.; Phang, J.K.; Oo, L.J.Y.; Blalock, D.V.; Chew, E.H.; Yap, K.Z.; Tan, C.Y.K.; Yoon, S.; et al. Measurement Properties of Existing Patient-Reported Outcome Measures on Medication Adherence: Systematic Review. J. Med. Internet Res. 2020, 22, e19179. [Google Scholar] [CrossRef]
- Naqvi, A.A.; Hassali, M.A.; Rizvi, M.; Zehra, A.; Iffat, W.; Haseeb, A.; Jamshed, S. Development and Validation of a Novel General Medication Adherence Scale (GMAS) for Chronic Illness Patients in Pakistan. Front. Pharmacol. 2018, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Sangha, O.; Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003, 49, 156–163. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- WMA. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrb. Wiss. Ethik 2009, 14, 233–238. [Google Scholar]
- Pati, S.; Swain, S.; Metsemakers, J.; Knottnerus, J.A.; van den Akker, M. Pattern and severity of multimorbidity among patients attending primary care settings in Odisha, India. PLoS ONE 2017, 12, e0183966. [Google Scholar] [CrossRef]
- Mphekgwana, P.M.; Malema, N.; Monyeki, K.D.; Mothiba, T.M.; Makgahlela, M.; Kgatla, N.; Makgato, I.; Sodi, T. Hypertension Prevalence and Determinants among Black South African Adults in Semi-Urban and Rural Areas. Int. J. Environ. Res. Public Health 2020, 17, 7463. [Google Scholar] [CrossRef]
- Lloyd-Sherlock, P.; Beard, J.; Minicuci, N.; Ebrahim, S.; Chatterji, S. Hypertension among older adults in low-and middle-income countries: Prevalence, awareness and control. Int. J. Epidemiol. 2014, 43, 116–128. [Google Scholar] [CrossRef]
- Bertram, M.Y.; Jaswal, A.V.; Van Wyk, V.P.; Levitt, N.S.; Hofman, K.J. The non-fatal disease burden caused by type 2 diabetes in South Africa, 2009. Glob. Health Action 2013, 6, 19244. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.; Norman, R.; Pieterse, D.; Levitt, N.S. Estimating the burden of disease attributable to diabetes in South Africa in 2000. S. Afr. Med. J. 2007, 97, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Pillay-van Wyk, V.; Turawa, E.; Levitt, N.; Kengne, A.P.; Bradshaw, D. Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5868. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Mutyambizi, C.; Pavlova, M.; Hongoro, C.; Groot, W. Inequalities and factors associated with adherence to diabetes self-care practices amongst patients at two public hospitals in Gauteng, South Africa. BMC Endocr. Disord. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Olivé, F.X.; Ali, S.A.; Made, F.; Kyobutungi, C.; Nonterah, E.; Micklesfield, L.; Alberts, M.; Boua, R.; Hazelhurst, S.; Debpuur, C.; et al. Regional and Sex Differences in the Prevalence and Awareness of Hypertension: An H3Africa AWI-Gen Study Across 6 Sites in Sub-Saharan Africa. Glob. Heart 2017, 12, 81–90. [Google Scholar] [CrossRef]
- Modjadji, P.; Salane, M.C.; Mokwena, K.E.; Mudau, T.S.; Mphekgwana, P.M. Utility of Obesity Indicators for Predicting Hypertension among Older Persons in Limpopo Province, South Africa. Appl. Sci. 2022, 12, 4697. [Google Scholar] [CrossRef]
- Stieglitz, L.-M.; Bärnighausen, T.; Leyna, G.H.; Kazonda, P.; Killewo, J.; Rohr, J.K.; Kohler, S. Patterns of comorbidity and multimorbidity among middle-aged and elderly women in peri-urban Tanzania. J. Multimorb. Comorbidity 2022, 12, 26335565221076254. [Google Scholar] [CrossRef]
- Mabaso, M.; Makola, L.; Naidoo, I.; Mlangeni, L.L.; Jooste, S.; Simbayi, L. HIV prevalence in South Africa through gender and racial lenses: Results from the 2012 population-based national household survey. Int. J. Equity Health 2019, 18, 167. [Google Scholar] [CrossRef]
- Moosa, A.; Gengiah, T.N.; Lewis, L.; Naidoo, K. Long-term adherence to antiretroviral therapy in a South African adult patient cohort: A retrospective study. BMC Infect. Dis. 2019, 19, 775. [Google Scholar] [CrossRef] [PubMed]
- Kubjane, M.; Osman, M.; Boulle, A.; Johnson, L.F. The impact of HIV and tuberculosis interventions on South African adult tuberculosis trends, 1990–2019: A mathematical modeling analysis. Int. Soc. Infect. Dis. 2022, 122, 811–819. [Google Scholar] [CrossRef]
- Roomaney, R.A.; van Wyk, B.; Turawa, E.B.; Pillay-van Wyk, V. Prevalence of multimorbidity in South Africa: A systematic review protocol. BMJ Open 2020, 10, e042889. [Google Scholar] [CrossRef]
- Agborsangaya, C.B.; Lau, D.; Lahtinen, M.; Cooke, T.; Johnson, J.A. Multimorbidity prevalence and patterns across socioeconomic determinants: A cross-sectional survey. BMC Public Health 2012, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.; Rudisill, C.; Bhattarai, N.; Gulliford, M. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J. Health Serv. Res. Policy 2013, 18, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Moin, J.S.; Moineddin, R.; Upshur, R.E.G. Measuring the association between marginalization and multimorbidity in Ontario, Canada: A cross-sectional study. J. Comorbidity 2018, 8, 2235042X18814939. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, J.; Epping, J.; Sperlich, S.; Eberhard, S.; Stahmeyer, J.T.; Geyer, S. Widening inequalities in multimorbidity? Time trends among the working population between 2005 and 2015 based on German health insurance data. Int. J. Equity Health 2018, 17, 103. [Google Scholar] [CrossRef]
- Schiøtz, M.L.; Stockmarr, A.; Høst, D.; Glümer, C.; Frølich, A. Social disparities in the prevalence of multimorbidity—A register-based population study. BMC Public Health 2017, 17, 422. [Google Scholar] [CrossRef]
- Arpey, N.C.; Gaglioti, A.H.; Rosenbaum, M.E. How Socioeconomic Status Affects Patient Perceptions of Health Care: A Qualitative Study. J. Prim. Care Community Health 2017, 8, 169–175. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P. Socio-demographic Determinants of Overweight and Obesity Among Mothers of Primary School Children Living in a Rural Health and Demographic Surveillance System Site, South Africa. Open Public Health J. 2020, 13, 518–528. [Google Scholar] [CrossRef]
- Mphekgwana, P.M.; Sono-Setati, M.E.; Mokgophi, T.V.; Kifle, Y.G.; Madiba, S.; Modjadji, P. Retrospective Analysis of the Outcome of Hospitalized COVID-19 Patients with Coexisting Metabolic Syndrome and HIV Using Multinomial Logistic Regression. Int. J. Environ. Res. Public Health 2023, 20, 5799. [Google Scholar] [CrossRef]
- Weimann, A.; Dai, D.; Oni, T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: A comparison between 2008 and 2012. Soc. Sci. Med. 2016, 163, 144–156. [Google Scholar] [CrossRef]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lim, N.-K.; Choi, S.-J.; Park, H.-Y. Hypertension is an independent risk factor for type 2 diabetes: The Korean genome and epidemiology study. Hypertens. Res. 2015, 38, 783–789. [Google Scholar] [CrossRef]
- Kushitor, S.B.; Sanuade, O.A.; Baatiema, L.; Kushitor, M.K.; Afrifa-Anane, E.K.; Awuah, R.B. Non-communicable disease comorbidities in KwaZulu-Natal Province, South Africa. S. Afr. Med. J. 2021, 111, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mata-Cases M, Franch-Nadal J, Real J, Cedenilla M, Mauricio D: Prevalence and coprevalence of chronic comorbid conditions in patients with type 2 diabetes in Catalonia: A population-based cross-sectional study. BMJ Open. 2019, 9, e031281.
- Luijks, H.; Schermer, T.; Bor, H.; van Weel, C.; Lagro-Janssen, T.; Biermans, M.; de Grauw, W. Prevalence and incidence density rates of chronic comorbidity in type 2 diabetes patients: An exploratory cohort study. BMC Med. 2012, 10, 128. [Google Scholar] [CrossRef]
- Iglay, K.; Hannachi, H.; Joseph Howie, P.; Xu, J.; Li, X.; Engel, S.S.; Moore, L.M.; Rajpathak, S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016, 32, 1243–1252. [Google Scholar] [CrossRef]
- Lalkhen, H.; Mash, R. Multimorbidity in non-communicable diseases in South African primary healthcare. S. Afr. Med. J. 2015, 105, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Banjare, P.; Pradhan, J. Socio-economic inequalities in the prevalence of multi-morbidity among the rural elderly in Bargarh District of Odisha (India). PLoS ONE 2014, 9, e97832. [Google Scholar] [CrossRef] [PubMed]
- Nimako, B.A.; Baiden, F.; Sackey, S.O.; Binka, F. Multimorbidity of chronic diseases among adult patients presenting to an inner-city clinic in Ghana. Glob. Health 2013, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Ziegler, D. Risk factors and comorbidities in diabetic neuropathy: An update 2015. Rev. Diabet. Stud. RDS 2015, 12, 48. [Google Scholar] [CrossRef]
- Ryan, B.L.; Bray Jenkyn, K.; Shariff, S.Z.; Allen, B.; Glazier, R.H.; Zwarenstein, M.; Fortin, M.; Stewart, M. Beyond the grey tsunami: A cross-sectional population-based study of multimorbidity in Ontario. Can. J. Public Health 2018, 109, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Enslin, D.; Mallya, P. Factors influencing treatment adherence in hypertension and HIV management in South Africa: A Comparative Literature Review. S. Afr. Fam. Pract. 2022, 64, 5434. [Google Scholar] [CrossRef] [PubMed]
- WHO. Multimorbidity: Technical Series on Safer Primary Care; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/10665/252275/1/9789241511650-eng.pdf (accessed on 7 April 2023).
- WHO/UN. Monitoring Health for the SDGs; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/data/gho/data/themes/sustainable-development-goals (accessed on 11 April 2023).
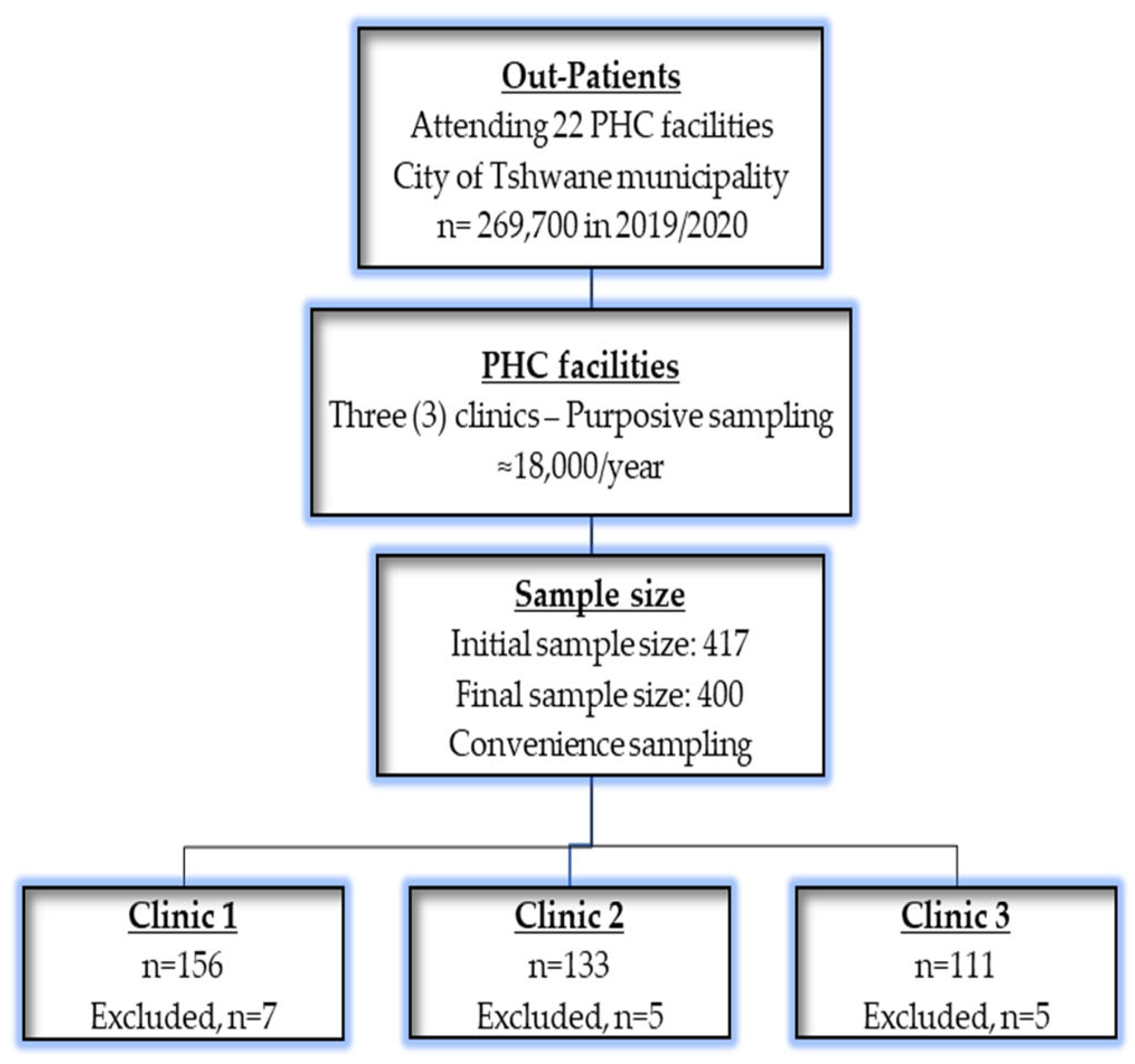
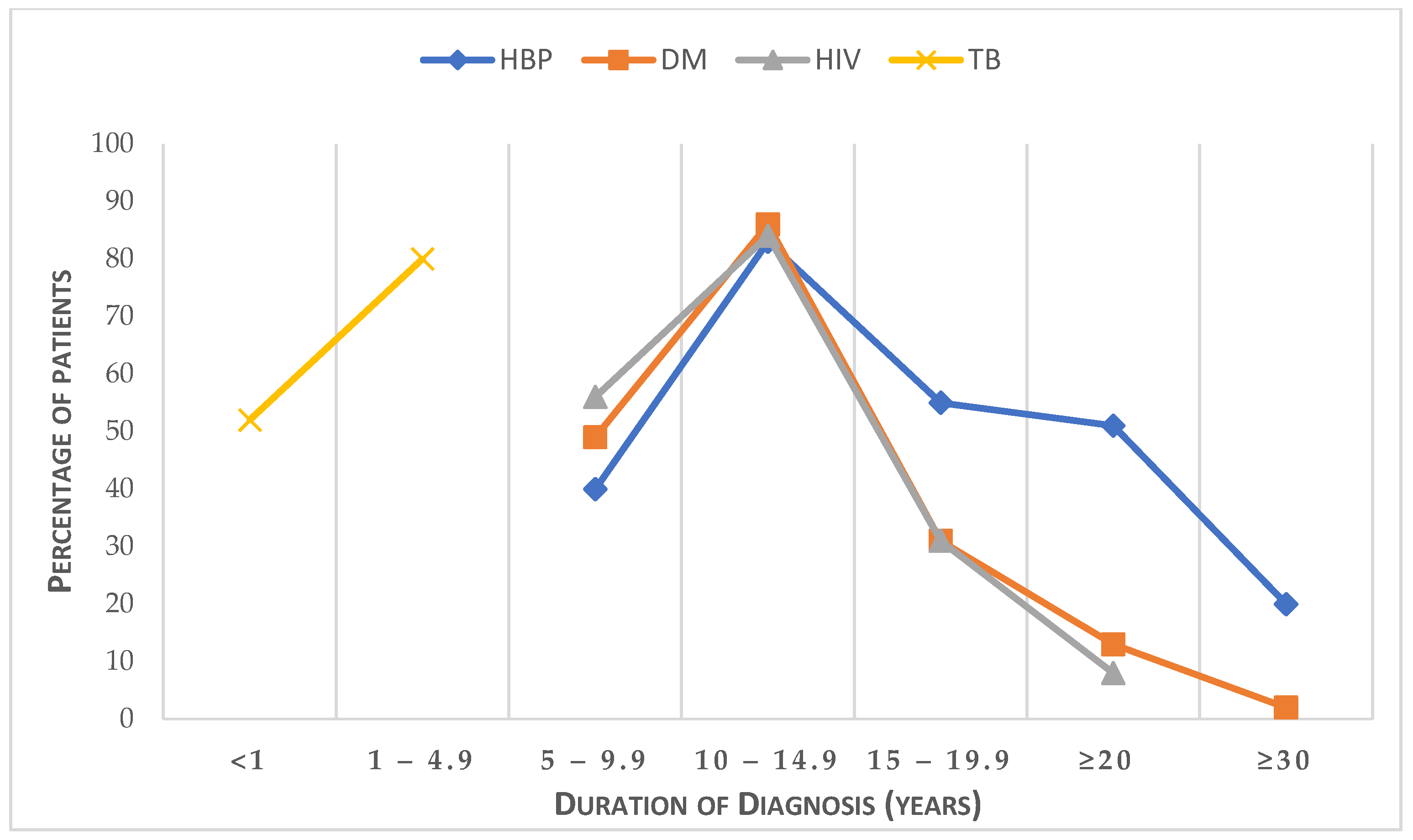
| Variables | Categories | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Sex | Male | 167 | 42 |
| Female | 233 | 58 | |
| Age groups | 18–34 | 86 | 30 |
| 35–59 | 133 | 47 | |
| ≥60 | 183 | 23 | |
| Race | Africans | 247 | 62 |
| White | 56 | 14 | |
| Coloureds | 48 | 12 | |
| Asian | 49 | 12 | |
| Place of Residence | Informal settlement | 108 | 27 |
| Peri-urban (i.e., township) | 133 | 33 | |
| Urban | 162 | 41 | |
| Marital status | Cohabiting | 79 | 20 |
| Single | 93 | 23 | |
| Married | 143 | 36 | |
| Divorced | 85 | 21 | |
| Level of Education | Primary | 74 | 19 |
| Secondary | 173 | 43 | |
| Completed grade 12 | 55 | 14 | |
| Tertiary | 98 | 25 | |
| Employed | No | 149 | 37 |
| Yes | 160 | 40 | |
| Pensioner | 91 | 23 | |
| BMI (kg/m2) | Normal | 8 | 2 |
| Underweight | 160 | 40 | |
| Overweight | 92 | 23 | |
| Obese | 140 | 35 | |
| Waist circumference | Normal | 49 | 12 |
| Abdominal obesity | 351 | 88 | |
| Waist-to-hip ratio | Normal | 239 | 60 |
| Abdominal obesity | 161 | 40 | |
| Waist-to-height ratio | Normal | 298 | 75 |
| Abdominal obesity | 102 | 25 | |
| Household income/month | $266.85 | 54 | 14 |
| $266.85–$533.80 | 172 | 43 | |
| $533.80–$800.40 | 73 | 18 | |
| >$800.40 | 101 | 25 | |
| Regular exercise | No | 257 | 64 |
| Yes | 143 | 34 | |
| Salt intake | No | 154 | 39 |
| Sometimes | 36 | 9 | |
| Yes | 210 | 53 | |
| Current alcohol use | No | 111 | 28 |
| Yes | 289 | 72 | |
| current cigarette smoking | No | 188 | 47 |
| Yes | 212 | 53 |
| Variables | Categories | n (%) |
|---|---|---|
| First diagnosed condition * | Hypertension | 197 (49) |
| HIV | 148 (37) | |
| Diabetes | 38 (10) | |
| Lung disease (i.e., asthma/TB) | 15 (4) | |
| Hypercholesterolemia | 2 (1) | |
| Hypertension | No | 151 (38) |
| Yes | 249 (62) | |
| Hypertension therapy | Mono | 54 (22) |
| Bi therapy | 89 (36) | |
| ≥3 therapies | 106 (42) | |
| Have blood pressure monitor | No | 213 (86) |
| Yes | 36 (14) | |
| Diabetes | No | 219 (55) |
| Yes | 181 (45) | |
| Diabetes therapy | Mono | 148 (82) |
| Bi therapy | 30 (10) | |
| 3 therapies | 3 (2) | |
| Have glucose meter | No | 66 (36) |
| Yes | 116 (64) | |
| HIV | Negative | 223 (56) |
| Positive | 177 (44) | |
| Taking ART (HIV) | No | 1 (1) |
| Yes | 176 (99) | |
| TB treatment | No | 0(0) |
| Yes | 132 (100) | |
| TB | Negative | 268 (67) |
| Positive | 132 (33) | |
| Other chronic conditions | None | 243 (61) |
| High cholesterol | 77 (18) | |
| Gout | 50 (13) | |
| Lung diseases, | 18 (5) | |
| High cholesterol/gout | 7 (1.75) | |
| High cholesterol/cardiac problems | 3 (0.75) | |
| Anaemia | 1 (0.25) | |
| Prostate cancer | 1 (0.25) | |
| Hospitalization due to: | Hypertension (yes) | 249 (62) |
| Diabetes (yes) | 22 (12) | |
| HIV-related problems (yes) | 18 (10) | |
| TB (yes) | 0 | |
| Medication adherence ** | Adherence | 297 (74) |
| Non-adherence | 103 (26) |
| Variables | Categories | n (%) |
|---|---|---|
| Multimorbidity classes | Concordant | 281 (72) |
| Discordant | 110 (28) | |
| Morbidity patterns | Single chronic conditions | 9 (2) |
| Two conditions | 300 (75) | |
| Three conditions | 80 (20) | |
| Four conditions | 11 (3) | |
| Two conditions | HIV and TB | 98 (25) |
| Hypertension and diabetes | 67 (17) | |
| Hypertension and Gout | 36 (9) | |
| Hypertension and HIV | 28 (7) | |
| Hypertension and hypercholesterolemia | 23 (6) | |
| Diabetes and HIV | 13 (3) | |
| Three conditions | Hypertension, diabetes, and hypercholesterolemia | 36 (9) |
| Hypertension, diabetes, and gout | 10 (3) | |
| Hypertension, diabetes, and HIV | 6 (2) | |
| Hypertension, diabetes, and asthma | 6 (2) | |
| Four conditions | Hypertension, diabetes, HIV, and hypercholesterolemia | 7 (2) |
| Variables | Males | Females | p | ≤35 | 35–59 | ≥60 | p |
|---|---|---|---|---|---|---|---|
| Years | Years | Years | |||||
| Hypertension | 0.061 | ≤0.0001 | |||||
| No | 72 (43) | 79 (34) | 50 (71) | 91 (29) | 10 (11) | ||
| Yes | 95 (57) | 154 (66) | 20 (29) | 145 (61) | 84 (89) | ||
| Diabetes | 0.081 | ≤0.0001 | |||||
| No | 100 (60) | 119 (51) | 61 (87) | 124 (53) | 34 (36) | ||
| Yes | 67 (40) | 114 (49) | 9 (13) | 112 (47) | 60 (64) | ||
| HIV | 0.213 | ≤0.0001 | |||||
| No | 87 (52) | 136 (58) | 11 (16) | 129 (55) | 83 (88) | ||
| Yes | 80 (48) | 97 (42) | 59 (84) | 107 (45) | 11 (12) | ||
| TB | ≤0.0001 | ≤0.0001 | |||||
| No | 94 (56) | 174 (75) | 26 (37) | 156 (66) | 86 (91) | ||
| Yes | 73 (44) | 59 (25) | 44 (63) | 80 (54) | 8 (9) | ||
| Multimorbidity class | 0.006 | 0.021 | |||||
| Concordant | 132 (79) | 149 (67) | 45 (70) | 158 (68) | 78 (83) | ||
| Discordant | 35 (21) | 75 (33) | 19 (30) | 75 (32) | 16 (17) | ||
| Morbidity patterns | 0.005 | ≤0.0001 | |||||
| Single | 0 | 9 (4) | 6 (9) | 3 (1) | 0 | ||
| Two | 131 (78) | 169 (73) | 58 (83) | 183 (78) | 59 (63) | ||
| Three | 28 (17) | 52 (22) | 6 (9) | 44 (19) | 30 (32) | ||
| Four | 8 (5) | 3 (1) | 0 | 6 (3) | 5 (5) | ||
| Medication | 0.164 | 0.003 | |||||
| Adherence | 118 (71) | 179 (77) | 43 (61) | 174 (74) | 0 (85) | ||
| Non-adherence | 49 (29) | 54 (23) | 27 (39) | 65 (26) | 14 (15) |
| Multimorbidity | COR (95%CI) | p-Value | AOR (95%CI) | p-Value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤35 | 1 | 1 | ||
| >35 | 7.77 (1.90–31.75) | 0.004 | 14.95 (3.28–68.19) | ≤0.0001 |
| Income ($) | ||||
| <$533.80 | 1 | 1 | ||
| ≥533.80 | 0.38 (0.09–1.53) | 0.172 | 0.16 (0.03–0.71) | 0.017 |
| Medication Adherence | COR (95%CI) | p-Value | AOR (95%CI) | p-Value |
|---|---|---|---|---|
| Age (years) | ||||
| <35 | 1 | |||
| 35–59 | 1.88 (1.31–2.71) | 0.001 | 2.15 (1.00–4.60) | 0.051 |
| ≥60 | 1.53 (1.02–2.32) | 0.042 | 3.20 (1.03–9.93) | 0.049 |
| Marital status | ||||
| Single | 1 | |||
| Cohabiting | 1.76 (0.95–3.27) | 0.072 | 2.17 (1.02–4.63) | 0.045 |
| Married | 3.78 (2.06–6.96) | ≤0.0001 | 5.01 (1.97–12.69) | 0.001 |
| Divorced | 8.48 (3.62–19.87) | ≤0.0001 | 8.84 (3.07–25.78) | ≤0.0001 |
| Education | ||||
| No education/primary | 1 | |||
| Secondary | 2.34 (1.33–4.11) | 0.003 | 3.06 (1.50–6.23) | 0.002 |
| Grade 12 | 5.37 (2.19–12.69) | ≤0.0001 | 9.81 (3.35–28.77) | ≤0.0001 |
| Tertiary | 6.43 (3.02–13.71) | ≤0.0001 | 9.40 (3.39–26.82) | ≤0.0001 |
| Household size | ||||
| <5 members | 1 | |||
| ≥5 members | 0.76 (0.48–1.19) | 0.231 | 0.28 (0.15–0.55) | ≤0.0001 |
| Current cigarette smoking | ||||
| No | 1 | |||
| Yes | 0.30 (0.18–0.40) | ≤0.0001 | 0.42 (0.31–0.76) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhwanazi, T.W.; Modjadji, P.; Mokgalaboni, K.; Madiba, S.; Roomaney, R.A. Multimorbidity, Treatment, and Determinants among Chronic Patients Attending Primary Health Facilities in Tshwane, South Africa. Diseases 2023, 11, 129. https://doi.org/10.3390/diseases11040129
Mkhwanazi TW, Modjadji P, Mokgalaboni K, Madiba S, Roomaney RA. Multimorbidity, Treatment, and Determinants among Chronic Patients Attending Primary Health Facilities in Tshwane, South Africa. Diseases. 2023; 11(4):129. https://doi.org/10.3390/diseases11040129
Chicago/Turabian StyleMkhwanazi, Thandiwe Wendy, Perpetua Modjadji, Kabelo Mokgalaboni, Sphiwe Madiba, and Rifqah Abeeda Roomaney. 2023. "Multimorbidity, Treatment, and Determinants among Chronic Patients Attending Primary Health Facilities in Tshwane, South Africa" Diseases 11, no. 4: 129. https://doi.org/10.3390/diseases11040129
APA StyleMkhwanazi, T. W., Modjadji, P., Mokgalaboni, K., Madiba, S., & Roomaney, R. A. (2023). Multimorbidity, Treatment, and Determinants among Chronic Patients Attending Primary Health Facilities in Tshwane, South Africa. Diseases, 11(4), 129. https://doi.org/10.3390/diseases11040129








