Abstract
Special populations, particularly pregnant women, are uniquely susceptible to infectious diseases due to alterations in their immunological, respiratory, and cardiovascular systems during gestation. Influenza infections during the perinatal period have been associated with more severe maternal and perinatal outcomes, underscoring the critical importance of vaccination data for pregnant women. According to the World Health Organization (WHO), all pregnant women and those of childbearing age should receive the inactivated influenza vaccine, irrespective of their pregnancy stage. This study aimed to elucidate factors influencing neonatal antibody presence following maternal influenza vaccination. Conducted through convenience sampling in Athens, Greece, this study involved 78 pregnant women who received flu vaccinations. The participants completed questionnaires covering demographics, obstetric history, attitudes toward influenza vaccination, and knowledge about the influenza virus and pregnancy vaccination. Blood samples were collected from 83 neonates to assess IgG antibody presence. Five of the surveyed women had twin pregnancies. The statistical analysis employed IBM SPSS-Statistics version 26.0. This study revealed the presence of positive influenza A and B antibodies in neonates following maternal immunization. Furthermore, it identified factors such as the gestational week and timing of vaccination during pregnancy that influenced the transfer of antibodies from mother to fetus. These findings offer valuable insights for healthcare professionals to provide informed recommendations on influenza vaccination during pregnancy and empower expectant mothers to make informed decisions about the benefits of immunization.
1. Introduction
Special populations, such as pregnant women and children, are susceptible to severe viral infections due to pregnancy-induced changes in the immune system, which is adapted to tolerate the presence of a semi-allogenic fetus [1]. Consequently, viral infections during pregnancy pose a significant risk, leading to severe maternal illness, elevated maternal mortality, and various pregnancy complications, including premature labor, spontaneous abortion, and fetal congenital abnormalities, particularly affecting the cardiovascular and central nervous systems [2].
Immunization has emerged as a pivotal strategy for safeguarding both maternal and neonatal health during pregnancy [3]. International health authorities, including the World Health Organization (WHO), the Advisory Committee on Immunization Practices (ACIP), and the American College of Obstetricians and Gynecologists (ACOG), strongly recommend that all pregnant women and women of childbearing age receive the inactivated influenza vaccine, regardless of their pregnancy stage [4,5]. The Centers for Disease Control and Prevention (CDC) further advise that all pregnant or potentially pregnant women receive a licensed, age-appropriate inactivated influenza vaccine or the recombinant quadrivalent influenza vaccine during the influenza season [6].
In Greece, as in many European countries, comprehensive antenatal care programs recommend immunization for women planning to become pregnant or already expecting [7]. Several retrospective studies have demonstrated the safety of influenza vaccines during pregnancy. Recent systematic reviews endorsed by the WHO have unequivocally refuted any association between influenza vaccination and adverse effects on pregnant women, including risks of miscarriage, fetal death, maternal mortality, preterm birth, or fetal congenital abnormalities [8,9,10]. Maternal influenza vaccination is particularly crucial because it passes maternal antibodies to the developing fetus through the placenta, offering protection to the newborn against influenza, a disease for which there are currently no approved vaccines for infants under six months of age [11].
The World Health Organization (WHO) annually provides recommendations on the influenza strains to be included in vaccines for the forthcoming northern hemisphere flu season. Vaccine compositions are updated accordingly based on WHO and EU guidance. Seasonal flu vaccines typically contain influenza A-H1N1, influenza AH3N2, and influenza B virus (IIV3). The quadrivalent IIV influenza vaccine now includes an additional B virus strain to enhance protection. Quadrivalent inactivated influenza vaccines were first licensed in 2012.
Following influenza vaccination, the body initiates antibody production approximately two weeks later, thereby offering protection against the influenza strains used in vaccine production. Seasonal flu vaccines are designed to protect against the influenza strains projected to be most prevalent in the upcoming flu season.
Immunoglobulin G (IgG) constitutes the primary antibody class, accounting for approximately 75% of human serum antibodies and featuring the neonatal Fc receptor (FcRn) expressed on syncytiotrophoblast cells within endosomes. This receptor is responsible for the transplacental transfer of passive humoral immunity from mother to fetus [12]. FcRn’s role in transporting IgG across the placenta involves binding maternal IgG in moderately acidic endosomes within the villous tree, where normal pH is restored, facilitating its transport to the basal plasma membrane and subsequent release from the FcRn [13]. FcRn expression commences after the 13th week of pregnancy. Between weeks 17 and 22, only 10% of maternal antibody concentration is present in fetal blood, with a notable increase in transfer efficiency observed thereafter. The majority of transfers transpire during the third trimester, especially between 28 and 32 weeks, although various factors may influence this process. Intriguingly, cord blood levels of IgG increase significantly after the 36th week of pregnancy, yet many aspects of IgG transport across the placenta remain poorly understood.
Several factors have been identified that influence the transfer of placental antibodies. These factors encompass the mother’s overall and specific antibody levels, IgG subclass, maternal health conditions during pregnancy (e.g., HIV infection), placental pathologies, maternal hypergammaglobulinemia, and the nature of the antigen itself, particularly its immunogenicity for thymus-dependent antigens [14]. Notably, maternal age, weight, parity, and delivery method do not impact placental antibody transmission. Furthermore, the immunization of full-term and preterm newborns poses challenges due to their underdeveloped immune systems and limited immunological responses to vaccine antigens [15,16].
This study aims to determine the optimal timing for influenza vaccination during pregnancy by investigating factors that may affect transplacental antibody transfer, as evidenced by the presence of antibodies in neonates.
2. Materials and Methods
The Clinical Research and Ethics Committees’ boards of the Elena Venizelou and the Alexandra Public Hospitals approved this study (T59-Μ10/16-09-2020) as a minimal risk that could not practicably be performed without the waiver of consent. Prior to the commencement of this study, written informed consent was obtained from all participating mothers of neonates.
2.1. Settings and Study Population Inclusion and Exclusion Criteria
Population: This was an institutional review board-approved cohort study of women who delivered their newborns at the Elena Venizelou and Alexandra Public Hospitals in Athens, Greece, and had received the quadrivalent influenza vaccine (IIV4) in the time period up to 6 months before pregnancy until birth. Vaccinated women, ≥18 years of age, fluent in the Greek language, who gave birth at the above hospitals between 1 December 2020 and 31 August 2022, were eligible for this study. A total of 78 women who received the flu vaccination during pregnancy, reported no significant complications or illnesses during pregnancy, and delivered within the study period were finally enrolled.
Gravidas were excluded if they were vaccinated before conception or during the postpartum period, those who did not give informed consent to take part in the research, and did not give their permission for the neonates’ blood sera collection. Only the participants who fully answered the questionnaires were included in our study. Vaccine administration was ascertained via a search of the electronic medical record (EMR) system. Neonatal blood sera were available for antibody detection in 83 newborns.
2.2. Sample Collection and Preparation
Prior to full study participant and biological sample deidentification, demographic data (i.e., age, level of education, family status, etc.) and obstetric clinical history data (such as previous pregnancies, miscarriage history, etc.) were collected from self-administered questionnaires.
Two sections constituted the questionnaire. Section 1 included items on the demographic characteristics and obstetric clinical history of women participating in the research. The knowledge and attitudes of women toward the influenza virus and the influenza vaccination were covered in Section 2 (some representative questions were “what is influenza?”, “what is the recommended flu vaccine for pregnant women in Greece?”, etc.).
Preterm delivery was defined as less than 37 gestational weeks, and term delivery was defined as 37 or more gestational weeks.
2.3. Sampling Procedure and Antibody Detection
Upon the neonates’ first day of life or within 10 days post-birth, blood samples were meticulously collected from 83 neonates (5 out of the 78 women had twin pregnancies). Both twins from each of the five pairs were included in all analyses. Blood samples were collected in Clot Activator tubes. All blood samples were processed within 4 h, and serum was stored at 80 °C until further investigation.
The sera were subjected to qualitative analysis for the presence of IgG class antibodies against influenza virus A and influenza virus B using two commercially available enzyme-linked immunosorbent assays (ELISAs), namely the Demeditec Influenza Virus A IgG ELISA (DEINFG0290) and the Demeditec Influenza Virus B IgG ELISA (DEINFG0300), following the relevant instructions for use (IFUs) provided by the manufacturer.
Briefly, coated enzyme-linked immunosorbent assay plates (Demeditec Diagnostics GmbH, Kiel, Germany) were meticulously incubated with appropriately diluted sera samples for 1 h ± 5 min at 37 ± 1 °C, then washed three times with 300 μL of washing buffer. Subsequently, 100 μL of conjugate was dispensed into all wells, with the exception of the substrate blank well, and the plates were once again incubated for 30 min at room temperature. After an additional three washing steps, Sure Blue 3,3′,5,5′-tetramethylbenzidine substrate (TMB) was added to each well, and the plates were incubated in the dark at room temperature (20–25 °C) for exactly 15 min, leading to a color change due to enzymatic reactions. A total of 100 μL of 0.2 mol/L sulfuric acid (stop solution) was added to each well to stop the reaction. Finally, the absorbance at 450/620 nm was measured within 30 min. All sera were tested in duplicate, and the mean absorbance values were calculated. The results were classified as negative (<9 U), equivocal (9–11 U), and positive (>11 U) in accordance with the manufacturer’s instructions.
2.4. Statistical Analysis
The Kolmogorov–Smirnov test was used to determine whether the distribution of the variables was normal. For continuous variables with two groups that were normally distributed, the t-test was used. We also used the Kruskal–Wallis test for non-normally distributed variables with three groups. In addition, continuous variables are displayed as mean (standard deviation). Categorical variables have been analyzed and presented as absolute numbers (frequency percent) using Fisher’s exact or chi-square tests; p-values under 0.05 were regarded as significant. IBM SPSS Statistics version 26.0 (IBM, Armonk, NY, USA) was used for the statistical analysis.
3. Results
3.1. Demographics, Characteristics, and Obstetric History of Enrolled Pregnant Women
During the study period, a total of 78 women gave birth, with five experiencing twin deliveries. The largest proportion of participants (31 individuals, 39.7%) fell within the 35–40 years age group, and the overwhelming majority were of Greek ethnicity (71 participants, 91%). In terms of education, a substantial portion (46 individuals, 59%) held bachelor’s degrees, while with regard to family status, most participants (67 individuals, 85.9%) were married. Additionally, a significant number of participants (37 individuals, 47.4%) were employed in the private sector.
Concerning obstetric history, a notable proportion of pregnant women (34 participants, 43%) had no prior pregnancies. Ten individuals (12.8%) reported a history of miscarriage, while 14 individuals (17.9%) experienced premature birth. Moreover, 20 women (25.6%) had a history of chronic diseases encompassing conditions such as type 1 diabetes, gestational diabetes, thrombophilia, hypothyroidism, and systemic lupus erythematosus. Furthermore, 31 participants (39.7%) reported close contact with individuals at high risk for flu complications within their households, and 15 (19.2%) reported similar contact with high-risk individuals at their workplaces.
Regarding the timing of flu vaccination during the current pregnancy, the majority of participants (31 individuals, 39.7%) received the flu vaccine during the first trimester, with all vaccinations occurring during the flu season.
Table 1 presents a comprehensive overview of the demographic characteristics and obstetric history of the study participants.

Table 1.
Demographics, characteristics, and obstetric history of pregnant women enrolled in the study.
3.2. Knowledge and Attitudes of Enrolled Pregnant Women Regarding the Influenza Virus and Influenza Vaccine
A noteworthy majority of participants exhibited a commendable level of awareness regarding the communicable nature of influenza (77 participants, 98.7%). Furthermore, a substantial portion acknowledged the potential severity of flu complications, recognizing the need for hospitalization (62 participants, 79.5%). Importantly, a considerable number of participants were cognizant of the heightened vulnerability of pregnant women to flu-related complications when compared to their non-pregnant counterparts (53 participants, 67.9%). Almost all participants (77, 98.7%) demonstrated an awareness of the recommendation and accessibility of free flu vaccinations for pregnant women in Greece, concurrently harboring the belief in the safety of administering the flu vaccine during pregnancy.
Concerning their past vaccination behavior, 27 participants (34.6%) reported having received the seasonal flu vaccine within the window of 6 months to 5 years preceding the current pregnancy. Furthermore, a substantial proportion (70, 89.7%) indicated that they had been adequately informed about the flu vaccine and the procedural aspects of obtaining it from healthcare professionals during the present pregnancy.
For a comprehensive overview of the knowledge and attitudes of pregnant women concerning the influenza virus and influenza vaccine, kindly refer to Table 2.

Table 2.
Knowledge and attitudes of pregnant women about influenza virus and influenza vaccine.
3.3. Association between Maternal Characteristics and Their Attitudes towards Influenza Vaccination
The present study aimed to investigate the association between pregnant women’s characteristics and their attitudes towards vaccination against influenza before pregnancy. A statistically significant disparity was evident in the proportion of participants who, within the timeframe of 6 months to 5 years before the current pregnancy, reported contact with high-risk individuals in their workplace and subsequently received a flu vaccine, in contrast to those who neither reported such contact nor received vaccination (9 out of 15 (60%) and 18 out of 63 (28.6%), respectively; p = 0.033). A comprehensive summary of these associations is provided in Table 3.

Table 3.
Association between maternal characteristics and their attitudes towards influenza vaccination.
3.4. Pregnancies and Neonates’ Characteristics
Out of the 78 pregnancies observed, a minority (five, 6.4%) constituted twin pregnancies. Predominantly, deliveries were administered through Caesarean section (53, 67.9%). Among the neonates, the female sex was more prevalent (43, 51.8%). Additionally, a significant proportion of neonates (66, 79.5%) were delivered within the gestational age range of 37 to 42 weeks, and the majority (73, 88%) exhibited an appropriate birth weight.
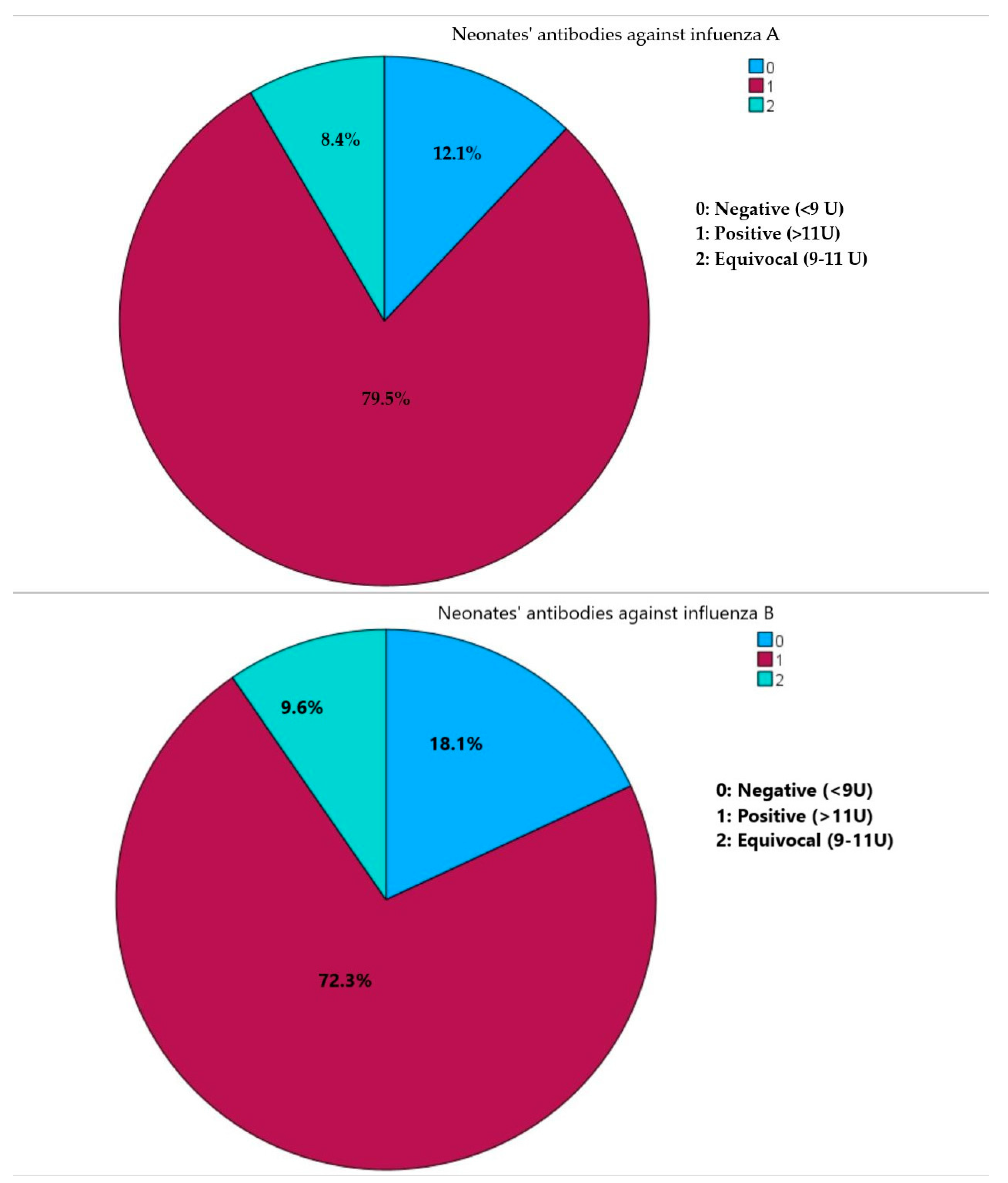
Sample collection primarily occurred during the flu season, accounting for 46 (55.4%) samplings. In terms of qualitative antibody assessment, a substantial number of neonates exhibited positive antibodies against both influenza A (66, 79.5%) and influenza B (60, 72.3%). Furthermore, the majority of neonates (50, 60.2%) demonstrated positivity for both influenza A and B, while merely three neonates (0.04%) tested negative for both influenza A and B (Figure 1).
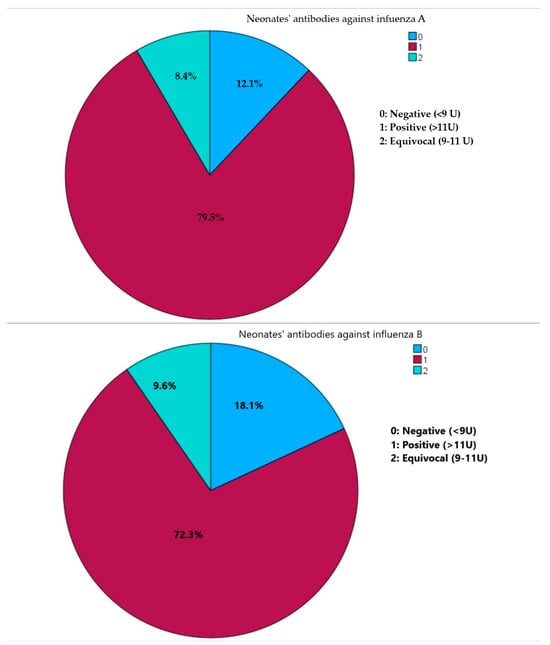
Figure 1.
Presence of neonates’ IgG class antibodies against influenza virus A and influenza virus B.
Table 4 provides a comprehensive overview of the characteristics pertaining to the pregnancies and neonates.

Table 4.
Characteristics of the pregnancies and neonates.
3.5. Association of Maternal and Neonatal Characteristics with the Presence of Neonates’ IgG Class Antibodies against Influenza Virus A and B
This study delved into the potential links between maternal and neonatal characteristics and the presence of antibodies against influenza A and influenza B. Our findings revealed a notably higher prevalence of positive results for influenza A antibodies within the neonates born between the 37th and 42nd week of gestation (p = 0.021), as well as among women vaccinated during the second trimester of pregnancy (p = 0.041). Furthermore, we observed a statistically significant association between the duration between vaccination and blood sampling and the presence of antibodies against influenza B. Participants who tested positive for influenza B antibodies exhibited a significantly shorter period between vaccination and blood sampling. These associations are displayed in Table 5 and Table 6.

Table 5.
Association of pregnant women’s and neonates’ characteristics with the presence of antibodies against influenza A.

Table 6.
Association of pregnant women’s and neonates’ characteristics with the presence of antibodies against influenza B.
3.6. Concordant IgG Detection in Twin Neonates
In our study, a total of five twin pregnancies were included, constituting a relatively small sample size. However, this limited cohort exhibited a noteworthy trend, where the twin neonates consistently displayed concordant detection rates for both influenza viruses. This observation implies a remarkable similarity in their immunological profiles. The results are displayed in Table 7.

Table 7.
Concordant IgG detection results among twin neonates.
4. Discussion
Influenza poses a significant threat to pregnant individuals due to pregnancy-induced changes in their immune system. As a result, pregnant women are more susceptible to severe illness and complications from influenza compared to those who are not pregnant [2]. Our study was conducted in the depths of the COVID-19 pandemic-related health system crisis. However, during the COVID-19 pandemic, influenza vaccination was promoted in Greece as a necessary public health measure to manage the COVID-19 pandemic. This included facilitating differential diagnosis and preventing the overloading of hospitals and health services associated with influenza infections. The National Public Health Organization in Greece recommends vaccinating pregnant women with mRNA COVID-19 vaccines at the same time as the general population. Despite the fact that some studies highlight that the COVID-19 pandemic has led to more positive intentions for influenza vaccination globally, we did not record data regarding the COVID-19 vaccine in our study population [17].
Our survey study revealed that pregnant participants were well aware of the risks associated with contracting influenza during pregnancy, consistent with previous research showing that the majority of pregnant individuals recognize the heightened danger posed by influenza during pregnancy compared to non-pregnant individuals [18]. This underscores the seriousness of influenza’s impact, as evidenced by the 2009 H1N1 influenza A pandemic, which demonstrated the propensity for severe illness, hospitalization, intensive care unit admissions, and unfortunately, fatalities among pregnant individuals [19]. The third trimester of pregnancy is associated with a higher risk of severe influenza-related outcomes, including preterm labor and a low birth weight, as demonstrated in multiple previous studies [20]. Maternal influenza infection also increases the risk of neonatal complications such as a low birth weight and stillbirth, emphasizing the importance of influenza vaccination during pregnancy. This is especially crucial as there is currently no approved influenza vaccine for infants younger than six months [21].
In a cross-sectional study conducted in Kenya, 68.3% of participants considered the flu vaccine safe during pregnancy, and 60.4% believed that it would protect their baby from infection. In our study, 97% of participants viewed the flu vaccine as safe during pregnancy. In Greece and most European countries, influenza vaccination is recommended and provided free of charge to all pregnant women. However, in some countries like Kenya, only tetanus toxoid is recommended as a maternal vaccine, while influenza vaccines are available in the private sector. Another survey in Kenya found that 83.7% of participants would choose to receive the influenza vaccine if it were offered for free [22].
Unlike most previous studies that administered flu shots in the second or third trimester, our study allowed vaccination in any trimester, with a significant number of participants receiving the vaccine during the first trimester. This shift may be due to increased awareness among healthcare professionals that influenza vaccination is safe at any trimester [23,24]. Healthcare providers’ recommendations also play a crucial role in pregnant women’s decision to receive vaccinations, as demonstrated in previous studies [24,25].
Our study included participants who received the quadrivalent influenza vaccine (IIV4) during pregnancy. This resulted in similar antibody responses against both influenza A and B strains, differing from previous research where antibody levels against influenza B were lower due to the use of trivalent vaccines [26].
Our findings align with the notion that the transfer of maternal antibodies is most robust in the third trimester of pregnancy [16]. We detected the highest rate of positive results for influenza A among neonates born during this period. Additionally, our study found no statistically significant associations between the demographic and clinical characteristics of pregnant individuals and the presence of antibodies against influenza A and B. Some studies have demonstrated that ethnicity can influence immune responses to vaccination. More specifically, a recent study showed that African Americans aged 30 to 40 have higher levels of H1N1 virus antibodies than Caucasians of the same age [27]. In our study, the majority of participants had Greek ethnicity, and this is reason why we elected not to investigate this association. According to previous studies, the majority of IgG is acquired by the fetus during the last four weeks of pregnancy. It i1111s noteworthy that after the 36th week of gestation, cord blood levels rapidly surge [16,28]. This fact confirms our findings where the highest positive results for influenza A were observed in neonates born in the 37th–42nd week of pregnancy (p = 0.021). Positive antibodies were also detected in 70.5% of preterm infants (36–31 weeks of gestation). Furthermore, other studies associated newborn weights of less than 2.5 kg with a lower antibody transfer [29,30]. In our study, birth weight did not show a statistically significant association with the presence of antibodies in our sample.
Additionally, our study revealed that the highest proportion of positive results for influenza A were found in the neonates of women vaccinated during the second trimester (p = 0.041). Recent research also supports the impact of vaccination trimester and birth season on antibody titers, with notably higher levels observed in cord blood when mothers were vaccinated during the second or third trimesters [31].
Our study’s strengths include a high response rate, lending credibility to our findings and making them representative. Peripheral blood samples, chosen for their accuracy in measuring antibody values, were used instead of umbilical cord blood. Obtaining maternal consent was challenging, but our results are valuable for neonates born from pregnancies with limited placental capacity for transferring protective antibodies.
Despite the robust response rate, limitations persist, including an uneven distribution of participants across pregnancy trimesters due to the study’s limited sample size. Furthermore, the study could not definitively exclude subclinical influenza cases, and the data collection process relied on non-standardized questionnaires. Additionally, the absence of maternal blood samples prevented the correlation of IgG antibody presence or titers between pregnant women and their neonates. The lack of a quantitative assay to determine antibody titers represents another significant limitation, constraining the depth of our analytical capabilities. Nevertheless, our study supports the hypothesis that maternal immunization through vaccination during pregnancy enhances neonatal immunization against specific pathogens, such as influenza.
Since infants under six months of age cannot receive influenza vaccinations, enhancing humoral immunity through the transplacental transmission of maternal antibodies emerges as a vital preventative measure during pregnancy. Understanding the kinetics of vertically conveyed immunity is crucial, as it provides neonates with a layer of defense against infections like influenza during their early stages of life. Risks associated with influenza infection are neglected; thus, vaccination remains as the primary prevention strategy. Transplacental antibody transport begins early in pregnancy, reaching its peak in the final four weeks of gestation. Consequently, all pregnant individuals, regardless of gestational stage, should be eligible for complimentary influenza vaccination through their respective country’s National Immunization Program. Health policymakers and obstetric care providers should strongly advocate for influenza vaccinations.
5. Conclusions
Pregnancy orchestrates intricate trimester-specific immunological adjustments that are vital for safeguarding the well-being of the maternal–newborn dyad. This inquiry illuminates the determinants impacting neonatal antibody acquisition following maternal influenza vaccination. The profound comprehension of these factors governing placental IgG antibody transference assumes paramount significance, offering avenues for therapeutic manipulation to confer early life advantages to neonates. In summation, amid the escalating global prevalence of infectious maladies, particularly in resource-limited regions, enhancing neonatal survival rates and refining the paradigm of prenatal vaccination is an imperative mandate.
Author Contributions
Conceptualization, C.T. and A.L.; data curation, C.T.; formal analysis, V.E.G.; experiments, P.V.D. and G.A.S.; investigation, C.T.; methodology, A.L. and C.T.; project administration, C.T.; supervision, A.L., A.B. and G.D.; writing—original draft, C.T. and A.S.; writing—review and editing, C.T. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Elena Venizelou and Alexandra Hospitals Clinical Research and Ethics Committee, T59-Μ10/16-09-2020 (protocol code T59-M10—16 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets produced and analyzed in the present study are not openly accessible to the public due to privacy and confidentiality concerns. Nevertheless, they can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robinson, D.P.; Klein, S.L. Pregnancy and Pregnancy-Associated Hormones Alter Immune Responses and Disease Pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Walensky, R.P.; Jernigan, D.B.; Bunnell, R.; Layden, J.; Kent, C.K.; Gottardy, A.J.; Leahy, M.A.; Martinroe, J.C.; Spriggs, S.R.; Yang, T.; et al. Morbidity and Mortality Weekly Report Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season Centers for Disease Control and Prevention MMWR. Recomm. Rep. 2021, 70, 1–28. [Google Scholar]
- American College of Obstetricians and Gynecologists. Influenza Vaccination during Pregnancy: Committee Opinion No 732. 2018. Available online: https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Influenza-Vaccination-During-Pregnancy (accessed on 5 October 2020).
- Center Of Disease Control and Prevention (CDC). Key Facts about Seasonal Flu Vaccine. 2020. Available online: https://www.cdc.gov/flu/prevent/keyfacts.htm (accessed on 16 December 2020).
- Bödeker, B.; Walter, D.; Reiter, S.; Wichmann, O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine 2014, 32, 4131–4139. [Google Scholar] [CrossRef]
- Fell, D.; Platt, R.; Lanes, A.; Wilson, K.; Kaufman, J.; Basso, O.; Buckeridge, D. Fetal death and preterm birth associated with maternal influenza vaccination: Systematic review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 17–26. [Google Scholar] [CrossRef]
- Fell, D.B.; Sprague, A.E.; Liu, N.; Yasseen, A.S.; Wen, S.-W.; Smith, G.; Walker, M.C.; Ontario, F.B.O.R.N. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am. J. Public Health 2012, 102, e33–e40. [Google Scholar] [CrossRef]
- Omer, S.B.; Goodman, D.; Steinhoff, M.C.; Rochat, R.; Klugman, K.P.; Stoll, B.J.; Ramakrishnan, U. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: A retrospective cohort study. PLoS Med. 2011, 8, e1000441. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD). Available online: https://www.cdc.gov/ncird/index.html (accessed on 30 October 2022).
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Abu-Raya, B.T.; Kollmann, R.; Marchant, A.; MacGillivray, D.M. The immune system of HIV-exposed uninfected infants. Front. Immunol. 2016, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Yao, F.; Chen, K. Immunization of pregnant women: Future of early infant protection. Hum. Vaccin. Immunother. 2015, 11, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, Q.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Lim, N.A.; Chin, Y.H.; Ng, Y.P.M.; Amin, Z. Effect of COVID-19 Pandemic on Influenza Vaccination Intention: A Meta-Analysis and Systematic Review. Vaccines 2022, 10, 606. [Google Scholar] [CrossRef]
- Napolitano, F.; Napolitano, P.; Angelillo, I.F. Seasonal influenza vaccination in pregnant women: Knowledge, attitudes, and behaviors in Italy. BMC Infect. Dis. 2017, 17, 48. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef]
- Mosby, L.G.; Rasmussen, S.A.; Jamieson, D.J. 2009 pandemic influenza A (H1N1) in pregnancy: A systematic review of the literature. Am. J. Obstet. Gynecol. 2011, 205, 10–18. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Kuwanda, L.; Weinberg, A.; Hugo, A.; Jones, S.; Adrian, P.V.; van Niekerk, N.; Treurnicht, F.; Ortiz, J.R.; et al. Maternal Flu Trial (Matflu) Team. Influenza vaccination of pregnant women and protection of their infants. N. Engl. J. Med. 2014, 371, 918–931. [Google Scholar] [CrossRef]
- Otieno, N.A.; Nyawanda, B.; Otiato, F.; Adero, M.; Wairimu, W.N.; Atito, R.; Wilson, A.D.; Gonzalez-Casanova, I.; Malik, F.A.; Verani, J.R.; et al. Knowledge and attitudes towards influenza and influenza vaccination among pregnant women in Kenya. Vaccine 2020, 38, 6832–6838. [Google Scholar] [CrossRef]
- Taskou, C.; Sarantaki, A.; Beloukas, A.; Georgakopoulou, V.; Daskalakis, G.; Papalexis, P.; Lykeridou, A. Knowledge and Attitudes of Healthcare Professionals Regarding Perinatal Influenza Vaccination during the COVID-19 Pandemic. Vaccines 2023, 11, 168. [Google Scholar] [CrossRef]
- Kong, K.L.; Chu, S.; Giles, M.L.; Aust, N.Z.J. Factors influencing the uptake of influenza vaccine vary among different groups in the hard-to-reach population. Aust. N. Z. J. Public Health 2020, 44, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Clarke, M.; Koehler, A.; Watson, M.; Marshall, H. Factors associated with uptake of influenza and pertussis vaccines among pregnant women in South Australia. PLoS ONE 2018, 13, e0197867. [Google Scholar] [CrossRef] [PubMed]
- Ledlie, S.; Gandhi-Banga, S.; Shrestha, A.; Moore, T.M.; Khromava, A. Exposure to quadrivalent influenza vaccine during pregnancy: Results from a global pregnancy registry. Influenza Other Respir. Viruses 2022, 16, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kurupati, R.; Kossenkov, A.; Haut, L.; Kannan, S.; Xiang, Z.; Li, Y.; Doyle, S.; Liu, Q.; Schmader, K.; Showe, L.; et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget 2016, 7, 62898–62911. [Google Scholar] [CrossRef] [PubMed]
- Doroudchi, M.; Samsami-Dehaghani, A.; Emad, K.; Ghaderi, A. Placental transfer of rubella-specific IgG in fullterm and preterm newborns. Int. J. Gynaecol. Obstet. 2003, 81, 157–162. [Google Scholar] [CrossRef]
- Yildiz, M.; Kara, M.; Sutcu, M.; Mese, S.; Demircili, M.E.; Sivrikoz, T.S.; Torun, S.H.; Agacfidan, A.; Coban, A.; Unuvar, E.; et al. Evaluation of respiratory syncytial virus IgG antibody dynamics in mother-infant pairs cohort. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2020, 39, 1279–1286. [Google Scholar] [CrossRef]
- Atwell, J.E.; Lutz, C.S.; Sparrow, E.G.; Feikin, D.R. Biological factors that may impair transplacental transfer of RSV antibodies: Implications for maternal immunization policy and research priorities for low- and middle-income countries. Vaccine 2022, 40, 4361–4370. [Google Scholar] [CrossRef]
- Zhong, Z.; Haltalli, M.; Holder, B.; Rice, T.; Donaldson, B.; O’driscoll, M.; Le-Doare, K.; Kampmann, B.; Tregoning, J.S. The impact of timing of maternal influenza immunization on infant antibody levels at birth. Clin. Exp. Immunol. 2019, 195, 139–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).