The Impact of Climate Change on Immunity and Gut Microbiota in the Development of Disease
Abstract
:1. Introduction
2. Climate Change in Health and Disease
3. Climate Change and Sex Differences
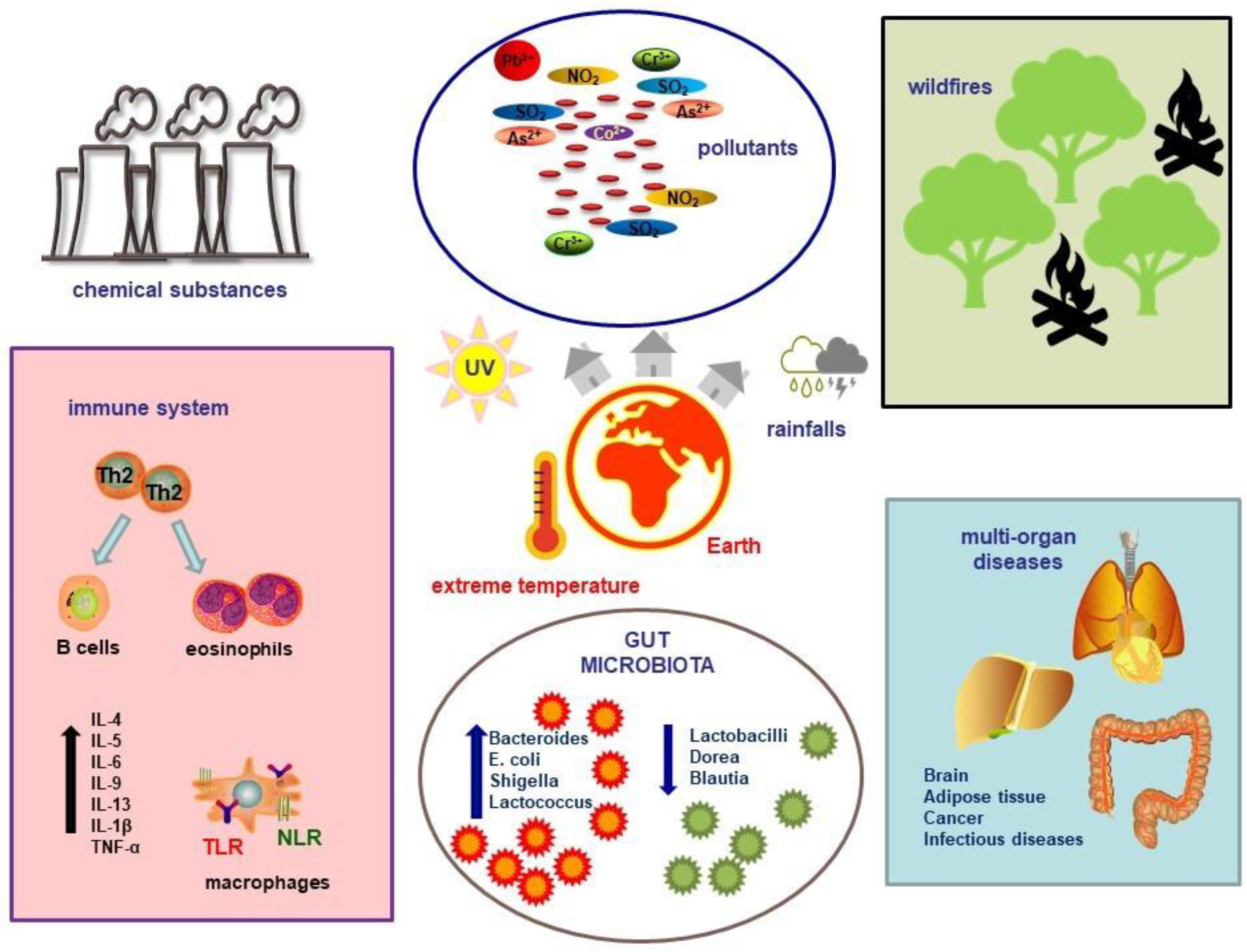
4. Climate Change and the Immune System
4.1. Global Warming
4.2. Fires, Storms, and Pollutants
5. Climate Change and the Gut Microbiome
5.1. Heat
5.2. Cold
5.3. Pollution
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- United Nations. What Is Climate Change? Available online: https://www.un.org/en/climatechange/what-is-climate-change (accessed on 14 April 2024).
- IPCC. Climate Change Widespread, Rapid, and Intensifying—IPCC. Available online: https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/ (accessed on 14 April 2024).
- Climate Change. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 13 April 2024).
- Ray, C.; Ming, X. Climate Change and Human Health: A Review of Allergies, Autoimmunity and the Microbiome. Int. J. Environ. Res. Public Health 2020, 17, 4814. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M.M.B., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- WHO. We Must Fight One of the World’s Biggest Health Threats: Climate Change. Available online: https://www.who.int/news-room/commentaries/detail/we-must-fight-one-of-the-world-s-biggest-health-threats-climate-change (accessed on 17 May 2024).
- Skevaki, C.; Nadeau, K.C.; Rothenberg, M.E.; Alahmad, B.; Mmbaga, B.T.; Masenga, G.G.; Sampath, V.; Christiani, D.C.; Haahtela, T.; Renz, H. Impact of Climate Change on Immune Responses and Barrier Defense. J. Allergy Clin. Immunol. 2024, 153, 1194–1205. [Google Scholar] [CrossRef]
- Haahtela, T. A Biodiversity Hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef]
- Gunawan, W.B.; Abadi, M.N.P.; Fadhillah, F.S.; Nurkolis, F.; Pramono, A. The Interlink between Climate Changes, Gut Microbiota, and Aging Processes. Hum. Nutr. Metab. 2023, 32, 200193. [Google Scholar] [CrossRef]
- Mattoo, R.; Mallikarjuna, S. Soil Microbiome Influences Human Health in the Context of Climate Change. Future Microbiol. 2023, 18, 845–859. [Google Scholar] [CrossRef]
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of Gut Barrier and Gut Microbiota in Food Restriction, Food Deprivation and Protein-Energy Wasting. Clin. Nutr. 2015, 34, 341–349. [Google Scholar] [CrossRef]
- Madhogaria, B.; Bhowmik, P.; Kundu, A. Correlation between Human Gut Microbiome and Diseases. Infect. Med. 2022, 1, 180–191. [Google Scholar] [CrossRef]
- White, S.; Montgomery, H. The Need for Radical Climate Interventions: Six Years to Secure Humanity’s ‘Liveable Future’. Anaesthesia 2024, 79, 232–236. [Google Scholar] [CrossRef]
- Filippini, T.; Paduano, S.; Veneri, F.; Barbolini, G.; Fiore, G.; Vinceti, M. Adverse Human Health Effects of Climate Change: An Update. Ann. Ig. 2024, 36, 281–291. [Google Scholar] [CrossRef]
- Rocque, R.J.; Beaudoin, C.; Ndjaboue, R.; Cameron, L.; Poirier-Bergeron, L.; Poulin-Rheault, R.-A.; Fallon, C.; Tricco, A.C.; Witteman, H.O. Health Effects of Climate Change: An Overview of Systematic Reviews. BMJ Open 2021, 11, e046333. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Impact on Human Health of Climate Changes. Eur. J. Intern. Med. 2015, 26, 1–5. [Google Scholar] [CrossRef]
- Bayram, H.; Rice, M.B.; Abdalati, W.; Akpinar Elci, M.; Mirsaeidi, M.; Annesi-Maesano, I.; Pinkerton, K.E.; Balmes, J.R. Impact of Global Climate Change on Pulmonary Health: Susceptible and Vulnerable Populations. Ann. Am. Thorac. Soc. 2023, 20, 1088–1095. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Vicedo-Cabrera, A.M.; Hoffmann, B.; Melén, E. Climate Change and Respiratory Disease: Clinical Guidance for Healthcare Professionals. Breathe 2023, 19, 220222. [Google Scholar] [CrossRef]
- Berg, C.D.; Schiller, J.H.; Boffetta, P.; Cai, J.; Connolly, C.; Kerpel-Fronius, A.; Kitts, A.B.; Lam, D.C.L.; Mohan, A.; Myers, R.; et al. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J. Thorac. Oncol. 2023, 18, 1277–1289. [Google Scholar] [CrossRef]
- Guo, B.; Gao, Q.; Pei, L.; Guo, T.; Wang, Y.; Wu, H.; Zhang, W.; Chen, M. Exploring the Association of PM2.5 with Lung Cancer Incidence under Different Climate Zones and Socioeconomic Conditions from 2006 to 2016 in China. Environ. Sci. Pollut. Res. 2023, 30, 126165–126177. [Google Scholar] [CrossRef]
- Aitken, W.W.; Brown, S.C.; Comellas, A.P. Climate Change and Cardiovascular Health. J. Am. Heart Assoc. 2022, 11, e027847. [Google Scholar] [CrossRef]
- Lee, B.-J.; Kim, B.; Lee, K. Air Pollution Exposure and Cardiovascular Disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef]
- Li, M.; Shaw, B.A.; Zhang, W.; Vásquez, E.; Lin, S. Impact of Extremely Hot Days on Emergency Department Visits for Cardiovascular Disease among Older Adults in New York State. Int. J. Environ. Res. Public Health 2019, 16, 2119. [Google Scholar] [CrossRef]
- Bernard, P.; Chevance, G.; Kingsbury, C.; Baillot, A.; Romain, A.-J.; Molinier, V.; Gadais, T.; Dancause, K.N. Climate Change, Physical Activity and Sport: A Systematic Review. Sports Med. 2021, 51, 1041–1059. [Google Scholar] [CrossRef]
- Sadeghi, A.; Leddin, D.; Malekzadeh, R. Mini Review: The Impact of Climate Change on Gastrointestinal Health. Middle East. J. Dig. Dis. 2023, 15, 72–75. [Google Scholar] [CrossRef]
- Walldorf, J.; Mezger, N.C.S.; Weber, L.; Knothe, A.; Klose, M.; Moritz, S.; Kantelhardt, E.J.; Feller, S.; Schlitt, A.; Greinert, R.; et al. Klimakrise: Welche gastroenterologischen Folgeerkrankungen dieses medizinischen Notfalls sollten wir kennen? Z. Gastroenterol. 2023, 61, 1608–1617. [Google Scholar] [CrossRef]
- Hales, S. Climate Change, Extreme Rainfall Events, Drinking Water and Enteric Disease. Rev. Environ. Health 2019, 34, 1–3. [Google Scholar] [CrossRef]
- Mora, C.; Counsell, C.W.W.; Bielecki, C.R.; Louis, L.V. Twenty-Seven Ways a Heat Wave Can Kill You: Deadly Heat in the Era of Climate Change. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004233. [Google Scholar] [CrossRef]
- Donnelly, M.C.; Talley, N.J. Effects of Climate Change on Digestive Health and Preventative Measures. Gut 2023, 72, 2199–2201. [Google Scholar] [CrossRef]
- Gregory, P.J.; Ingram, J.S.I.; Brklacich, M. Climate Change and Food Security. Phil. Trans. R. Soc. B 2005, 360, 2139–2148. [Google Scholar] [CrossRef]
- Owino, V.; Kumwenda, C.; Ekesa, B.; Parker, M.E.; Ewoldt, L.; Roos, N.; Lee, W.T.; Tome, D. The Impact of Climate Change on Food Systems, Diet Quality, Nutrition, and Health Outcomes: A Narrative Review. Front. Clim. 2022, 4, 941842. [Google Scholar] [CrossRef]
- United Nations. Food and Climate Change: Healthy Diets for a Healthier Planet. Available online: https://www.un.org/en/climatechange/science/climate-issues/food (accessed on 20 May 2024).
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Aziz, T.; Naveed, M.; Makhdoom, S.I.; Ali, U.; Mughal, M.S.; Sarwar, A.; Khan, A.A.; Zhennai, Y.; Sameeh, M.Y.; Dablool, A.S.; et al. Genome Investigation and Functional Annotation of Lactiplantibacillus Plantarum YW11 Revealing Streptin and Ruminococcin-A as Potent Nutritive Bacteriocins against Gut Symbiotic Pathogens. Molecules 2023, 28, 491. [Google Scholar] [CrossRef]
- Aziz, T.; Khan, A.A.; Tzora, A.; Voidarou, C.; Skoufos, I. Dietary Implications of the Bidirectional Relationship between the Gut Microflora and Inflammatory Diseases with Special Emphasis on Irritable Bowel Disease: Current and Future Perspective. Nutrients 2023, 15, 2956. [Google Scholar] [CrossRef]
- Van De Vuurst, P.; Escobar, L.E. Climate Change and Infectious Disease: A Review of Evidence and Research Trends. Infect. Dis. Poverty 2023, 12, 51. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; Von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Yu, P.; Xu, R.; Yang, Z.; Ye, T.; Liu, Y.; Li, S.; Abramson, M.J.; Kimlin, M.; Guo, Y. Cancer and Ongoing Climate Change: Who Are the Most Affected? ACS Environ. Au 2023, 3, 5–11. [Google Scholar] [CrossRef]
- Nogueira, L.; Florez, N. The Impact of Climate Change on Global Oncology. Hematol. Oncol. Clin. N. Am. 2024, 38, 105–121. [Google Scholar] [CrossRef]
- Friedrich, M.J. Determining Health Effects of Hazardous Materials Released During Hurricane Harvey. JAMA 2017, 318, 2283. [Google Scholar] [CrossRef]
- Hill, W.; Lim, E.L.; Weeden, C.E.; Lee, C.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Evans, E.J.; Moore, D.A.; et al. Lung Adenocarcinoma Promotion by Air Pollutants. Nature 2023, 616, 159–167. [Google Scholar] [CrossRef]
- Navarro, K.M.; Kleinman, M.T.; Mackay, C.E.; Reinhardt, T.E.; Balmes, J.R.; Broyles, G.A.; Ottmar, R.D.; Naher, L.P.; Domitrovich, J.W. Wildland Firefighter Smoke Exposure and Risk of Lung Cancer and Cardiovascular Disease Mortality. Environ. Res. 2019, 173, 462–468. [Google Scholar] [CrossRef]
- Parker, E.R. The Influence of Climate Change on Skin Cancer Incidence—A Review of the Evidence. Int. J. Women Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Weadick, C.S.; Keogh, R.J.; Carroll, H.K.; Boldrin, S.; Mulroe, E.; Murphy, L.; Sheils, B.; Barry, A.; O’Reilly, S. Climate Toxicity: An Increasingly Relevant Clinical Issue in Cancer Care. J. Cancer Policy 2023, 35, 100410. [Google Scholar] [CrossRef]
- Walinski, A.; Sander, J.; Gerlinger, G.; Clemens, V.; Meyer-Lindenberg, A.; Heinz, A. The Effects of Climate Change on Mental Health. Dtsch. Ärztebl. Int. 2023, 120, 117–124. [Google Scholar] [CrossRef]
- Lawrance, E.L.; Thompson, R.; Newberry Le Vay, J.; Page, L.; Jennings, N. The Impact of Climate Change on Mental Health and Emotional Wellbeing: A Narrative Review of Current Evidence, and Its Implications. Int. Rev. Psychiatry 2022, 34, 443–498. [Google Scholar] [CrossRef]
- Explainer: How Gender Inequality and Climate Change Are Interconnected. Available online: https://www.unwomen.org/en/news-stories/explainer/2022/02/explainer-how-gender-inequality-and-climate-change-are-interconnected (accessed on 15 April 2024).
- Van Daalen, K.; Jung, L.; Dhatt, R.; Phelan, A.L. Climate Change and Gender-Based Health Disparities. Lancet Planet. Health 2020, 4, e44–e45. [Google Scholar] [CrossRef]
- Desai, Z.; Zhang, Y. Climate Change and Women’s Health: A Scoping Review. GeoHealth 2021, 5, e2021GH000386. [Google Scholar] [CrossRef]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef]
- Morton, A.; Teasdale, S. Physiological Changes in Pregnancy and Their Influence on the Endocrine Investigation. Clin. Endocrinol. 2022, 96, 3–11. [Google Scholar] [CrossRef]
- Jiao, A.; Sun, Y.; Avila, C.; Chiu, V.; Slezak, J.; Sacks, D.A.; Abatzoglou, J.T.; Molitor, J.; Chen, J.-C.; Benmarhnia, T.; et al. Analysis of Heat Exposure During Pregnancy and Severe Maternal Morbidity. JAMA Netw. Open 2023, 6, e2332780. [Google Scholar] [CrossRef]
- Wyrwoll, C.S. RISING STARS: The Heat Is on: How Does Heat Exposure Cause Pregnancy Complications? J. Endocrinol. 2023, 259, e230030. [Google Scholar] [CrossRef]
- Yüzen, D.; Graf, I.; Diemert, A.; Arck, P.C. Climate Change and Pregnancy Complications: From Hormones to the Immune Response. Front. Endocrinol. 2023, 14, 1149284. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.B.; Arora, T.; Singh, S.; Singh, R. Critical Review on Emerging Health Effects Associated with the Indoor Air Quality and Its Sustainable Management. Sci. Total Environ. 2023, 872, 162163. [Google Scholar] [CrossRef]
- Liu, G.; Sun, B.; Yu, L.; Chen, J.; Han, B.; Li, Y.; Chen, J. The Gender-Based Differences in Vulnerability to Ambient Air Pollution and Cerebrovascular Disease Mortality: Evidences Based on 26781 Deaths. Glob. Heart 2020, 15, 46. [Google Scholar] [CrossRef]
- Kim, H.; Noh, J.; Noh, Y.; Oh, S.S.; Koh, S.-B.; Kim, C. Gender Difference in the Effects of Outdoor Air Pollution on Cognitive Function Among Elderly in Korea. Front. Public Health 2019, 7, 375. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Struniawski, K.; Pogorzelski, S.; Bachórzewska-Gajewska, H.; Dobrzycki, S. Gender Differences in Association between Air Pollution and Daily Mortality in the Capital of the Green Lungs of Poland–Population-Based Study with 2,953,000 Person-Years of Follow-Up. J. Clin. Med. 2020, 9, 2351. [Google Scholar] [CrossRef]
- Demir, R.; Yalazı, R.Ö.; Dinç, A. The Relationship between Women’s Climate Change Awareness and Concerns about Climate Change in Turkiye. Public Health Nurs. 2024, 41, 215–220. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Isidori, A.M.; Hasenmajer, V.; Sciarra, F.; Venneri, M.A. Environmental Impact on Immune System. In Environmental Endocrinology and Endocrine Disruptors; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Endocrinology; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–33. ISBN 978-3-030-38366-4. [Google Scholar]
- Swaminathan, A.; Lucas, R.; Harley, D.; McMichael, A. Will Global Climate Change Alter Fundamental Human Immune Reactivity: Implications for Child Health? Children 2014, 1, 403–423. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, S.; Yang, H.; Li, Y.; Zhao, Y.; Li, Y.; Yang, Q. Spatiotemporal Variation in Heatwaves and Elderly Population Exposure across China. Sci. Total Environ. 2024, 917, 170245. [Google Scholar] [CrossRef]
- Extreme Heat: Preparing for the Heat Waves of the Future. Available online: https://www.ifrc.org/document/extreme-heat-preparing-heat-waves-future (accessed on 5 April 2024).
- Maloney, E.; Duffy, D. Deciphering the Relationship between Temperature and Immunity. Discov. Immunol. 2024, 3, kyae001. [Google Scholar] [CrossRef]
- Ghulam Mohyuddin, S.; Khan, I.; Zada, A.; Qamar, A.; Arbab, A.A.I.; Ma, X.; Yu, Z.; Liu, X.-X.; Yong, Y.-H.; Ju, X.H.; et al. Influence of Heat Stress on Intestinal Epithelial Barrier Function, Tight Junction Protein, and Immune and Reproductive Physiology. BioMed Res. Int. 2022, 2022, 8547379. [Google Scholar] [CrossRef]
- Cantet, J.M.; Yu, Z.; Ríus, A.G. Heat Stress-Mediated Activation of Immune–Inflammatory Pathways. Antibiotics 2021, 10, 1285. [Google Scholar] [CrossRef]
- Presbitero, A.; Melnikov, V.R.; Krzhizhanovskaya, V.V.; Sloot, P.M.A. A Unifying Model to Estimate the Effect of Heat Stress in the Human Innate Immunity during Physical Activities. Sci. Rep. 2021, 11, 16688. [Google Scholar] [CrossRef]
- Billaud, M.; Larbret, F.; Czerucka, D. Impact of Rising Seawater Temperature on a Phagocytic Cell Population during V. Parahaemolyticus Infection in the Sea Anemone E. Pallida. Front. Immunol. 2023, 14, 1292410. [Google Scholar] [CrossRef]
- Azizan, A.; Venter, L.; Zhang, J.; Young, T.; Ericson, J.A.; Delorme, N.J.; Ragg, N.L.C.; Alfaro, A.C. Interactive Effects of Elevated Temperature and Photobacterium swingsii Infection on the Survival and Immune Response of Marine Mussels (Perna canaliculus): A Summer Mortality Scenario. Mar. Environ. Res. 2024, 196, 106392. [Google Scholar] [CrossRef]
- Folkertsma, R.; Charbonnel, N.; Henttonen, H.; Heroldová, M.; Huitu, O.; Kotlík, P.; Manzo, E.; Paijmans, J.L.A.; Plantard, O.; Sándor, A.D.; et al. Genomic Signatures of Climate Adaptation in Bank Voles. Ecol. Evol. 2024, 14, e10886. [Google Scholar] [CrossRef]
- Kim, H.; Jo, J.-H.; Lee, H.-G.; Park, W.; Lee, H.-K.; Park, J.-E.; Shin, D. Inflammatory Response in Dairy Cows Caused by Heat Stress and Biological Mechanisms for Maintaining Homeostasis. PLoS ONE 2024, 19, e0300719. [Google Scholar] [CrossRef]
- Martin, L.E.; Hillyer, J.F. Higher Temperature Accelerates the Aging-Dependent Weakening of the Melanization Immune Response in Mosquitoes. PLoS Pathog. 2024, 20, e1011935. [Google Scholar] [CrossRef]
- Hinchliffe, A.; Alguacil, J.; Bijoux, W.; Kogevinas, M.; Menegaux, F.; Parent, M.-E.; Pérez Gomez, B.; Uuksulainen, S.; Turner, M.C. Occupational Heat Exposure and Prostate Cancer Risk: A Pooled Analysis of Case-Control Studies. Environ. Res. 2023, 216, 114592. [Google Scholar] [CrossRef]
- Hinchliffe, A.; Kogevinas, M.; Pérez-Gómez, B.; Ardanaz, E.; Amiano, P.; Marcos-Delgado, A.; Castaño-Vinyals, G.; Llorca, J.; Moreno, V.; Alguacil, J.; et al. Occupational Heat Exposure and Breast Cancer Risk in the MCC-Spain Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 364–372. [Google Scholar] [CrossRef]
- Calapre, L.; Gray, E.S.; Ziman, M. Heat Stress: A Risk Factor for Skin Carcinogenesis. Cancer Lett. 2013, 337, 35–40. [Google Scholar] [CrossRef]
- Aguilera, J.; Ibarra-Mejia, G.; Johnson, M. Editorial: The Impact of Climate Change on Allergic Disease. Front. Allergy 2023, 4, 1246899. [Google Scholar] [CrossRef]
- Martikainen, M.-V.; Tossavainen, T.; Hannukka, N.; Roponen, M. Pollen, Respiratory Viruses, and Climate Change: Synergistic Effects on Human Health. Environ. Res. 2023, 219, 115149. [Google Scholar] [CrossRef]
- Singh, A.B.; Kumar, P. Climate Change and Allergic Diseases: An Overview. Front. Allergy 2022, 3, 964987. [Google Scholar] [CrossRef]
- Lee, A.S.; Aguilera, J.; Efobi, J.A.; Jung, Y.S.; Seastedt, H.; Shah, M.M.; Yang, E.; Konvinse, K.; Utz, P.J.; Sampath, V.; et al. Climate Change and Public Health: The Effects of Global Warming on the Risk of Allergies and Autoimmune Diseases. EMBO Rep. 2023, 24, e56821. [Google Scholar] [CrossRef]
- Herceg, Z. Epigenetic Mechanisms as an Interface Between the Environment and Genome. In Hypoxia; Roach, R.C., Hackett, P.H., Wagner, P.D., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2016; Volume 903, pp. 3–15. ISBN 978-1-4899-7676-5. [Google Scholar]
- Çelebi Sözener, Z.; Treffeisen, E.R.; Özdel Öztürk, B.; Schneider, L.C. Global Warming and Implications for Epithelial Barrier Disruption and Respiratory and Dermatologic Allergic Diseases. J. Allergy Clin. Immunol. 2023, 152, 1033–1046. [Google Scholar] [CrossRef]
- Albery, G.F.; Turilli, I.; Joseph, M.B.; Foley, J.; Frere, C.H.; Bansal, S. From Flames to Inflammation: How Wildfires Affect Patterns of Wildlife Disease. Fire Ecol. 2021, 17, 23. [Google Scholar] [CrossRef]
- Noah, T.L.; Worden, C.P.; Rebuli, M.E.; Jaspers, I. The Effects of Wildfire Smoke on Asthma and Allergy. Curr. Allergy Asthma Rep. 2023, 23, 375–387. [Google Scholar] [CrossRef]
- Prunicki, M.; Kelsey, R.; Lee, J.; Zhou, X.; Smith, E.; Haddad, F.; Wu, J.; Nadeau, K. The Impact of Prescribed Fire versus Wildfire on the Immune and Cardiovascular Systems of Children. Allergy 2019, 74, 1989–1991. [Google Scholar] [CrossRef]
- Lwin, K.S.; Tobias, A.; Chua, P.L.; Yuan, L.; Thawonmas, R.; Ith, S.; Htay, Z.W.; Yu, L.S.; Yamasaki, L.; Roqué, M.; et al. Effects of Desert Dust and Sandstorms on Human Health: A Scoping Review. GeoHealth 2023, 7, e2022GH000728. [Google Scholar] [CrossRef]
- Sampath, V.; Aguilera, J.; Prunicki, M.; Nadeau, K.C. Mechanisms of Climate Change and Related Air Pollution on the Immune System Leading to Allergic Disease and Asthma. Semin. Immunol. 2023, 67, 101765. [Google Scholar] [CrossRef]
- Sand and Dust Storm Frequency Increasing in Many World Regions, UN Warns. Available online: https://www.unccd.int/news-stories/press-releases/sand-and-dust-storm-frequency-increasing-many-world-regions-un-warns (accessed on 7 April 2024).
- Seastedt, H.; Nadeau, K. Factors by Which Global Warming Worsens Allergic Disease. Ann. Allergy Asthma Immunol. 2023, 131, 694–702. [Google Scholar] [CrossRef]
- Esmaeil, N.; Gharagozloo, M.; Rezaei, A.; Grunig, G. Dust Events, Pulmonary Diseases and Immune System. Am. J. Clin. Exp. Immunol. 2014, 3, 20–29. [Google Scholar]
- Bredeck, G.; Busch, M.; Rossi, A.; Stahlmecke, B.; Fomba, K.W.; Herrmann, H.; Schins, R.P.F. Inhalable Saharan Dust Induces Oxidative Stress, NLRP3 Inflammasome Activation, and Inflammatory Cytokine Release. Environ. Int. 2023, 172, 107732. [Google Scholar] [CrossRef]
- Li, W.; Wang, W. Causal Effects of Exposure to Ambient Air Pollution on Cancer Risk: Insights from Genetic Evidence. Sci. Total Environ. 2024, 912, 168843. [Google Scholar] [CrossRef]
- Wu, H.; Eckhardt, C.M.; Baccarelli, A.A. Molecular Mechanisms of Environmental Exposures and Human Disease. Nat. Rev. Genet. 2023, 24, 332–344. [Google Scholar] [CrossRef]
- Giammona, A.; Remedia, S.; Porro, D.; Lo Dico, A.; Bertoli, G. The Biological Interplay between Air Pollutants and miRNAs Regulation in Cancer. Front. Cell Dev. Biol. 2024, 12, 1343385. [Google Scholar] [CrossRef]
- Chauhan, A.; Gupta, S.K.; Liou, Y.-A. Rising Surface Ozone Due to Anthropogenic Activities and Its Impact on COVID-19 Related Deaths in Delhi, India. Heliyon 2023, 9, e14975. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Bromberg, P.A. Mechanisms of the Acute Effects of Inhaled Ozone in Humans. Biochim. Biophys. Acta (BBA)- Gen. Subj. 2016, 1860, 2771–2781. [Google Scholar] [CrossRef]
- González-Guevara, E.; Martínez-Lazcano, J.C.; Custodio, V.; Hernández-Cerón, M.; Rubio, C.; Paz, C. Exposure to Ozone Induces a Systemic Inflammatory Response: Possible Source of the Neurological Alterations Induced by This Gas. Inhal. Toxicol. 2014, 26, 485–491. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Li, G.; Wang, W.; Wang, K.; Wang, J.; Wei, C.; Li, Y.; Deng, F.; Baccarelli, A.A.; et al. Ozone Pollution and Hospital Admissions for Cardiovascular Events. Eur. Heart J. 2023, 44, 1622–1632. [Google Scholar] [CrossRef]
- Blum, W.E.H.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef]
- Ottman, N.; Ruokolainen, L.; Suomalainen, A.; Sinkko, H.; Karisola, P.; Lehtimäki, J.; Lehto, M.; Hanski, I.; Alenius, H.; Fyhrquist, N. Soil Exposure Modifies the Gut Microbiota and Supports Immune Tolerance in a Mouse Model. J. Allergy Clin. Immunol. 2019, 143, 1198–1206.e12. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M. Research Progress of Gut Microbiota and Frailty Syndrome. Open Med. 2021, 16, 1525–1536. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Risely, A.; Müller-Klein, N.; Schmid, D.W.; Wilhelm, K.; Clutton-Brock, T.H.; Manser, M.B.; Sommer, S. Climate Change Drives Loss of Bacterial Gut Mutualists at the Expense of Host Survival in Wild Meerkats. Glob. Chang. Biol. 2023, 29, 5816–5828. [Google Scholar] [CrossRef]
- Liu, S.; Wen, D.; Feng, C.; Yu, C.; Gu, Z.; Wang, L.; Zhang, Z.; Li, W.; Wu, S.; Liu, Y.; et al. Alteration of Gut Microbiota after Heat Acclimation May Reduce Organ Damage by Regulating Immune Factors during Heat Stress. Front. Microbiol. 2023, 14, 1114233. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Lei, R.; Xue, B.; Li, Y.; Tian, X.; Zhang, K.; Luo, B. Cold Exposure, Gut Microbiota, and Hypertension: A Mechanistic Study. Sci. Total Environ. 2022, 833, 155199. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Chen, Z.; Xu, J.; Nie, J.; Lu, J.; Ma, L.; Ji, H.; Yuan, J.; Xu, B. Cold Exposure Induces Intestinal Barrier Damage and Endoplasmic Reticulum Stress in the Colon via the SIRT1/Nrf2 Signaling Pathway. Front. Physiol. 2022, 13, 822348. [Google Scholar] [CrossRef]
- Zhou, E.; Zhang, L.; He, L.; Xiao, Y.; Zhang, K.; Luo, B. Cold Exposure, Gut Microbiota and Health Implications: A Narrative Review. Sci. Total Environ. 2024, 916, 170060. [Google Scholar] [CrossRef]
- Li, T.; Fang, J.; Tang, S.; Du, H.; Zhao, L.; Wang, Y.; Deng, F.; Liu, Y.; Du, Y.; Cui, L.; et al. PM2.5 Exposure Associated with Microbiota Gut-Brain Axis: Multi-Omics Mechanistic Implications from the BAPE Study. Innovation 2022, 3, 100213. [Google Scholar] [CrossRef]
- Yi, W.; Cheng, J.; Wei, Q.; Pan, R.; Song, S.; He, Y.; Tang, C.; Liu, X.; Zhou, Y.; Su, H. Effect of Temperature Stress on Gut-Brain Axis in Mice: Regulation of Intestinal Microbiome and Central NLRP3 Inflammasomes. Sci. Total Environ. 2021, 772, 144568. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhu, R.; Zhang, S.; Liu, S.; Wang, Y.; Wu, Y.; Xing, S.; Liao, X.; Mi, J. Porcine Gut Microbiota in Mediating Host Metabolic Adaptation to Cold Stress. NPJ Biofilms Microbiomes 2022, 8, 18. [Google Scholar] [CrossRef]
- Wen, J.; Bo, T.; Zhang, X.; Wang, Z.; Wang, D. Thermo-TRPs and Gut Microbiota Are Involved in Thermogenesis and Energy Metabolism during Low Temperature Exposure of Obese Mice. J. Exp. Biol. 2020, 223, jeb218974. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut Microbe Analysis Between Hyperthyroid and Healthy Individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Saffari, A.; Jacobs, J.; Baek, K.I.; Hough, G.; Larauche, M.H.; Ma, J.; Jen, N.; Moussaoui, N.; et al. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci. Rep. 2017, 7, 42906. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Li, Y.; Qin, Y.; Yu, L.; Li, R.; Chen, Y.; Xu, Y. Effects of PM2.5 Exposure during Gestation on Maternal Gut Microbiota and Pregnancy Outcomes. Chemosphere 2020, 247, 125879. [Google Scholar] [CrossRef]
- White, B.P.; Breakey, S.; Brown, M.J.; Smith, J.R.; Tarbet, A.; Nicholas, P.K.; Ros, A.M.V. Mental Health Impacts of Climate Change Among Vulnerable Populations Globally: An Integrative Review. Ann. Glob. Health 2023, 89, 66. [Google Scholar] [CrossRef]
- Galway, L.P.; Beery, T.; Jones-Casey, K.; Tasala, K. Mapping the Solastalgia Literature: A Scoping Review Study. Int. J. Environ. Res. Public Health 2019, 16, 2662. [Google Scholar] [CrossRef]
- Mental Health and Climate Change in Africa|BJPsych International|Cambridge Core. Available online: https://www.cambridge.org/core/journals/bjpsych-international/article/mental-health-and-climate-changein-africa/65A414598BA1D620F4208A9177EED94B (accessed on 16 April 2024).
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Moon, S.J.; Lee, Y.C.; Kim, T.J.; Kim, K.; Son, H.J. Effects of Temperature, Weather, Seasons, Atmosphere, and Climate on the Exacerbation of Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0279277. [Google Scholar] [CrossRef]
- Dalal, N.; Jalandra, R.; Bayal, N.; Yadav, A.K.; Harshulika; Sharma, M.; Makharia, G.K.; Kumar, P.; Singh, R.; Solanki, P.R.; et al. Gut Microbiota-Derived Metabolites in CRC Progression and Causation. J. Cancer Res. Clin. Oncol. 2021, 147, 3141–3155. [Google Scholar] [CrossRef]
- Nurkolis, F.; Mayulu, N.; Yasmine, N.; Puspaningtyas, D.S.; Taslim, N.A. Human Activities and Changes in the Gut Microbiome: A Perspective. Human Nutr. Metab. 2022, 30, 200165. [Google Scholar] [CrossRef]
- Kinney, P.L.; Ge, B.; Sampath, V.; Nadeau, K. Health-Based Strategies for Overcoming Barriers to Climate Change Adaptation and Mitigation. J. Allergy Clin. Immunol. 2023, 152, 1053–1059. [Google Scholar] [CrossRef]
- Rio, P.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Pollutants, Microbiota and Immune System: Frenemies within the Gut. Front. Public Health 2024, 12, 1285186. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Anitha, D.P.M.; Sellamuthu, P.S.; Emmanuel Rotimi, S. Probiotic Incorporation into Edible Packaging: A Recent Trend in Food Packaging. Biocatal. Agric. Biotechnol. 2023, 51, 102803. [Google Scholar] [CrossRef]

| Climate-Related Event | Health Consequence | References |
|---|---|---|
| Global warming | Cardiovascular diseases Gastrointestinal infections Hepatitis and hepatocyte necrosis Cancer | [23,25,26,27,28] |
| Air pollutants (PM, greenhouse gases) | Exacerbation of chronic respiratory disorders and allergic diseases Cardio-cerebrovascular diseases Gastrointestinal inflammation Cancer | [17,19,22,29] |
| Extreme weather events (floods, hurricanes, fires) | Post-traumatic stress disorder, affective disorders and anxiety Reduced physical activity | [24,47,48] |
| Changes in rainfall patterns | Changes in the distribution of vectors Infectious diseases | [37] |
| Food contamination and impaired food availability | Gastrointestinal and systemic inflammation Metabolic diseases Immune imbalance Cancer | [30,31] |
| Temperature | Alteration of GM | References |
|---|---|---|
| High temperature | Declines in soil biodiversity | [9] |
| Higher ratio Bacteroidetes-to-Firmicutes | [103] | |
| Increase in proportion of potentially pathogenic bacteria (Escherichia, Shigella, and Lactococcus) | [107] | |
| Increase of IL-10, IL-6, CXCL2, CCL4, CCL5, and CCL11 | [107] | |
| Increase of ALT, ALP, creatinine, LDH, α-HBDH, and cholinesterase | [107] | |
| Decrease in probiotics (Dorea, Blautia, and Lactobacillus) | [107] | |
| Cold temperature | Reduced richness and decreased α-diversity | [108] |
| Increased gut permeability, apoptosis (cell death), inflammation, and oxidative stress | [109] | |
| Secretion of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α | [109] | |
| Secretion of butyric acid with the activation of the cAMP-PKA-pCREB signaling pathway | [110] | |
| SCFAs and LPS can modulate local levels of T3 increased by cold temperatures | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rio, P.; Caldarelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. The Impact of Climate Change on Immunity and Gut Microbiota in the Development of Disease. Diseases 2024, 12, 118. https://doi.org/10.3390/diseases12060118
Rio P, Caldarelli M, Gasbarrini A, Gambassi G, Cianci R. The Impact of Climate Change on Immunity and Gut Microbiota in the Development of Disease. Diseases. 2024; 12(6):118. https://doi.org/10.3390/diseases12060118
Chicago/Turabian StyleRio, Pierluigi, Mario Caldarelli, Antonio Gasbarrini, Giovanni Gambassi, and Rossella Cianci. 2024. "The Impact of Climate Change on Immunity and Gut Microbiota in the Development of Disease" Diseases 12, no. 6: 118. https://doi.org/10.3390/diseases12060118
APA StyleRio, P., Caldarelli, M., Gasbarrini, A., Gambassi, G., & Cianci, R. (2024). The Impact of Climate Change on Immunity and Gut Microbiota in the Development of Disease. Diseases, 12(6), 118. https://doi.org/10.3390/diseases12060118









