Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Isolation of Immune Cells
2.3. Intracellular Staining and Flow Cytometry Analysis of Immune Cells
2.4. Measurement of Cytokines in Serum Samples
2.5. Statistics
3. Results
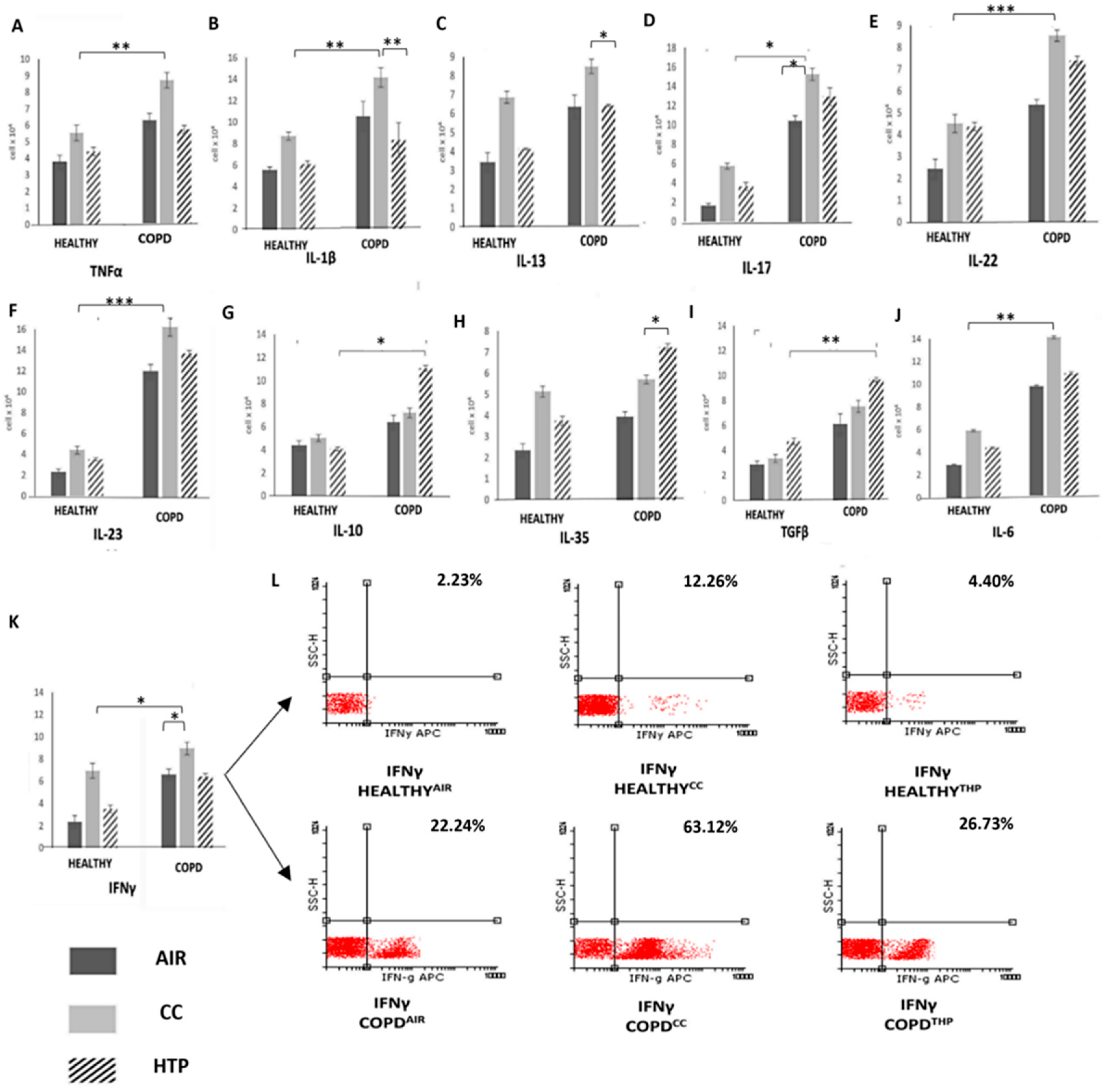
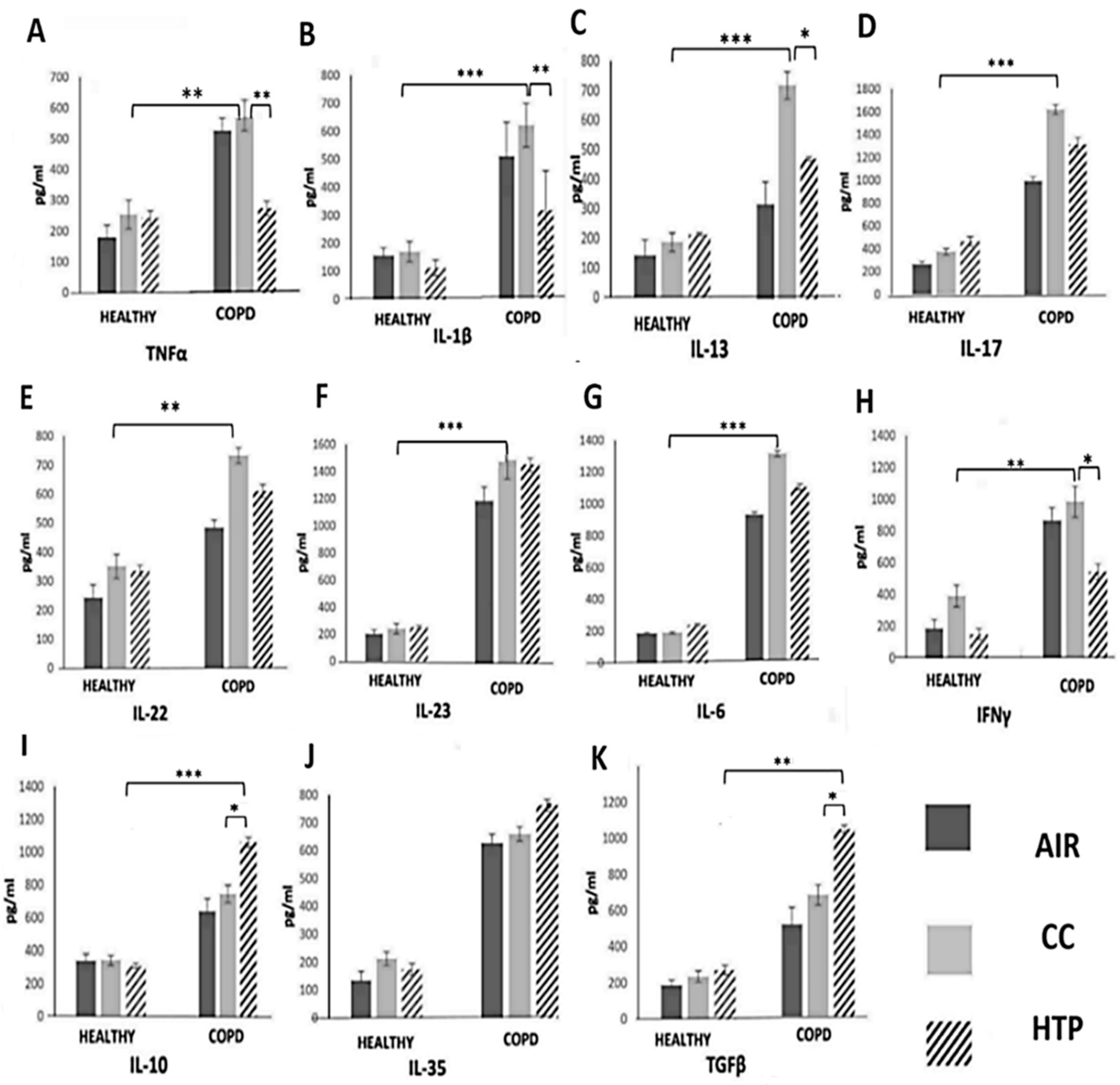
3.1. Continuous Use of Either CCs or HTPs Modulates Cytokine Production in Immune Cells of COPD Patients
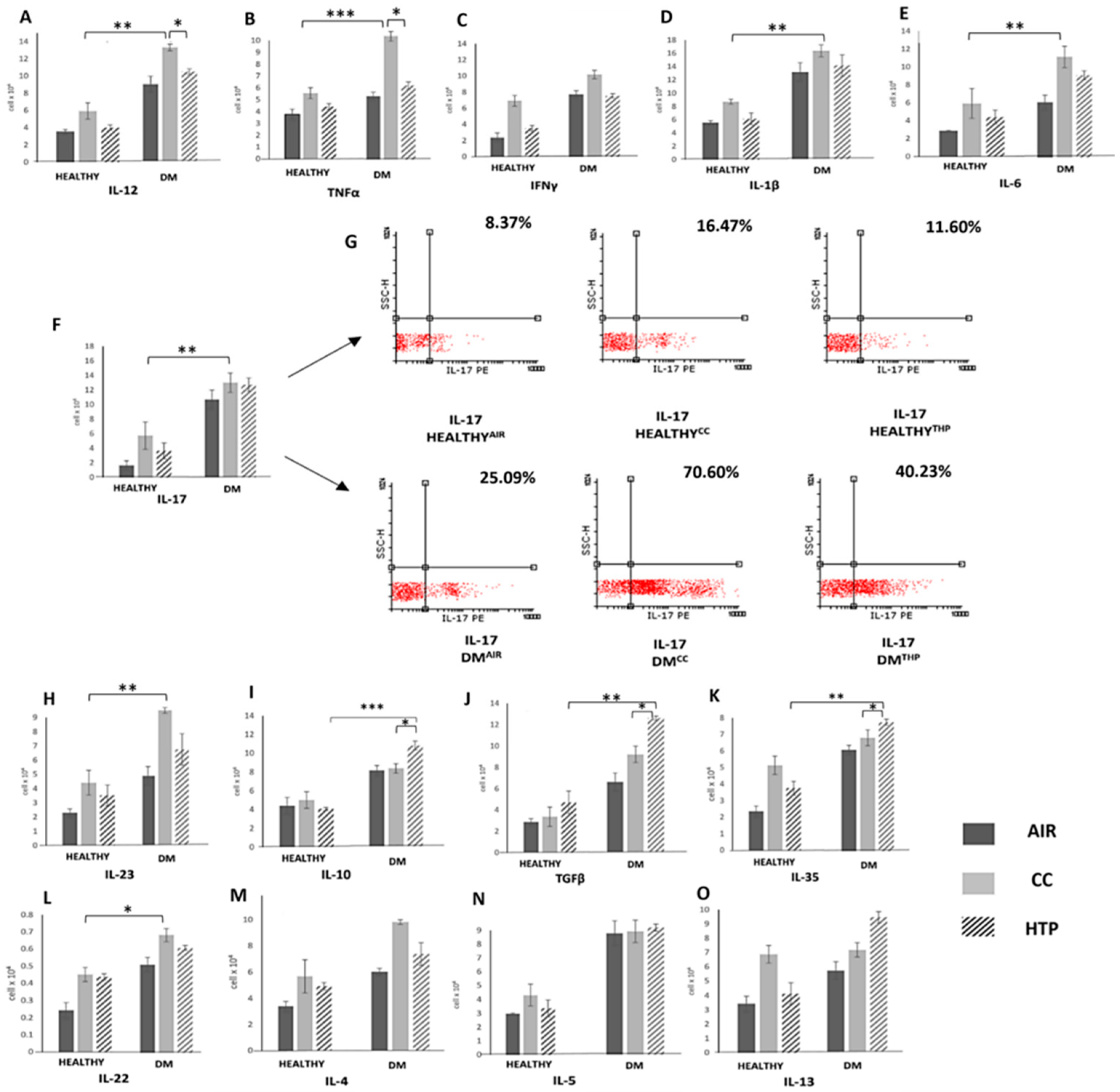
3.2. Compared to CCs, HTPs Had Weaker Capacity to Induce Production of Th1- and Th17-Related Cytokines in Immune Cells of DM Patients
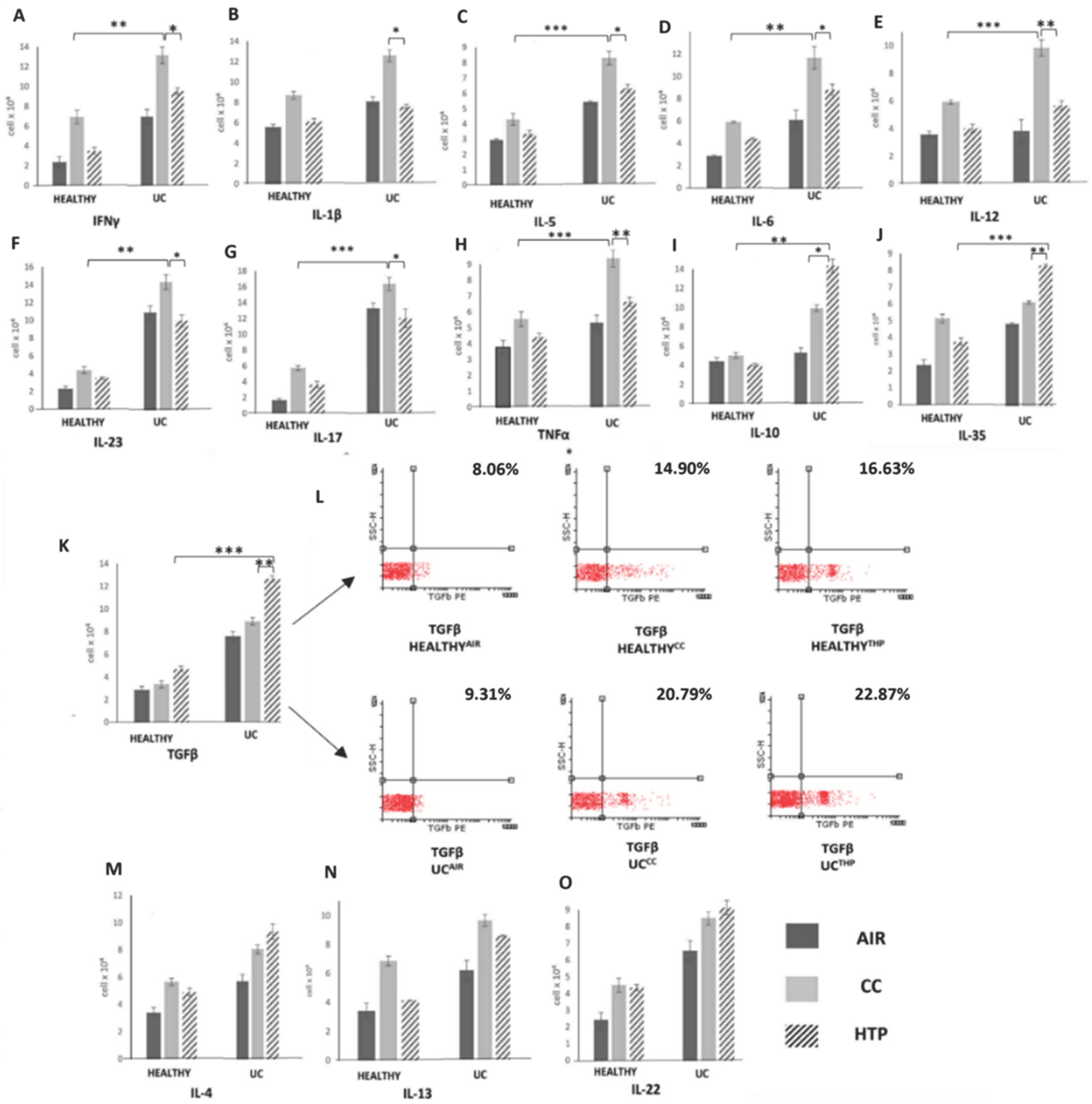
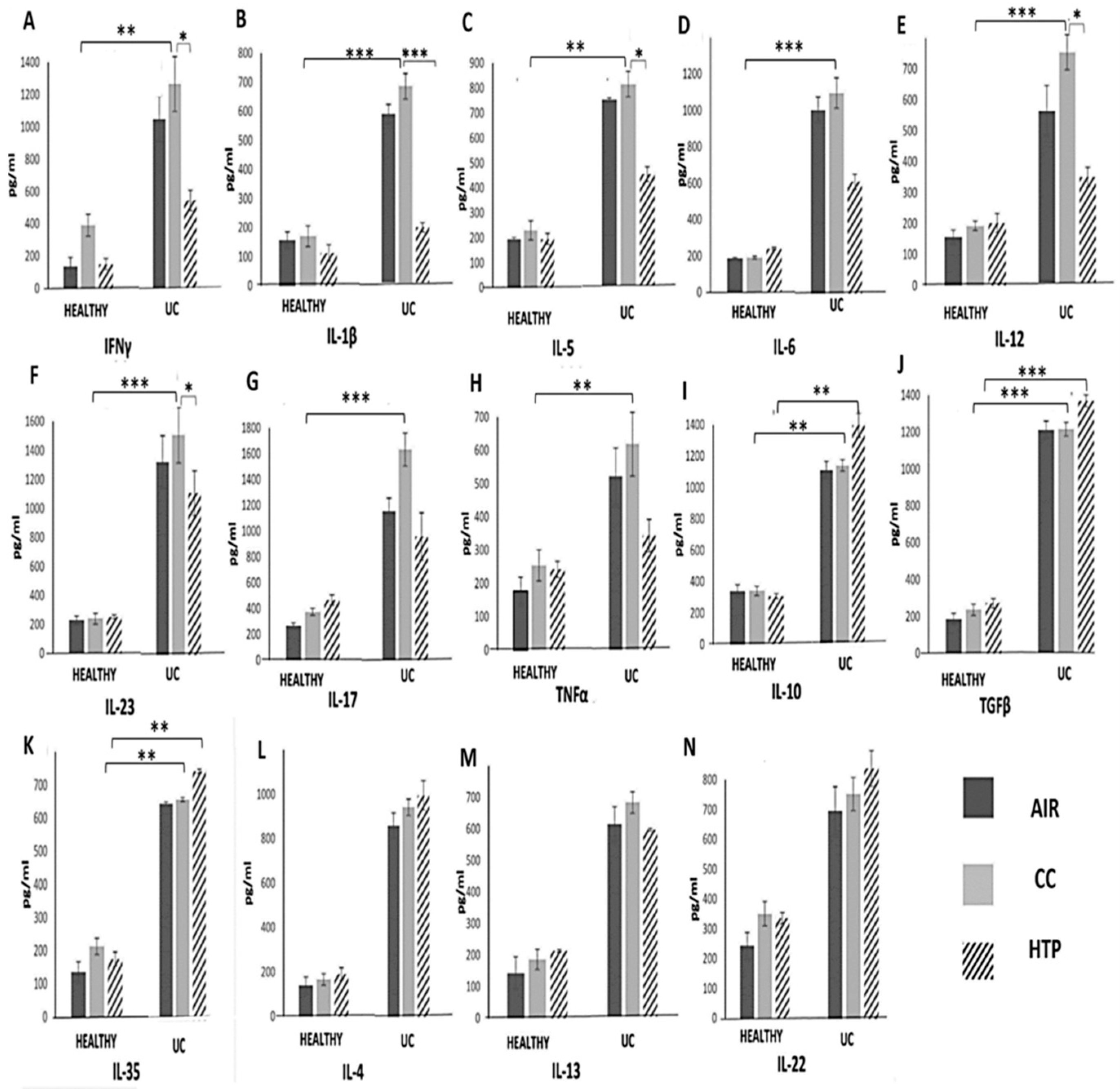
3.3. Compared to CCs, HTPs More Efficiently Induce Production of Immunosuppressive Cytokines in Immune Cells of UC Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Palomo, M.; Moreno-Castaño, A.B.; Salas, M.Q.; Escribano-Serrat, S.; Rovira, M.; Guillen-Olmos, E.; Fernandez, S.; Ventosa-Capell, H.; Youssef, L.; Crispi, F.; et al. Endothelial activation and damage as a common pathological substrate in different pathologies and cell therapy complications. Front. Med. 2023, 10, 1285898. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, M.; Jiang, D.; Su, Q.; Shi, J. The role of inflammation in autoimmune disease: A therapeutic target. Front. Immunol. 2023, 14, 1267091. [Google Scholar] [CrossRef] [PubMed]
- Kastratovic, N.; Markovic, V.; Harrell, C.R.; Arsenijevic, A.; Stojanovic, M.D.; Djonov, V.; Volarevic, V. Effects of combustible cigarettes and electronic nicotine delivery systems on the development and progression of chronic lung inflammation in mice. Nicotine Tob. Res. 2023, 26, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Miloradovic, D.; Stojanovic, M.D.; Harrell, C.R.; Polosa, R.; Rust, S.; Volti, G.L.; Caruso, M.; Jakovljevic, V.; Djonov, V.; et al. Cigarette smoke attenuates mesenchymal stem cell-based suppression of immune cell-driven acute liver failure. Toxicol. Lett. 2023, 385, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Emma, R.; Distefano, A.; Rust, S.; Poulas, K.; Giordano, A.; Volarevic, V.; Mesiakaris, K.; Boffo, S.; Arsenijevic, A.; et al. Comparative assessment of electronic nicotine delivery systems aerosol and cigarette smoke on endothelial cell migration: The Replica Project. Drug Test Anal. 2023, 15, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Liao, Z.; Tkach, M.; Tarwater, P.M.; Ostrowski, M.; Théry, C.; Witwer, K.W. Cigarette smoke-induced extracellular vesicles from dendritic cells alter T-cell activation and HIV replication. Toxicol. Lett. 2022, 360, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Schmalen, A.; Haines, S.; Wolff, M.; Ma, H.; Zhang, H.; Stoleriu, M.G.; Nowak, J.; Nakayama, M.; et al. Antiviral CD8+ T-cell immune responses are impaired by cigarette smoke and in COPD. Eur. Respir. J. 2023, 62, 2201374. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Hirschfeld, J.; Milward, M.R.; Cooper, P.R.; Wright, H.J.; Matthews, J.B.; Chapple, I.L.C. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J. Periodontal Res. 2018, 53, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.G.; O’Connor, T.P.; Crystal, R.G. Smoking-dependent reprogramming of alveolar macrophage polarization: Implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 2009, 183, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, T.H.; Benson, R.P.; Phipps, R.P.; Sime, P.J. High-dose but not low-dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am. J. Physiol.-Lung Cell Mol. Physiol. 2008, 295, L412–L421. [Google Scholar] [CrossRef][Green Version]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tob. Control 2019, 28, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef] [PubMed]
- St Helen, G.; Jacob Iii, P.; Nardone, N.; Benowitz, N.L. IQOS: Examination of Philip Morris International’s claim of reduced exposure. Tob. Control 2018, 27, s30–s36. [Google Scholar] [CrossRef] [PubMed]
- Kastratovic, N.; Cekerevac, I.; Sekerus, V.; Markovic, V.; Arsenijevic, A.; Volarevic, A.; Harrell, C.R.; Jakovljevic, V.; Djonov, V.; Volarevic, V. Effects of combustible cigarettes and heated tobacco products on immune cell-driven inflammation in chronic obstructive respiratory diseases. Toxicol. Sci. 2024, kfae068. [Google Scholar] [CrossRef]
- Acovic, A.; Simovic Markovic, B.; Gazdic, M.; Arsenijevic, A.; Jovicic, N.; Gajovic, N.; Jovanovic, M.; Zdravkovic, N.; Kanjevac, T.; Harrell, C.R.; et al. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap. Adv. Gastroenterol. 2018, 11, 1756284818793558. [Google Scholar] [CrossRef]
- Volarevic, V.; Zdravkovic, N.; Harrell, C.R.; Arsenijevic, N.; Fellabaum, C.; Djonov, V.; Lukic, M.L.; Simovic Markovic, B. Galectin-3 Regulates Indoleamine-2,3-dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and May Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 2019, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Mortazavi, D.; Konyalilar, N.; Aksoy, G.T.; Erkan, S.; Korkunc, S.K.; Kayalar, O.; Bayram, H.; Rahbarghazi, R. Forthcoming complications in recovered COVID-19 patients with COPD and asthma; possible therapeutic opportunities. Cell Commun. Signal 2022, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Burris, T.P. Th17 cells in Type 1 diabetes: A future perspective. Diabetes Manag. 2015, 5, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, K.H.; Postma, D.S.; Hop, W.C.; Wielders, P.L.; Schlösser, N.J.; Wouters, E.F.; COSMIC Study Group. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest 2008, 133, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.R.; Jang, J.; Park, S.M.; Ryu, S.M.; Cho, S.J.; Yang, S.R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.C.; MacDonald, A.S. Dendritic cells in lung immunopathology. Semin. Immunopathol. 2016, 38, 449–460. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. Dampening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed]
- Shyam Prasad Shetty, B.; Chaya, S.K.; Kumar, V.S.; Mahendra, M.; Jayaraj, B.S.; Lokesh, K.S.; Ganguly, K.; Mahesh, P.A. Inflammatory Biomarkers Interleukin 1 Beta (IL-1β) and Tumour Necrosis Factor Alpha (TNF-α) Are Differentially Elevated in Tobacco Smoke Associated COPD and Biomass Smoke Associated COPD. Toxics 2021, 9, 72. [Google Scholar] [CrossRef]
- Kubysheva, N.; Boldina, M.; Eliseeva, T.; Soodaeva, S.; Klimanov, I.; Khaletskaya, A.; Bayrasheva, V.; Solovyev, V.; Villa-Vargas, L.A.; Ramírez-Salinas, M.A.; et al. Relationship of Serum Levels of IL-17, IL-18, TNF-α, and Lung Function Parameters in Patients with COPD, Asthma-COPD Overlap, and Bronchial Asthma. Mediat. Inflamm. 2020, 2020, 4652898. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Poll, C.T.; Barnes, P.J.; Sturton, R.G. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am. J. Respir. Cell Mol. Biol. 2010, 43, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Biswas, S.; Singh, G.; Pal, A.; Ghosh, N.; Ojha, A.K.; Das, S.; Dutta, G.; Chaudhury, K. Immunological profiling and development of a sensing device for detection of IL-13 in COPD and asthma. Bioelectrochemistry 2022, 143, 107971. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Sundar, I.K.; Hwang, J.W.; Yull, F.E.; Blackwell, T.S.; Kinnula, V.L.; Bulger, M.; Yao, H.; Rahman, I. NF-κB inducing kinase, NIK mediates cigarette smoke/TNFα-induced histone acetylation and inflammation through differential activation of IKKs. PLoS ONE 2011, 6, e23488. [Google Scholar] [CrossRef] [PubMed]
- Kum, J.J.Y.; Howlett, C.J.; Khan, Z.A. Dysregulated transforming growth factor-beta mediates early bone marrow dysfunction in diabetes. Commun. Biol. 2022, 5, 1145. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef]
- Mahmoudzadeh, L.; Abtahi Froushani, S.M.; Ajami, M.; Mahmoudzadeh, M. Effect of Nicotine on Immune System Function. Adv. Pharm. Bull. 2023, 13, 69–78. [Google Scholar] [CrossRef]
- Nakata, Y.; Miura, K.; Yamasaki, N.; Ogata, S.; Miura, S.; Hosomi, N.; Kaminuma, O. Expression and Function of Nicotinic Acetylcholine Receptors in Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 1779. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Med. Cell. Longev. 2016, 2016, 1580967. [Google Scholar] [CrossRef] [PubMed]
- Soeteman-Hernández, L.G.; Bos, P.M.; Talhout, R. Tobacco smoke-related health effects induced by 1,3-butadiene and strategies for risk reduction. Toxicol. Sci. 2013, 136, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastratovic, N.; Zdravkovic, N.; Cekerevac, I.; Sekerus, V.; Harrell, C.R.; Mladenovic, V.; Djukic, A.; Volarevic, A.; Brankovic, M.; Gmizic, T.; et al. Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases. Diseases 2024, 12, 144. https://doi.org/10.3390/diseases12070144
Kastratovic N, Zdravkovic N, Cekerevac I, Sekerus V, Harrell CR, Mladenovic V, Djukic A, Volarevic A, Brankovic M, Gmizic T, et al. Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases. Diseases. 2024; 12(7):144. https://doi.org/10.3390/diseases12070144
Chicago/Turabian StyleKastratovic, Nikolina, Natasa Zdravkovic, Ivan Cekerevac, Vanesa Sekerus, Carl Randall Harrell, Violeta Mladenovic, Aleksandar Djukic, Ana Volarevic, Marija Brankovic, Tijana Gmizic, and et al. 2024. "Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases" Diseases 12, no. 7: 144. https://doi.org/10.3390/diseases12070144
APA StyleKastratovic, N., Zdravkovic, N., Cekerevac, I., Sekerus, V., Harrell, C. R., Mladenovic, V., Djukic, A., Volarevic, A., Brankovic, M., Gmizic, T., Zdravkovic, M., Bjekic-Macut, J., Zdravkovic, N., Djonov, V., & Volarevic, V. (2024). Effects of Combustible Cigarettes and Heated Tobacco Products on Systemic Inflammatory Response in Patients with Chronic Inflammatory Diseases. Diseases, 12(7), 144. https://doi.org/10.3390/diseases12070144









