Influence of Meteorological Parameters on the Prevalence of TEE Detected Left Atrial Appendage Thrombi
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Collective
2.2. Transesophageal Echocardiography
2.3. Meteorological Factors
- -
- Daily average temperature at 2 m altitude in °C;
- -
- Daily maximum temperature at 2 m altitude in °C;
- -
- Daily minimum temperature at 2 m altitude in °C;
- -
- Daily average relative humidity at 2 m altitude in %;
- -
- Daily average wind speed at 10 m altitude in m/s;
- -
- Daily maximum wind speed at 10 m altitude in m/s;
- -
- Daily sunshine duration in hours;
- -
- Daily precipitation amount in mm;
- -
- Daily mean air pressure at station altitude in hPA (hectoPascal);
- -
- Daily mean water vapor pressure in hPA.
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Temperature
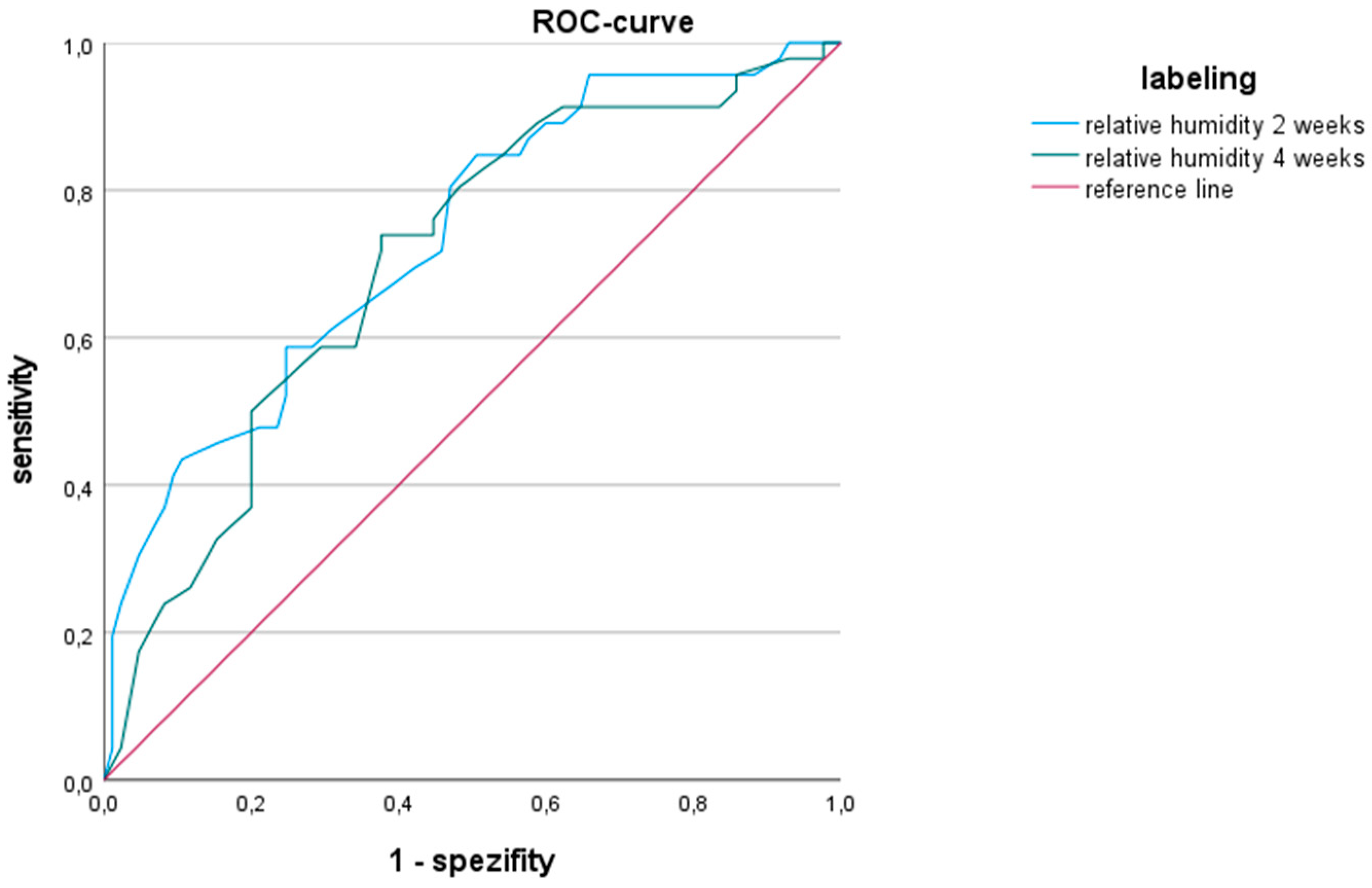
3.2. Relative Humidity
3.3. Wind Speed
3.4. Sunshine
3.5. Other Parameters
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cicci, K.R.; Maltby, A.; Clemens, K.K.; Vicedo-Cabrera, A.M.; Gunz, A.C.; Lavigne, É.; Wilk, P. High Temperatures and Cardiovascular-Related Morbidity: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 11243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzmenko, N.V.; Galagudza, M.M. Dependence of seasonal dynamics of hemorrhagic and ischemic strokes on the climate of a region: A meta-analysis. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ertl, M.; Beck, C.; Kühlbach, B.; Hartmann, J.; Hammel, G.; Straub, A.; Giemsa, E.; Seubert, S.; Philipp, A.; Traidl-Hoffmann, C.; et al. New Insights into Weather and Stroke: Influences of Specific Air Masses and Temperature Changes on Stroke Incidence. Cerebrovasc. Dis. 2019, 47, 275–284. [Google Scholar] [CrossRef]
- Di Blasi, C.; Renzi, M.; Michelozzi, P.; Donato, F.D.; Scortichini, M.; Davoli, M.; Forastiere, F.; Mannucci, P.M.; Stafoggia, M. Association between air temperature, air pollution and hospital admissions for pulmonary embolism and venous thrombosis in Italy. Eur. J. Intern. Med. 2022, 96, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, J.; Peng, L.; Li, H.; Ji, J.S.; Fang, W.; Yan, H.; Chen, J.; Wang, W.; Xiang, D.; et al. Non-optimum temperature increases risk and burden of acute myocardial infarction onset: A nationwide case-crossover study at hourly level in 324 Chinese cities. eClinicalMedicine 2022, 50, 101501. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, C.M.; Sugg, M.M.; Konrad, C.E., II; Waller, A. Impact of Extreme Heat Events on Emergency Department Visits in North Carolina (2007–2011). J. Community Health 2016, 41, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Gebhard, C.E.; Stähli, B.E.; Maafi, F.; Bertrand, M.J.; Wildi, K.; Fortier, A.; Galvan Onandia, Z.; Toma, A.; Zhang, Z.W.; et al. Weather and risk of ST-elevation myocardial infarction revisited: Impact on young women. PLoS ONE 2018, 13, e0195602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Auger, N.; Potter, B.J.; Smargiassi, A.; Bilodeau-Bertrand, M.; Paris, C.; Kosatsky, T. Association between quantity and duration of snowfall and risk of myocardial infarction. CMAJ 2017, 189, E235–E242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.; Sarnat, S.E.; Grundstein, A.J.; Winquist, A.; Chang, H.H. Time-series Analysis of Heat Waves and Emergency Department Visits in Atlanta, 1993 to 2012. Environ. Health Perspect. 2017, 125, 057009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guirguis, K.; Gershunov, A.; Tardy, A.; Basu, R. The Impact of Recent Heat Waves on Human Health in California. J. Appl. Meteorol. Clim. 2014, 53, 3–19. [Google Scholar] [CrossRef]
- Mukai, T.; Hosomi, N.; Tsunematsu, M.; Sueda, Y.; Shimoe, Y.; Ohshita, T.; Torii, T.; Aoki, S.; Nezu, T.; Maruyama, H.; et al. Various meteorological conditions exhibit both immediate and delayed influences on the risk of stroke events: The HEWS-stroke study. PLoS ONE 2017, 12, e0178223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bunker, A.; Wildenhain, J.; Vandenbergh, A.; Henschke, N.; Rocklöv, J.; Hajat, S.; Sauerborn, R. Effects of Air Temperature on Climate-Sensitive Mortality and Morbidity Outcomes in the Elderly; a Systematic Review and Meta-analysis of Epidemiological Evidence. eBioMedicine 2016, 6, 258–268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monin, A.; Didier, R.; Leclercq, T.; Chagué, F.; Rochette, L.; Danchin, N.; Zeller, M.; Fauchier, L.; Cochet, A.; Cottin, Y. Coronary artery embolism and acute coronary syndrome: A critical appraisal of existing data. Trends Cardiovasc. Med. 2024, 34, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Johannsen, S.S.; Wild, P.S.; Blankenberg, S. Prävalenz und Risikofaktoren von Vorhofflimmern in Deutschland: Daten aus der Gutenberg Health Study. Herz 2015, 40, 8–15. [Google Scholar] [CrossRef]
- Zhan, Y.; Joza, J.; Al Rawahi, M.; Barbosa, R.S.; Samuel, M.; Bernier, M.; Huynh, T.; Thanassoulis, G.; Essebag, V. Assessment and Management of the Left Atrial Appendage Thrombus in Patients With Nonvalvular Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Berlit, P. Ischämischer Schlaganfall. In Basiswissen Neurologie; Berlit, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–217. [Google Scholar]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Pathophysiology and classification of thrombosis. Nihon Rinsho Jpn. J. Clin. Med. 2014, 72, 1184–1190. [Google Scholar]
- Desai, Y.; Khraishah, H.; Alahmad, B. Heat and the Heart. Yale J. Biol. Med. 2023, 96, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.J.; Suarez, J. Detection and prevention of deep venous thrombosis. Drug Intell. Clin. Pharm. 1988, 22, 107–114. [Google Scholar] [CrossRef]
- Salam, A.; Kamran, S.; Bibi, R.; Korashy, H.M.; Parray, A.; Mannai, A.A.; Ansari, A.A.; Kanikicharla, K.K.; Gashi, A.Z.; Shuaib, A. Meteorological Factors and Seasonal Stroke Rates: A Four-year Comprehensive Study. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2019, 28, 2324–2331. [Google Scholar] [CrossRef]
- Pan, R.; Okada, A.; Yamana, H.; Yasunaga, H.; Kumazawa, R.; Matsui, H.; Fushimi, K.; Honda, Y.; Kim, Y. Association between ambient temperature and cause-specific cardiovascular disease admissions in Japan: A nationwide study. Environ. Res. 2023, 225, 115610. [Google Scholar] [CrossRef] [PubMed]
- Houdas, Y.; Deklunder, G.; Lecroart, J.L. Cold exposure and ischemic heart disease. Int. J. Sports Med. 1992, 13 (Suppl. 1), S179–S181. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, J.A.; Jessup, M. Understanding Heart Failure. Heart Fail. Clin. 2017, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pétersen, C.L.; Hansen, A.; Frandsen, E.; Strange, S.; Jonassen, O.; Nielsen, J.R.; Dige-Petersen, H.; Hesse, B. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am. Heart J. 1994, 128, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Amiya, S.; Nuruki, N.; Tanaka, Y.; Tofuku, K.; Fukuoka, Y.; Sata, N.; Kashima, K.; Tsubouchi, H. Relationship between weather and onset of acute myocardial infarction: Can days of frequent onset be predicted? J. Cardiol. 2009, 54, 231–237. [Google Scholar] [CrossRef]
- Bai, L.; Li, Q.; Wang, J.; Lavigne, E.; Gasparrini, A.; Copes, R.; Yagouti, A.; Burnett, R.T.; Goldberg, M.S.; Cakmak, S.; et al. Increased coronary heart disease and stroke hospitalisations from ambient temperatures in Ontario. Heart 2018, 104, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Maseri, A.; Beltrame, J.F.; Shimokawa, H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ. J. Off. J. Jpn. Circ. Soc. 2009, 73, 394–403. [Google Scholar]
- Liu, J.; Varghese, B.M.; Hansen, A.; Zhang, Y.; Driscoll, T.; Morgan, G.; Dear, K.; Gourley, M.; Capon, A.; Bi, P. Heat exposure and cardiovascular health outcomes: A systematic review and meta-analysis. Lancet Planet. Health 2022, 6, e484–e495. [Google Scholar] [CrossRef]
- Deutsche Akademie der Naturforscher Leopoldina. Klimawandel: Ursachen, Folgen und Handlungsmöglichkeiten; MyCoRe Community: Hamburg, Germany, 2021. [Google Scholar]
- Li, M.; Gu, S.; Bi, P.; Yang, J.; Liu, Q. Heat waves and morbidity: Current knowledge and further direction-a comprehensive literature review. Int. J. Environ. Res. Public Health 2015, 12, 5256–5283. [Google Scholar] [CrossRef]
| Patient Characteristics | LAA Thrombus Study (n = 131) |
|---|---|
| Median Age | 73 Jahre |
| Male gender | 64% |
| Average BMI | 28 kg/m2 |
| Heart failure | 62% |
| Arterial hypertension | 77% |
| Diabetes mellitus | 31% |
| State after apoplexy | 16% |
| Dyslipidemia | 37% |
| Diuretic medication | 58% |
| Antidiabetic medication | 18% |
| Anticoagulant medication | 76% |
| nOAK therapy alone | 30% |
| Antiplatelet agents alone | 16% |
| Coumarin alone | 13% |
| nOAK and antiplatelet agent | 12% |
| Meteorological Parameters Average 2 Weeks before Diagnosis | F-Months (85) | N-Months (46) | p-Value | Effect Strength, r |
|---|---|---|---|---|
| Average temperature (°C) | 10.8 ± 6.5 | 7.5 ± 7.3 | 0.012 | 0.22 |
| Temperature maximum (°C) | 15.8 ± 7.5 | 11.6 ± 8.5 | 0.005 | 0.25 |
| Temperature minimum (°C) | 5.9 ± 5.6 | 3.8 ± 6.4 | 0.057 | 0.17 |
| Absolute temperature difference (°C) | 10.0 ± 2.8 | 7.7 ± 2.9 | <0.001 | 0.33 |
| Relative humidity (%) | 75 ± 9 | 83 ± 9 | <0.001 | 0.39 |
| Mean wind speed (m/s) | 2.8 ± 0.6 | 2.8 ± 1.0 | 0.073 | 0.16 |
| Maximum wind speed (m/s) | 9.5 ± 1.7 | 8.5 ± 2.3 | 0.003 | 0.26 |
| Sunshine duration (h) | 6.0 ± 3.0 | 3.7 ± 2.6 | <0.001 | 0.35 |
| Precipitation amount (mm) | 2.0 ± 1.7 | 2.1 ± 1.6 | 0.506 | 0.06 |
| Mean air pressure (hPa) | 968 ± 12 | 966 ± 17 | 0.845 | 0.02 |
| Average vapor pressure (hPa) | 9.9 ± 3.3 | 9.3 ± 4.1 | 0.151 | 0.13 |
| Equivalent temperature (°C) | 27.0 ± 12.0 | 22.5 ± 14.1 | 0.034 | 0.19 |
| Meteorological Parameters Average 4 Weeks before Diagnosis | F-Months (85 ED) | N-Months (46 ED) | p-Value | Effect Strength, r |
|---|---|---|---|---|
| Average temperature (°C) | 10.1 ± 6.4 | 8.2 ± 7.3 | 0.090 | 0.15 |
| Temperature maximum (°C) | 15.1 ± 7.3 | 12.4 ± 8.5 | 0.058 | 0.17 |
| Temperature minimum (°C) | 5.3 ± 5.6 | 4.3 ± 6.3 | 0.225 | 0.11 |
| Absolute temperature difference (°C) | 9.7 ± 2.6 | 8.2 ± 2.7 | 0.004 | 0.25 |
| Relative humidity (%) | 76 ± 9 | 82 ± 8 | <0.001 | 0.33 |
| Mean wind speed (m/s) | 2.8 ±0.5 | 2.8 ± 0.8 | 0.075 | 0.16 |
| Maximum wind speed (m/s) | 9.3 ± 1.5 | 8.7 ± 2.0 | 0.004 | 0.25 |
| Sunshine duration (h) | 5.7 ± 2.7 | 4.2 ± 2.6 | 0.004 | 0.25 |
| Precipitation amount (mm) | 2.0 ± 1.3 | 1.9 ± 1.1 | 0.965 | <0.001 |
| Mean air pressure (hPa) | 968 ± 12 | 968 ± 14 | 0.851 | 0.02 |
| Average vapor pressure (hPa) | 9.6 ± 3.1 | 9.5 ± 4.0 | 0.472 | 0.06 |
| Equivalent temperature (°C) | 25.8 ± 11.5 | 23.5 ± 14.0 | 0.186 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecker, F.; Tiemann, K.; Lewalter, T.; Jilek, C. Influence of Meteorological Parameters on the Prevalence of TEE Detected Left Atrial Appendage Thrombi. Diseases 2024, 12, 151. https://doi.org/10.3390/diseases12070151
Lecker F, Tiemann K, Lewalter T, Jilek C. Influence of Meteorological Parameters on the Prevalence of TEE Detected Left Atrial Appendage Thrombi. Diseases. 2024; 12(7):151. https://doi.org/10.3390/diseases12070151
Chicago/Turabian StyleLecker, Franziska, Klaus Tiemann, Thorsten Lewalter, and Clemens Jilek. 2024. "Influence of Meteorological Parameters on the Prevalence of TEE Detected Left Atrial Appendage Thrombi" Diseases 12, no. 7: 151. https://doi.org/10.3390/diseases12070151
APA StyleLecker, F., Tiemann, K., Lewalter, T., & Jilek, C. (2024). Influence of Meteorological Parameters on the Prevalence of TEE Detected Left Atrial Appendage Thrombi. Diseases, 12(7), 151. https://doi.org/10.3390/diseases12070151







