Evaluating Immunologic and Illness Outcomes of SARS-CoV-2 Infection in Vaccinated and Unvaccinated Children Aged ≥ 5 Years, in a Multisite Longitudinal Cohort
Abstract
:Simple Summary
Abstract
1. Background
2. Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Blood Collection
2.5. Enzyme-Linked Immunosorbent Assay (EL–ISA)
2.6. Statistical Methods
2.7. Sensitivity Analysis
3. Results
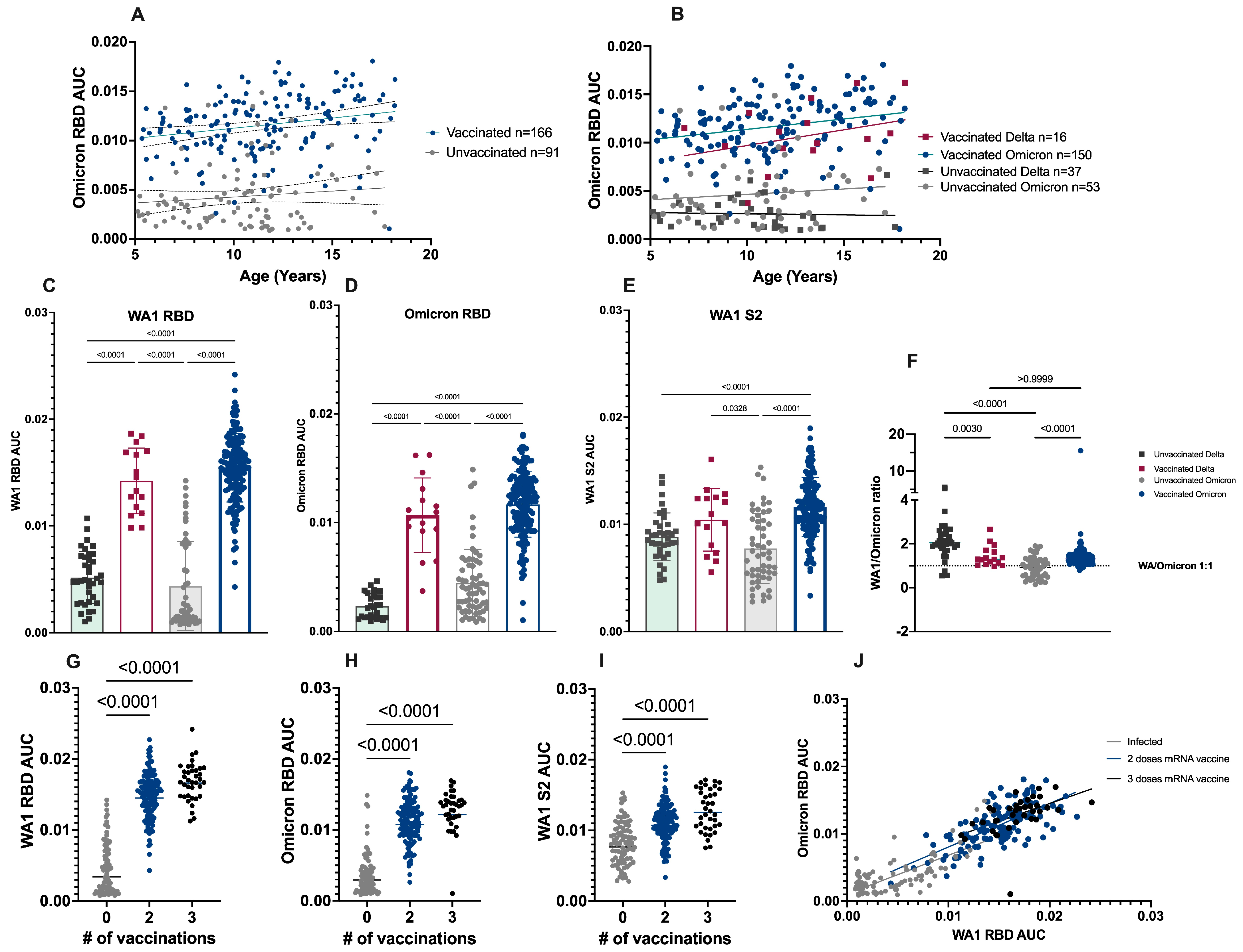
3.1. Association of Immune Response and Vaccination Status
3.2. Ratio of WA1 to Omicron Binding Antibodies
3.3. Antibody Response to Primary Infection by Number of COVID-19 Vaccine Doses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| AUC | Area under the curve |
| BA.2 | Omicron |
| BMI | Body Mass Index |
| CDC | Centers for Disease Control and Prevention |
| COVID-19 | Coronavirus disease 2019 |
| ELISA | Enzyme-linked immunosorbent assays |
| GMR | Geometric mean ratio |
| IRB | Institutional review board |
| OR | Odds Ratio |
| RBD | Receptor binding domain |
| rRT-PCR | Real-time reverse transcription polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| S2 | S2 region of spike protein |
| PROTECT | Pediatric Research Observing Trends and Exposures in COVID-19 Timelines |
| HEROES-RECOVER | Arizona Healthcare, Emergency Response, and Other Essential Workers Study and Research on the Epidemiology of SARS-CoV-2 in Essential Response Personnel |
| WA1 | Washington-1 |
| 95% CI | 95% confidence interval |
References
- Bonfante, F.; Costenaro, P.; Cantarutti, A.; Di Chiara, C.; Bortolami, A.; Petrara, M.R.; Carmona, F.; Pagliari, M.; Cosma, C.; Cozzani, S.; et al. Mild SARS-CoV-2 Infections and Neutralizing Antibody Titers. Pediatrics 2021, 148, e2021052173. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection among Children Aged 5–11 Years and Adolescents Aged 12–15 Years-PROTECT Cohort, July 2021-February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Sberna, G.; Meschi, S.; Gramigna, G.; Colavita, F.; Lapa, D.; Francalancia, M.; Bettini, A.; Capobianchi, M.R.; Puro, V.; et al. Differential Dynamics of SARS-CoV-2 Binding and Functional Antibodies upon BNT162b2 Vaccine: A 6-Month Follow-Up. Viruses 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Rotulo, G.A.; Palma, P. Understanding COVID-19 in children: Immune determinants and post-infection conditions. Pediatr. Res. 2023, 94, 434–442. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of Age with SARS-CoV-2 Antibody Response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef]
- Di Chiara, C.; Cantarutti, A.; Costenaro, P.; Donà, D.; Bonfante, F.; Cosma, C.; Ferrarese, M.; Cozzani, S.; Petrara, M.R.; Carmona, F.; et al. Long-term Immune Response to SARS-CoV-2 Infection among Children and Adults after Mild Infection. JAMA Netw. Open 2022, 5, e2221616. [Google Scholar] [CrossRef]
- Castilla, J.; Lecea, O.; Martin Salas, C.; Quilez, D.; Miqueleiz, A.; Trobajo-Sanmartin, C.; Navascues, A.; Martinez-Baz, I.; Casado, I.; Burgui, C.; et al. Seroprevalence of antibodies against SARS-CoV-2 and risk of COVID-19 in Navarre, Spain, May to July 2022. Eurosurveillance 2022, 27, 2200619. [Google Scholar] [CrossRef]
- Selva, K.J.; van de Sandt, C.E.; Lemke, M.M.; Lee, C.Y.; Shoffner, S.K.; Chua, B.Y.; Davis, S.K.; Nguyen, T.H.O.; Rowntree, L.C.; Hensen, L.; et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 2021, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Echevarría, A.; Sainz, T.; Falces-Romero, I.; de Felipe, B.; Escolano, L.; Alcolea, S.; Pertiñez, L.; Neth, O.; Calvo, C. Long-Term Persistence of Anti-SARS-CoV-2 Antibodies in a Pediatric Population. Pathogens 2021, 10, 700. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.D.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; St Denis, K.J.; Sheehan, M.L.; Chen, J.W.; Davis, J.P.; Lima, R.; Edlow, A.G.; et al. Durability and Cross-Reactivity of SARS-CoV-2 mRNA Vaccine in Adolescent Children. Vaccines 2022, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron—The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- de Gier, B.; Huiberts, A.J.; Hoeve, C.E.; den Hartog, G.; van Werkhoven, H.; van Binnendijk, R.; Hahné, S.J.M.; de Melker, H.E.; van den Hof, S.; Knol, M.J. Effects of COVID-19 vaccination and previous infection on Omicron SARS-CoV-2 infection and relation with serology. Nat. Commun. 2023, 14, 4793. [Google Scholar] [CrossRef] [PubMed]
- Raineri, A.; Radtke, T.; Rueegg, S.; Haile, S.R.; Menges, D.; Ballouz, T.; Ulyte, A.; Fehr, J.; Cornejo, D.L.; Pantaleo, G.; et al. Persistent humoral immune response in youth throughout the COVID-19 pandemic: Prospective school-based cohort study. Nat. Commun. 2023, 14, 7764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarupan, M.; Jantarabenjakul, W.; Jaruampornpan, P.; Subchartanan, J.; Phasomsap, C.; Sritammasiri, T.; Cartledge, S.; Suchartlikitwong, P.; Anugulruengkitt, S.; Kawichai, S.; et al. Long COVID and Hybrid Immunity among Children and Adolescents Post-Delta Variant Infection in Thailand. Vaccines 2023, 11, 884. [Google Scholar] [CrossRef]
- Zhong, Y.; Kang, A.Y.H.; Tay, C.J.X.; Li, H.E.; Elyana, N.; Tan, C.W.; Yap, W.C.; Lim, J.M.E.; Le Bert, N.; Chan, K.R.; et al. Correlates of protection against symptomatic SARS-CoV-2 in vaccinated children. Nat. Med. 2024, 30, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Rivers, P.; LeClair, L.B.; Jovel, K.S.; Rai, R.P.; Lowe, A.A.; Edwards, L.J.; Khan, S.M.; Mathenge, C.; Ferraris, M.; et al. Pediatric Research Observing Trends and Exposures in COVID-19 Timelines (PROTECT): Protocol for a Multisite Longitudinal Cohort Study. JMIR Res. Protoc. 2022, 11, e37929. [Google Scholar] [CrossRef] [PubMed]
- Lyski, Z.L.; Porter, C.; Uhrlaub, J.L.; Ellingson, K.D.; Jeddy, Z.; Gwynn, L.; Rivers, P.; Sprissler, R.; Hegmann, K.T.; Coughlin, M.; et al. Humoral Immune Response to mRNA COVID-19 Vaccination among Children 5–11 in a Multisite Prospective Cohort study, September 2021–September 2022. Open Forum Infect. Dis. 2023, 10, ofad431. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Fowlkes, A.L.; Wesley, M.G.; Kuntz, J.L.; Odean, M.J.; Caban-Martinez, A.J.; Dunnigan, K.; Phillips, A.L.; Grant, L.; Herring, M.K.; et al. Research on the Epidemiology of SARS-CoV-2 in Essential Response Personnel (RECOVER): Protocol for a Multisite Longitudinal Cohort Study. JMIR Res. Protoc. 2021, 10, e31574. [Google Scholar] [CrossRef] [PubMed]
- Lutrick, K.; Ellingson, K.D.; Baccam, Z.; Rivers, P.; Beitel, S.; Parker, J.; Hollister, J.; Sun, X.; Gerald, J.K.; Komatsu, K.; et al. COVID-19 Infection, Reinfection, and Vaccine Effectiveness in a Prospective Cohort of Arizona Frontline/Essential Workers: The AZ HEROES Research Protocol. JMIR Res. Protoc. 2021, 10, e28925. [Google Scholar] [CrossRef] [PubMed]
- Control, C.f.D. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 29 August 2023).
- Lambrou, A.S.; Shirk, P.; Steele, M.K.; Paul, P.; Paden, C.R.; Cadwell, B.; Reese, H.E.; Aoki, Y.; Hassell, N.; Zheng, X.Y.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants-United States, June 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. Available online: https://www.cdc.gov/covid/hcp/clinical-care/clinical-specimen-guidelines.html?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 23 January 2024).
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Asthagiri Arunkumar, G.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. medRxiv 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Yu, X.; Gilbert, P.B.; Hioe, C.E.; Zolla-Pazner, S.; Self, S.G. Statistical approaches to analyzing HIV-1 neutralizing antibody assay data. Stat. Biopharm. Res. 2012, 4, 1–13. [Google Scholar] [CrossRef]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef]
- Lipsitz, S.R.; Fitzmaurice, G.M.; Regenbogen, S.E.; Sinha, D.; Ibrahim, J.G.; Gawande, A.A. Bias correction for the proportional odds logistic regression model with application to a study of surgical complications. J. R. Stat. Soc. Ser. C Appl. Stat. 2013, 62, 233–250. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G.; Chakraborty, C. Hybrid immunity against COVID-19 in different countries with a special emphasis on the Indian scenario during the Omicron period. Int. Immunopharmacol. 2022, 108, 108766. [Google Scholar] [CrossRef]
- Files, J.K.; Sarkar, S.; Fram, T.R.; Boppana, S.; Sterrett, S.; Qin, K.; Bansal, A.; Long, D.M.; Sabbaj, S.; Kobie, J.J.; et al. Duration of post-COVID-19 symptoms is associated with sustained SARS-CoV-2-specific immune responses. JCI Insight 2021, 6, e151544. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination against Multisystem Inflammatory Syndrome in Children among Persons Aged 12–18 Years-United States, July-December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef]
- Puhach, O.; Meyer, B.; Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol. 2023, 21, 147–161. [Google Scholar] [CrossRef]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Naderi Ghale-Noie, Z.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filchakova, O.; Dossym, D.; Ilyas, A.; Kuanysheva, T.; Abdizhamil, A.; Bukasov, R. Review of COVID-19 testing and diagnostic methods. Talanta 2022, 244, 123409. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef]
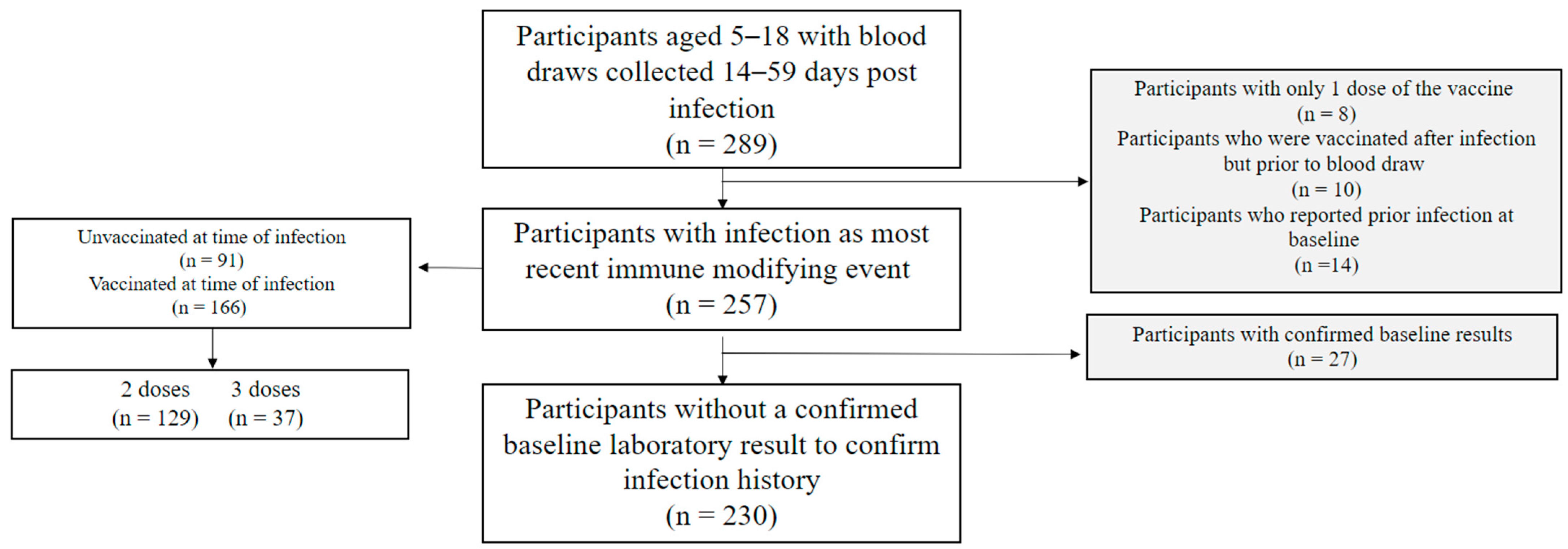
| Participant Characteristics n, % | Unvaccinated n (%) (Total = 91) | Vaccinated a n (%) (Total = 166) | Total (n = 157) | p-Value b |
|---|---|---|---|---|
| Variant | <0.001 * | |||
| Delta | 37 (40.7) | 16 (9.6) | 53 (20.6) | |
| Omicron | 54 (59.3) | 150 (90.4) | 204 (79.4) | |
| Gender b | 0.071 | |||
| Female | 36 (39.6) | 87 (52.4) | 123 (47.9) | |
| Male | 54 (59.3) | 79 (47.6) | 133 (51.8) | |
| Other Gender Identity | 1 (1.1) | 0 (0.0) | 1 (0.3) | |
| Study Site b | 0.020 * | |||
| Temple, TX | 7 (7.7) | 6 (3.6) | 13 (5.1) | |
| Tucson, AZ | 36 (39.6) | 91 (54.8) | 127 (49.4) | |
| Salt Lake City, UT | 15 (16.5) | 14 (8.4) | 29 (11.3) | |
| Miami, FL | 7 (7.7) | 4 (2.4) | 11 (4.3) | |
| Phoenix, AZ | 16 (17.6) | 38 (22.9) | 54 (21.0) | |
| Other places in AZ | 10 (10.9) | 13 (7.8) | 23 (8.9) | |
| Race/Ethnicity b | 0.229 | |||
| NH White | 60 (66.7) | 105 (63.3) | 165 (64.2) | |
| NH Black | 0 (0.0) | 4 (2.4) | 4 (1.6) | |
| NH Asian | 3 (3.0) | 1 (0.6) | 4 (1.6) | |
| Hispanic | 21 (21.8) | 42 (25.3) | 64 (24.9) | |
| Not listed/Refused | 7 (8.5) | 14 (8.4) | 21 (7.7) | |
| Weight Status b,c | 0.696 | |||
| Not overweight or obese | 81 (89.0) | 145 (87.4) | 226 (87.9) | |
| Overweight or obese | 10 (11.0) | 21 (12.7) | 31 (12.1) | |
| Symptom Status b | 0.982 | |||
| Asymptomatic | 16 (17.6) | 29 (17.5) | 45 (17.5) | |
| Symptomatic | 75 (82.4) | 137 (82.5) | 212 (82.5) | |
| Weeks of PCR positivity d mean, SD | 1.2 (1.0) | 1.6 (1.4) | 1.5 (1.3) | 0.028 * |
| Length of symptoms in days mean, SD | 9.2 (8.4) | 8.2 (7.7) | 8.6 (7.9) | 0.382 |
| Days spent in bed, mean, SD | 1.7 (1.8) | 1.7 (1.8) | 1.7 (1.8) | 0.785 |
| Fever e | 44 (47.8) | 55 (33.1) | 99 (38.5) | 0.021 * |
| Days from illness to blood draw mean, SD | 34.0 (9.9) | 33.3 (9.0) | 33.5 (9.3) | 0.605 |
| Comorbid Conditions f | 0.779 | |||
| 0 | 79 (86.8) | 142 (85.5) | 221 (86.0) | |
| 1+ | 12 (13.2) | 24 (14.5) | 36 (14.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porter, C.; Lyski, Z.L.; Uhrlaub, J.L.; Ellingson, K.D.; Jeddy, Z.; Gwynn, L.; Rivers, P.; Sprissler, R.; Hegmann, K.T.; Coughlin, M.M.; et al. Evaluating Immunologic and Illness Outcomes of SARS-CoV-2 Infection in Vaccinated and Unvaccinated Children Aged ≥ 5 Years, in a Multisite Longitudinal Cohort. Diseases 2024, 12, 171. https://doi.org/10.3390/diseases12080171
Porter C, Lyski ZL, Uhrlaub JL, Ellingson KD, Jeddy Z, Gwynn L, Rivers P, Sprissler R, Hegmann KT, Coughlin MM, et al. Evaluating Immunologic and Illness Outcomes of SARS-CoV-2 Infection in Vaccinated and Unvaccinated Children Aged ≥ 5 Years, in a Multisite Longitudinal Cohort. Diseases. 2024; 12(8):171. https://doi.org/10.3390/diseases12080171
Chicago/Turabian StylePorter, Cynthia, Zoe L. Lyski, Jennifer L. Uhrlaub, Katherine D. Ellingson, Zuha Jeddy, Lisa Gwynn, Patrick Rivers, Ryan Sprissler, Kurt T. Hegmann, Melissa M. Coughlin, and et al. 2024. "Evaluating Immunologic and Illness Outcomes of SARS-CoV-2 Infection in Vaccinated and Unvaccinated Children Aged ≥ 5 Years, in a Multisite Longitudinal Cohort" Diseases 12, no. 8: 171. https://doi.org/10.3390/diseases12080171
APA StylePorter, C., Lyski, Z. L., Uhrlaub, J. L., Ellingson, K. D., Jeddy, Z., Gwynn, L., Rivers, P., Sprissler, R., Hegmann, K. T., Coughlin, M. M., Fowlkes, A. L., Hollister, J., LeClair, L., Mak, J., Beitel, S. C., Fuller, S., Zheng, P. Q., Vaughan, M., Rai, R. P., ... Lutrick, K. (2024). Evaluating Immunologic and Illness Outcomes of SARS-CoV-2 Infection in Vaccinated and Unvaccinated Children Aged ≥ 5 Years, in a Multisite Longitudinal Cohort. Diseases, 12(8), 171. https://doi.org/10.3390/diseases12080171









