Osteocalcin, Osteopontin and RUNX2 Expression in Patients’ Leucocytes with Arteriosclerosis
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Expression of Leucocytic OC Transcripts Is Significantly Reduced in Patients with Arteriosclerotic Disease
3.2. Expression of Leukocytic OPN and RUNX2 Transcripts Is Significantly Increased in Patients with Arteriosclerotic Disease
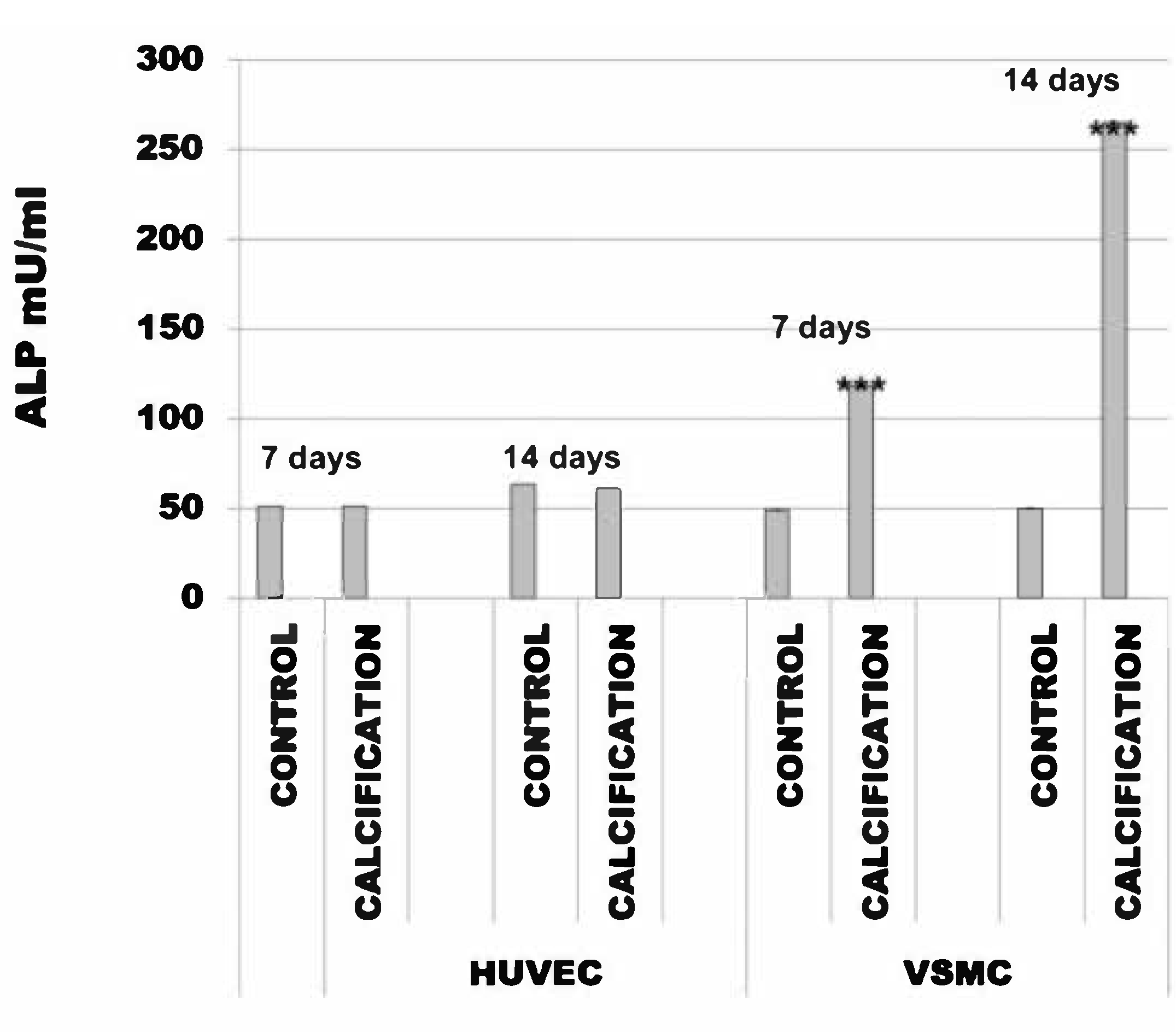
3.3. Alkaline Phosphatase Activity Was the Same in HUVEC and Increased in VSMC Cells Treated with Calcification Medium
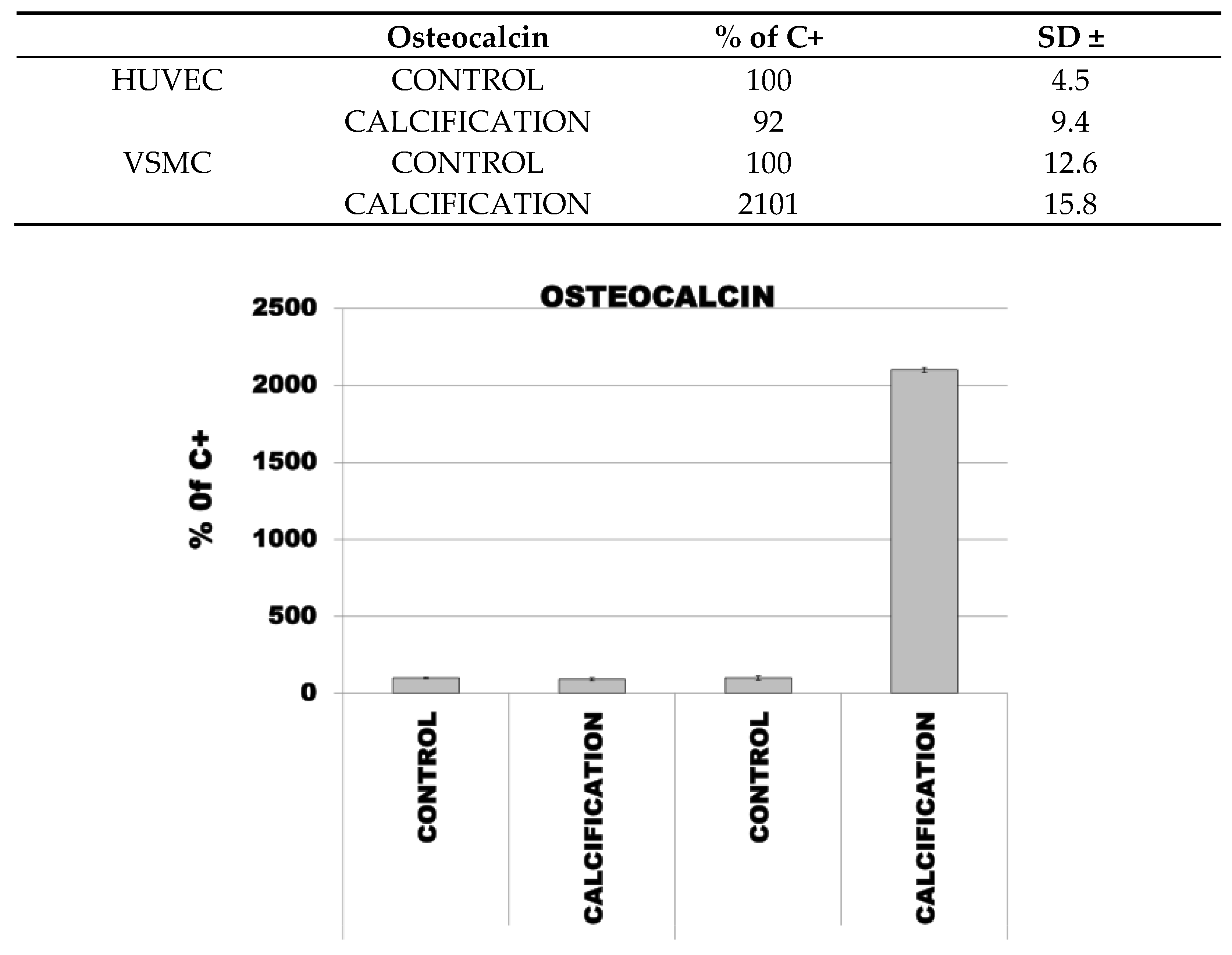
3.4. Induction of Calcification in HUVEC and VSMC Cells Led to Increased Expression of OC, OPN and RUNX2
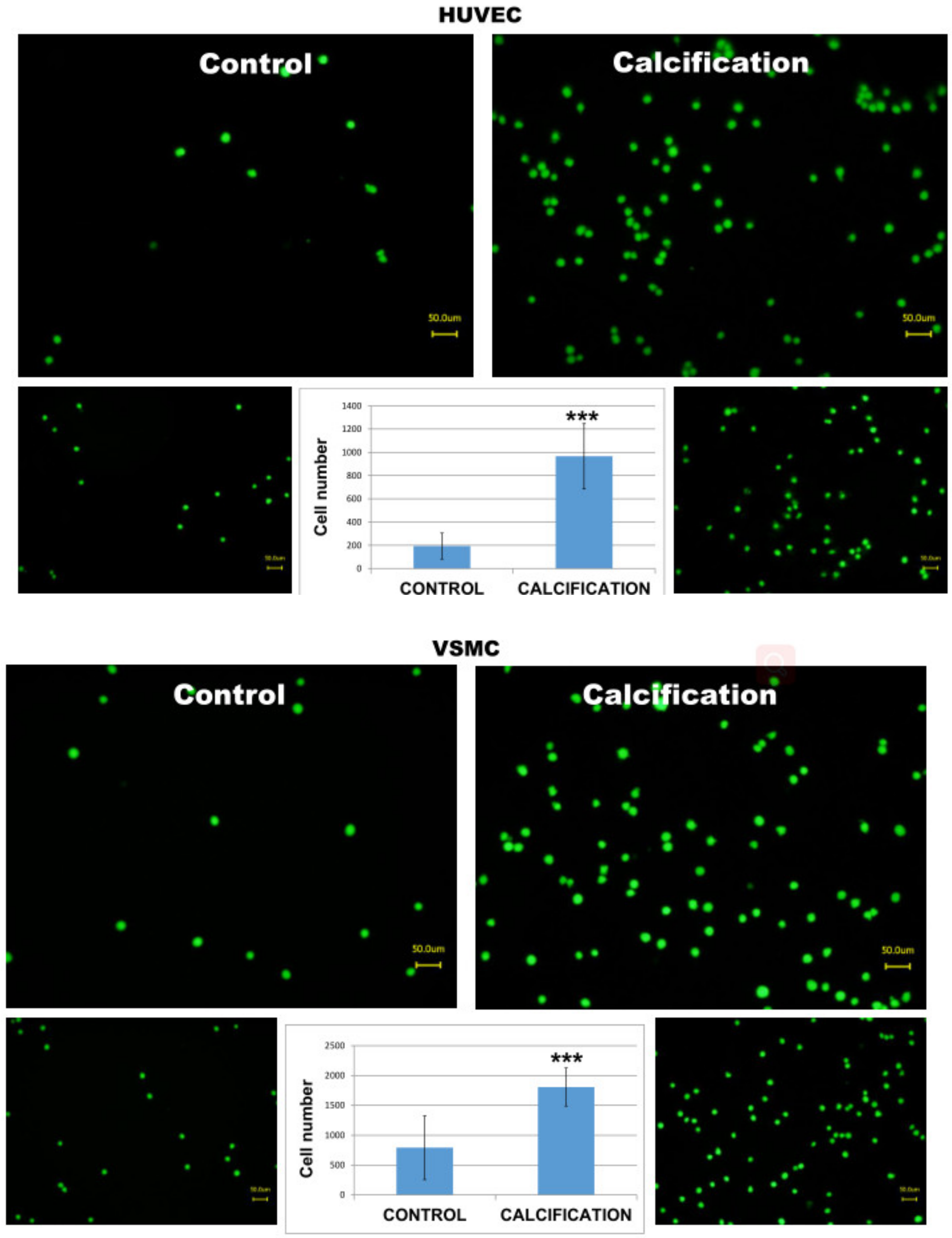
3.5. Adhesion of THP-1 Monocytes to Calcified HUVEC or VSMC Cells Was Higher in Calcified Cells as Compared to Controls
4. Discussion
4.1. Osteocalcin
4.2. Osteopontin
4.3. Runt-Related Transcription Factor 2
4.4. Alkaline Phosphatase Activity (ALP)
4.5. Monocytes
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability:
References
- Nicoll, R.; Henein, M.Y. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. IJC Hear Vessel. 2014, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 22 December 2020).
- Van Camp, G. Cardiovascular disease prevention. Acta Clin. Belg. 2014, 69, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.-D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Berger, J.M.; Singh, P.; Khrimian, L.; Morgan, D.A.; Chowdhury, S.; Arteaga-Solis, E.; Horvath, T.L.; Domingos, A.I.; Marsland, A.L.; Yadav, V.K.; et al. Mediation of the Acute Stress Response by the Skeleton. Cell Metab. 2019, 30, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ma, X.; Xiong, Q.; Zhang, X.; Shen, Y.; Bao, Y. Osteocalcin value to identify subclinical atherosclerosis over atherosclerotic cardiovascular disease (ASCVD) risk score in middle-aged and elderly Chinese asymptomatic men. Clin. Chem. Lab. Med. 2018, 56, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Chiba, S.; Rashid, M.M.; Okamoto, H.; Shiraiwa, H.; Kon, S.; Maeda, M.; Murakami, M.; Inobe, M.; Kitabatake, A.; Chambers, A.F.; et al. The role of osteopontin in the development of granulomatous lesions in lung. Microbiol. Immunol. 2000, 44, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. Osteopontin and cardiovascular system. Mol. Cell. Biochem. 2007, 300, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wolak, T. Osteopontin—A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.K.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Tyson, K.L.; Reynolds, J.L.; McNair, R.; Zhang, Q.; Weissberg, P.L.; Shanahan, C.M. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcification. Arter. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, W.; Chao, Y.; Zhang, F.; Wang, C. CTRP13 attenuates vascular calcification by regulating Runx2. FASEB J. 2019, 33, 9627–9637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizokami, A.; Kawakubo-Yasukochi, T.; Hirata, M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 2017, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An overview of osteocalcin progress. J. Bone Miner. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kord-Varkaneh, H.; Djafarian, K.; Khorshidi, M.; Shab-Bidar, S. Association between serum osteocalcin and body mass index: A systematic review and meta-analysis. Endocrine 2017, 58, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.N.; Rush, C.; Parr, A.; Van Campenhout, A.; Golledge, J. Osteocalcin positive mononuclear cells are associated with the severity of aortic calcification. Atherosclerosis 2010, 210, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin, J.; Gössl, M.; Matsuo, Y.; Cilluffo, R.R.; Flammer, A.J.; Loeffler, D.; Lennon, R.J.; Simari, R.D.; Spoon, D.B.; Erbel, R.; et al. Osteogenic monocytes within the coronary circulation and their association with plaque vulnerability in patients with early atherosclerosis. Int. J. Cardiol. 2015, 181, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Sandoval, J.C.; Casanova, I.; Villar-Tapia, J.A.; Gomez, F.E.; Cruz, C.; Correa-Rotter, R. Biomarkers Associated with Vascular Calcification in Peritoneal Dialysis. Perit. Dial. Int. 2016, 36, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.-J.; Kim, H.-S. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A.; Kremzer, A. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis 2013, 229, 475–481. [Google Scholar] [CrossRef]
- Tanaka, T.; Sato, H.; Doi, H.; Yoshida, C.A.; Shimizu, T.; Matsui, H.; Yamazaki, M.; Akiyama, H.; Kawai-Kowase, K.; Iso, T.; et al. Runx2 Represses Myocardin-Mediated Differentiation and Facilitates Osteogenic Conversion of Vascular Smooth Muscle Cells. Mol. Cell. Biol. 2007, 28, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Huseini, I.; Ashida, N.; Kimura, T. Deletion of IκB-Kinase β in Smooth Muscle Cells Induces Vascular Calcification Through β-Catenin–Runt-Related Transcription Factor 2 Signaling. J. Am. Heart Assoc. 2018, 7, e007405. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Huang, L.; Sun, Y.; Heath, J.M.; Wu, H.; Chen, Y. Inhibition of FOXO1/3 Promotes Vascular Calcification. Arter. Thromb. Vasc. Biol. 2015, 35, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, C.R.; Kuss, P.; Narisawa, S.; Yadav, M.C.; Nigro, J.; Wang, W.; Chhea, T.N.; Sergienko, E.A.; Kapoor, K.; Jackson, M.R.; et al. Pathophysiological Role of Vascular Smooth Muscle Alkaline Phosphatase in Medial Artery Calcification. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 824–836. [Google Scholar] [CrossRef] [Green Version]
- Savinov, A.Y.; Salehi, M.; Yadav, M.C.; Radichev, I.; Milla ́n, J.L.; Savinova, O.V. Transgenic Overexpression of Tissue-Nonspecific Alkaline Phosphatase (TNAP) in Vascular Endothelium Results in Generalized Arterial Calcification. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2015, 4, e002499. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, F.; Corbo, A.; Salehi, M.; Yadav, M.C.; Salman, S.; Petrosian, D.; Rashidbaigi, O.J.; Chait, J.; Kuruvilla, J.; Plummer, M.; et al. Overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in endothelial cells accelerates coronary artery disease in a mouse model of familial hypercholesterolemia. PLoS ONE 2017, 12, e0186426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbin, O.; Regelmann, A.G.; Ramkhelawon, B.; Weinstein, E.G.; Moore, K.J.; Alexandropoulos, K.; Ramkelawon, B. Monocyte Adhesion and Plaque Recruitment During Atherosclerosis Development Is Regulated by the Adapter Protein Chat-H/SHEP1. Arter. Thromb. Vasc. Biol. 2016, 36, 1791–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita-Okubo, S.; Kim-Kaneyama, J.-R.; Lei, X.-F.; Fu, W.-G.; Ohnishi, K.; Takeya, M.; Miyauchi, A.; Honda, H.; Itabe, H.; Miyazaki, T. Role of Hic-5 in the formation of microvilli-like structures and the monocyte–endothelial interaction that accelerates atherosclerosis. Cardiovasc. Res. 2015, 105, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primer | Sequence | Product Size |
|---|---|---|
| RUNX2 | Sense-5′-CCCTGAACTCTGCACCAAGT-3′ Antisense-5′-GGCTCAGGTAGGAGGGGTAA-3′ | 120 bp |
| Osteopontin | s GCCGAGGTGATAGTGTGGTT as AACGGGGATGGCCTTGTATG | 149 bp |
| ßActin | s agg cac cag ggc gtg at as gcc cac ata gga atc ctt ctg ac | 51 bp |
| GAPDH | s acc cag aag act gtg gat gg as ttc tag acg gca ggt cag gt | 233 bp |
| 18S | s gtt ggt gga gcg att tgt ctg g as agg gca ggg act taa tca acg c | 151 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukkat, J.; Hoang-Vu, C.; Trojanowicz, B.; Rebelo, A. Osteocalcin, Osteopontin and RUNX2 Expression in Patients’ Leucocytes with Arteriosclerosis. Diseases 2021, 9, 19. https://doi.org/10.3390/diseases9010019
Ukkat J, Hoang-Vu C, Trojanowicz B, Rebelo A. Osteocalcin, Osteopontin and RUNX2 Expression in Patients’ Leucocytes with Arteriosclerosis. Diseases. 2021; 9(1):19. https://doi.org/10.3390/diseases9010019
Chicago/Turabian StyleUkkat, Jörg, Cuong Hoang-Vu, Bogusz Trojanowicz, and Artur Rebelo. 2021. "Osteocalcin, Osteopontin and RUNX2 Expression in Patients’ Leucocytes with Arteriosclerosis" Diseases 9, no. 1: 19. https://doi.org/10.3390/diseases9010019






