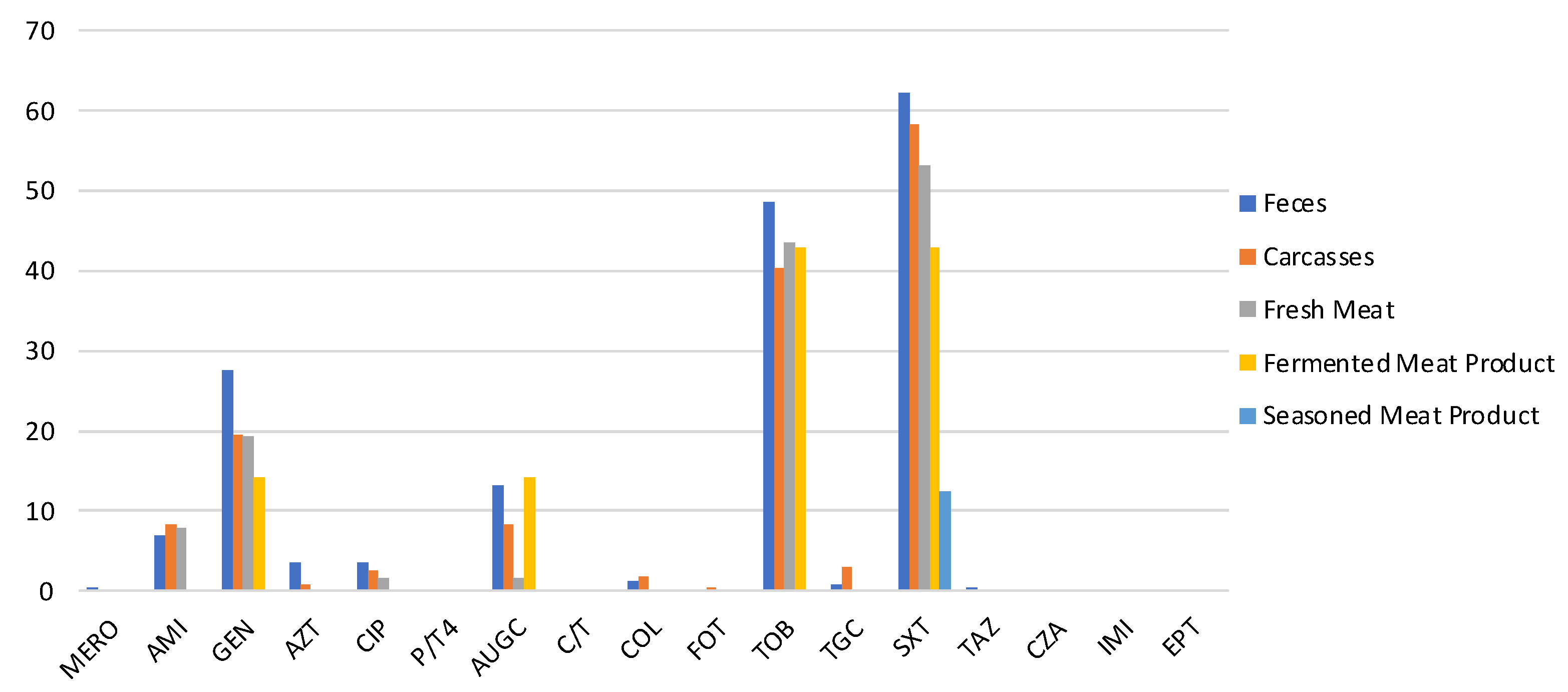Abstract
Antimicrobial resistance (AMR) is a public health risk that needs to be faced from a One Health perspective that includes humans, animals, and environmental health. The food production chain has been identified as a possible route of transmission of AMR bacteria to humans. The most critical phenomenon is related to Critically Important Antimicrobial (CIA) resistance. β-lactams antibiotics (cephalosporin of 3rd, 4th generation, carbapenem, monobactams, and penicillins), quinolones, aminoglycosides, polymyxin, and glycylcyclines were the CIAs chosen in this study. Samples derived from all the stages of the pork food production chain were collected, including pig feces, carcasses, and pork food products (fresh meat, fermented, and seasoned). Escherichia coli were isolated, and AMR and MDR profiles were evaluated. Enterobacterial Repetitive Intragenic Consensus (ERIC-PCR) was used to evaluate phylogenetic similarities. Data showed that 50% of phenotypical AMR observed in the entire pork food chain were related phylogenetically. The contamination of fresh meat, in half of the cases, was not directly related to contamination from feces or carcasses. Despite this, some similarities were found between feces and carcasses. In group analysis, phylogenetic similarities were detected in a 3/36 cluster (8.3%). Nevertheless, further studies are needed to improve consumer risk communication and access to clear and reliable information and health concerns on food labels.
1. Introduction
Antimicrobial resistance (AMR) is one of the most significant public health risks that the world currently faces [1]. Decreased sensitivity of microorganisms to commonly used drugs increasingly affects human, animal, and environmental health [2]. The indiscriminate use and misuse of antimicrobials and unprescribed animal feed additives to improve production can have a large impact on the emergence and dissemination of AMR in the food animal industry [3]. In fact, many studies suggest that antimicrobials used in food-producing animals may be directly related to the increase in antimicrobial resistance [4,5,6].
Actively monitoring the emergence and the spread of AMR is essential, as is understanding the underlying molecular mechanisms involved, to develop novel strategies to combat AMR [7,8]. The possible consequences of not safeguarding antimicrobial use have been reported as having a negative impact on health, the economy, and the industry sector [2].
The monitoring of AMR in the food chain was established in 2003 by the Directive on the monitoring of zoonoses and zoonotic agents [9]. The European Commission, under scientifical guidance from the European Food Safety Authority (EFSA), has provided valuable information and decisions for the establishment of targeted animal populations or food categories and bacterial hazards [10].
Escherichia coli (E. coli) is a gram-negative bacterium that, although often considered a commensal, can be pathogenic and present both a clinical and an epidemiological challenge [11]. It is commonly found in the intestinal tract of humans and warm-blooded animals, and its ability to acquire multidrug resistance makes it a good bioindicator for monitoring AMR [12].
Pork and poultry meat have been identified as a source of transfer of antimicrobial-resistant microorganisms to humans, and a recent study estimated the probability of 1.5% exposure to resistant E. coli through meat consumption [13]. Food of animal origin may be contaminated with antimicrobial-resistant bacteria in many ways. Contamination easily occurs during slaughter and food processing. Antimicrobial resistance can also be transferred from one microorganism to another (conjugation) during food manipulation, thus allowing the spread of AMR caused by different food matrices [13]. In fact, the increasing demand for raw meat carries the risk of ingesting live, non-stressed antimicrobial-resistant bacterial cells [14]. Different food processing and preservation techniques can extend the products’ shelf life thanks to inhibiting their bacterial flora. Despite this, stressed or sub-lethally damaged bacteria cells can be a source of free bacterial DNA, including the eventual presence of antimicrobial resistance genes [13,15].
The World Health Organization (WHO) has ranked antimicrobial agents as Critically Important Antimicrobials (CIAs) to human health, defining risk management related to the use of these drugs [16]. The antimicrobial classes analyzed in the present study belong to CIAs and are β-lactams antibiotics (cephalosporin of 3rd, 4th generation, carbapenem, monobactams, and penicillins), quinolones, aminoglycosides, polymiyxin, and glycylcyclines and were chosen because of their classification as high or highest priority molecules. This classification is based on the availability of alternative antimicrobial therapies to treat serious bacterial infections, particularly in bacteria that may be transmitted to humans by a non-human source and bacteria that can acquire resistance genes from non-human sources [17]. From 2010 to 2020, veterinary antimicrobials sales decreased by 30%, also due to a national action plan introduced in 2017 to reduce the use of CIAs by 10% in three years [18,19]. Despite this, Italy is one of the EU countries with the highest veterinary antimicrobials sales, after Germany, Spain, the United Kingdom, France, and Poland [20].
The present study focuses on the pork food chain from a farm-to-fork perspective. Pigs’ feces, carcasses, and pork food products (fresh meat, fermented and seasoned) from the same pigs were analyzed along the entire food-producing chain. E. coli were isolated and tested phenotypically to evaluate their AMR and MDR profiles to five different antimicrobial classes. Similar profiles were then tested using Enterobacterial Repetitive Intragenic Consensus (ERIC-PCR). ERIC is an intragenic repetitive unit based on the amplification of regions between the ERIC sequences that differentiate bacterial strains according to variations in their location. It is adaptable to a wide range of bacterial species, and ERIC sequences were first described in E. coli [21]. This technique was used to evaluate the phylogenetic similarities and the potential involvement of food production steps in transmitting antimicrobial-resistant bacteria to consumers.
2. Results
2.1. Escherichia coli Isolation
Collected samples were processed for E. coli isolation. E. coli were isolated from all carcass, fresh meat, and fermented meat product samples: 225 strains from carcasses, 62 strains from fresh meat, and 7 strains from fermented meat products. From fecal and seasoned meat product samples, 243/245 and 8/15 E. coli were isolated, respectively.
2.2. Antimicrobial Resistance Evaluation
All strains were tested for their susceptibility to 17 antimicrobials. The antimicrobial resistances most detected were against SXT, TOB, and GEN, as shown in Figure 1.

Figure 1.
Prevalence of antimicrobial-resistant E. coli isolated from feces, carcasses, and meat products. (MERO = meropenem, AUGC= amoxicillin/clavulanic, C/T4 = ceftolozane/tazobactam, FOT = cefotaxime, TAZ = ceftazidime, CZA = ceftazidime/tazobactam, IMI = imipenem, ETP = ertapenem, AZT = aztreonam, AMI = amikacin, GEN = gentamicin, TOB = tobramycin, CIP = ciprofloxacin, COL = colistin, TGC = tigecycline, SXT = sulphamethoxazole/trimethoprim).
In particular, 62.1% (CI 95% = 56–68.2) of E. coli isolated from feces, 58.2% (CI 95% = 51.8–64.6) of carcasses strain, and 48% (CI 95% = 36.8–59.2) of pork meat products E. coli were resistant to SXT. TOB resistance was observed in 48.6% (CI 95% = 42.3–54.9), 40.4% (CI 95% = 34–46.8), and 39% (CI 95% = 28–50) of E. coli isolated from feces, carcasses, and pork meat products, respectively. GEN resistance was detected in 27.6% (CI 95% =22–33.2), 19.5% (CI 95% = 14.3–24.7), and 16.9% (CI 95% = 8.5–25.3) of E. coli isolated along the food chain (feces, carcasses, and meat products). From feces, only one strain (0.4%) was non-susceptible to MERO, and one (0.4%) was resistant to TAZ. Moreover, only one strain isolated from carcasses (0.4%) was resistant to FOT. Despite the presence of resistant isolates from feces and carcasses, strains isolated from pork meat products were not resistant to AZT, TGC, or COL (Figure 1). All strains detected along the food chain were susceptible to C/T, P/T4, CZA, IMI, and EPT.
MDR patterns were evaluated from a farm-to-fork perspective. Particularly, MDR profiles were frequently found in fecal isolates, a few in carcasses, but never in pork meat products. The MDR patterns were: (i) AUGC-TOB-SXT (10/243 fecal E. coli and 4/225 carcasses E. coli); (ii) GEN-AUGC-TOB-SXT (7/243 fecal E. coli and 1/225 carcasses E. coli); (iii) AMI-AUGC-TOB-SXT (1/243 fecal E. coli); (iv) GEN-CIP-AUGC-TOB-SXT (2/243 fecal E. coli and 1/225 carcasses E. coli); (v) AMI-GEN-AUGC-TOB-SXT (1/243 fecal E. coli and 2/225 carcasses E. coli); (vi) AZT-CIP-COL-TOB-TGC-SXT-TAZ (1/243 fecal E. coli); (vii) GEN-CIP-TOB-SXT (2/243 fecal E. coli); (viii) GEN-CIP-AUGC-TOB-SXT (1/225 carcasses E. coli); ix) CIP-TOB-SXT (1/240 fecal E. coli); (x) GEN-AUGC-SXT (5/243 fecal E. coli); (xi) MERO-AMI-AZT (1/243 fecal E. coli); (xii) CIP-AUGC-SXT (1/243 fecal E. coli); (xiii) CIP-TGC-SXT (1/225 carcasses E. coli).
Moreover, other antimicrobial patterns were evaluated along the food chain (feces, carcasses, meat products). SXT, TOB, CIP, AUGC, SXT-TOB, GEN-TOB, GEN-TOB-SXT, and AMI-GEN-TOB-SXT were considered along the same food chains (Table 1) or in groups of animals that showed the same resistance profile along the food chains evaluated (Table 2). Considering the 12 pork food chains reported in Table 1, phylogenetic analysis was performed on all the E. coli.

Table 1.
E. coli AMR pattern isolated in the entire food chain (feces, carcasses, and pork meat products of the same pig). The enumeration of “Pig” is independent and related to each farm.

Table 2.
E. coli AMR pattern detected in groups of pigs along the different food chains. The enumeration of “Pig” is independent and related to each farm.
On farm A, resistance to SXT was detected in feces, fresh meat, and seasoned meat product isolates derived from Pig 3. Moreover, on the same farm, SXT resistance was detected in fecal, carcasses, and fresh meat E. coli of Pig 8. On farms C and G, E. coli resistant to SXT were found on each farm in carcass samples and their related fresh meat samples (Pig 44 and Pig 57, respectively).
The SXT-TOB resistance pattern was the most frequently found. In fact, on farms B and H, resistant E. coli strains were found in the carcasses and fresh meat samples of Pig 34 and Pig 9, respectively (as shown in Table 1). On farm C, one E. coli isolated from feces (Pig 27) and one from the carcass (Pig 21) have the same SXT-TOB pattern as their fresh meat isolates (Pig 27 and 21, respectively). On farm G, 2 fecal E. coli strains (Pig 51 and Pig 52) showed the same AMR pattern of their meat products (fresh meat and fermented product), and in Pig 55, SXT-TOB resistant strains were found along all the food chain. The AMI-GEN-TOB-SXT pattern was found in E. coli isolated from feces and fresh meat belonging to Pig 46 on farm F (Table 1).
As reported in Table 2, 15 groups of pigs were selected for their identical antimicrobial-resistant profile along the food chain. SXT-resistant strains belonging to the same group were identified on farm B. Resistance against TOB was detected in only one group of isolates from farm H (Table 2). CIP and AUGC resistances were detected in carcasses and fresh meat E. coli only on farm D—groups 3 and 4.
Three different groups were considered on farms A (group 3), D (group 1), and E (group 1) for their SXT-TOB resistance along the food chain (Table 2). The GEN-TOB pattern was found only in one group of samples belonging to farm C, while the GEN-TOB-SXT pattern was detected in E. coli of farms A, B, C, D, G, and H (Table 2). The AMI-GEN-TOB-SXT pattern was found in one group of samples on farm G.
Other AMR patterns were detected in E. coli isolates but were not considered for phylogenetic analysis because they did not harbor the same resistances along the food chain and/or food chain groups.
Data obtained were compared with information derived from electronic prescriptions collected for the four months before slaughtering, but no antimicrobial treatments were administrated to the pigs selected in that period.
2.3. Phylogenetic Analysis of Antimicrobial Resistant Bacteria
A total of 26 E. coli were tested on farm A, 17 isolates on farm B, 16 on farm C, 23 on farm D, 14 on farm E, 2 on farm F, 17 on farm G, and 8 on farm H. Phylogentic analysis performed by ERIC-PCR revealed the presence of 36 clusters.
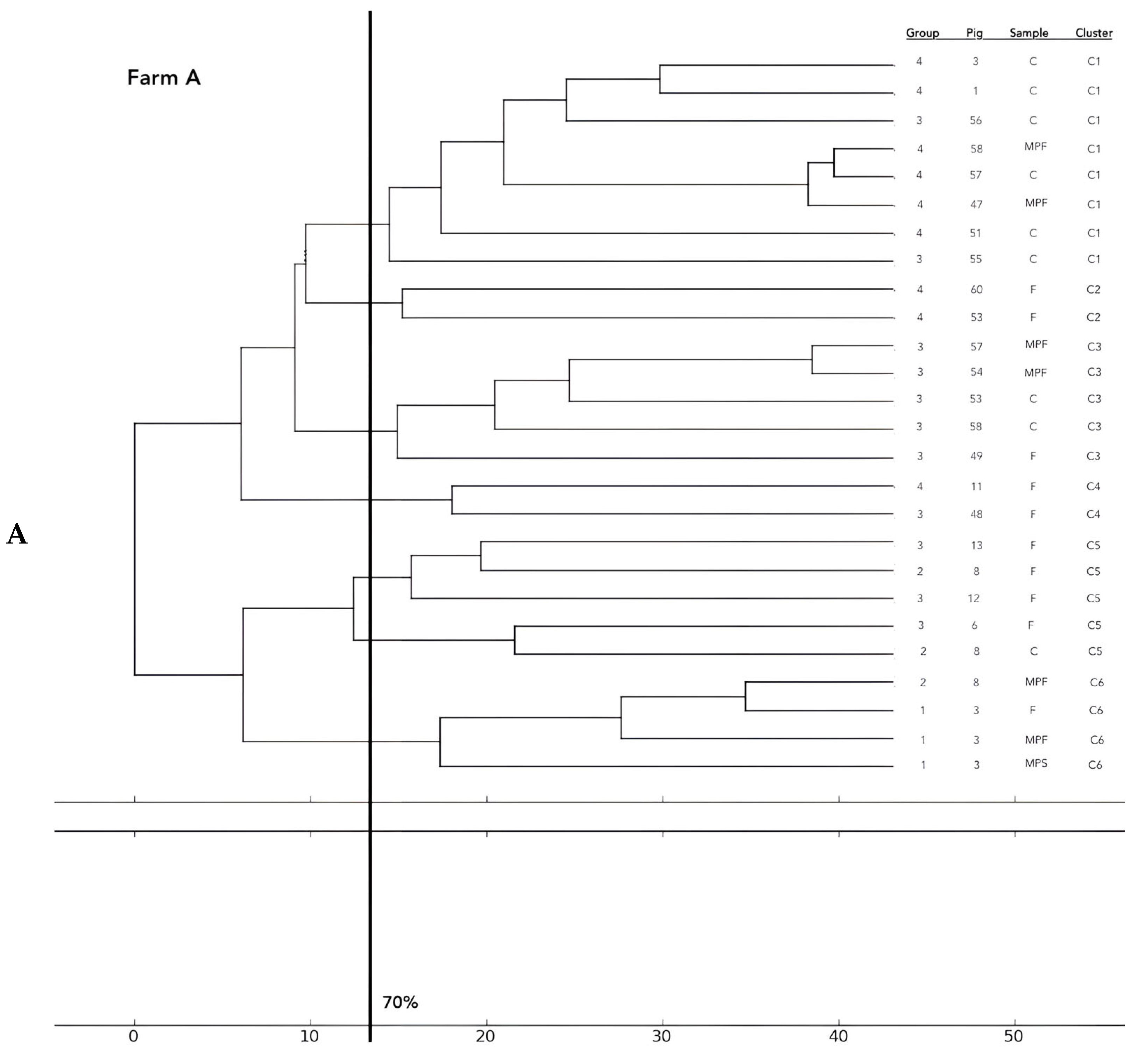
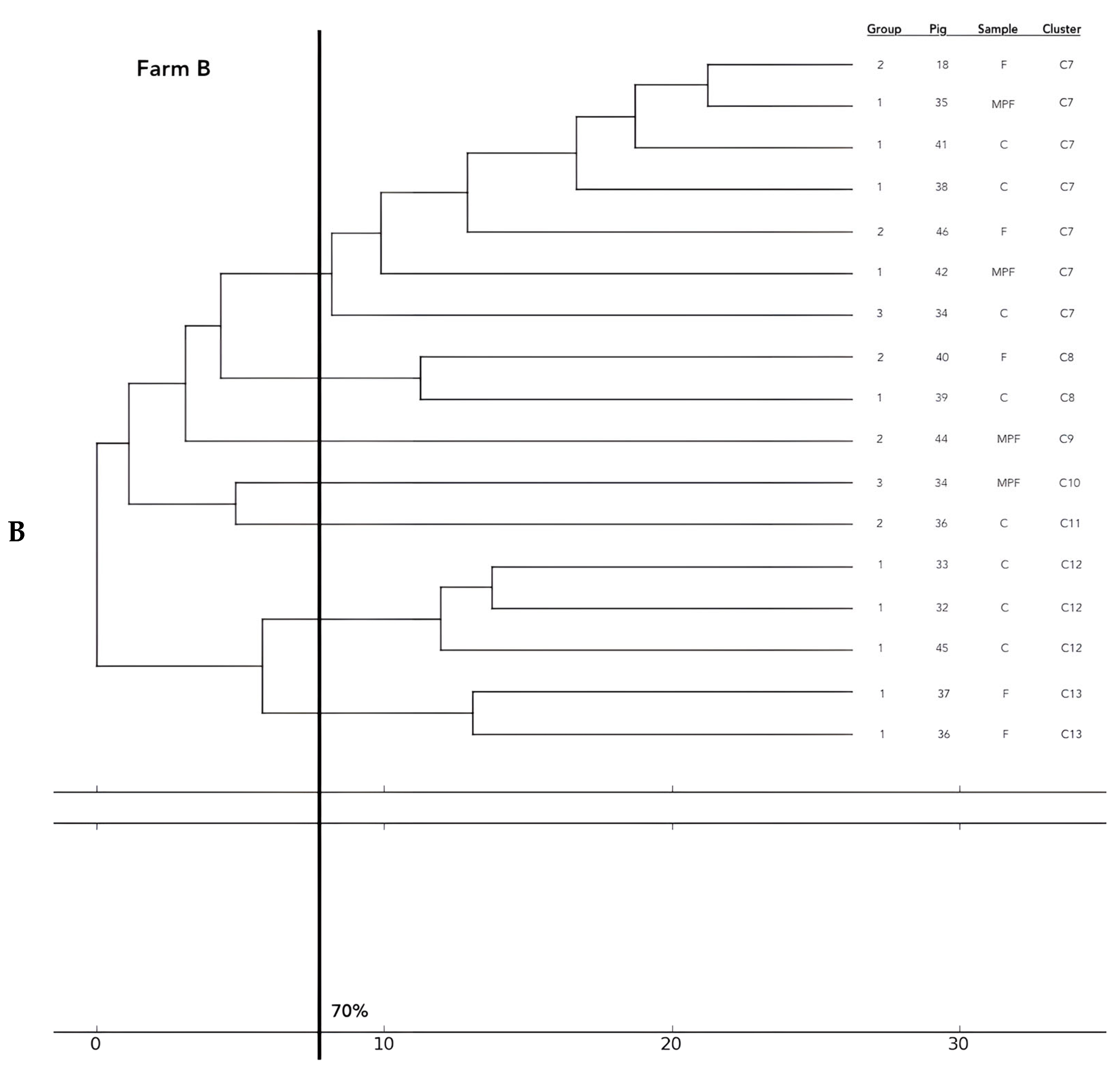
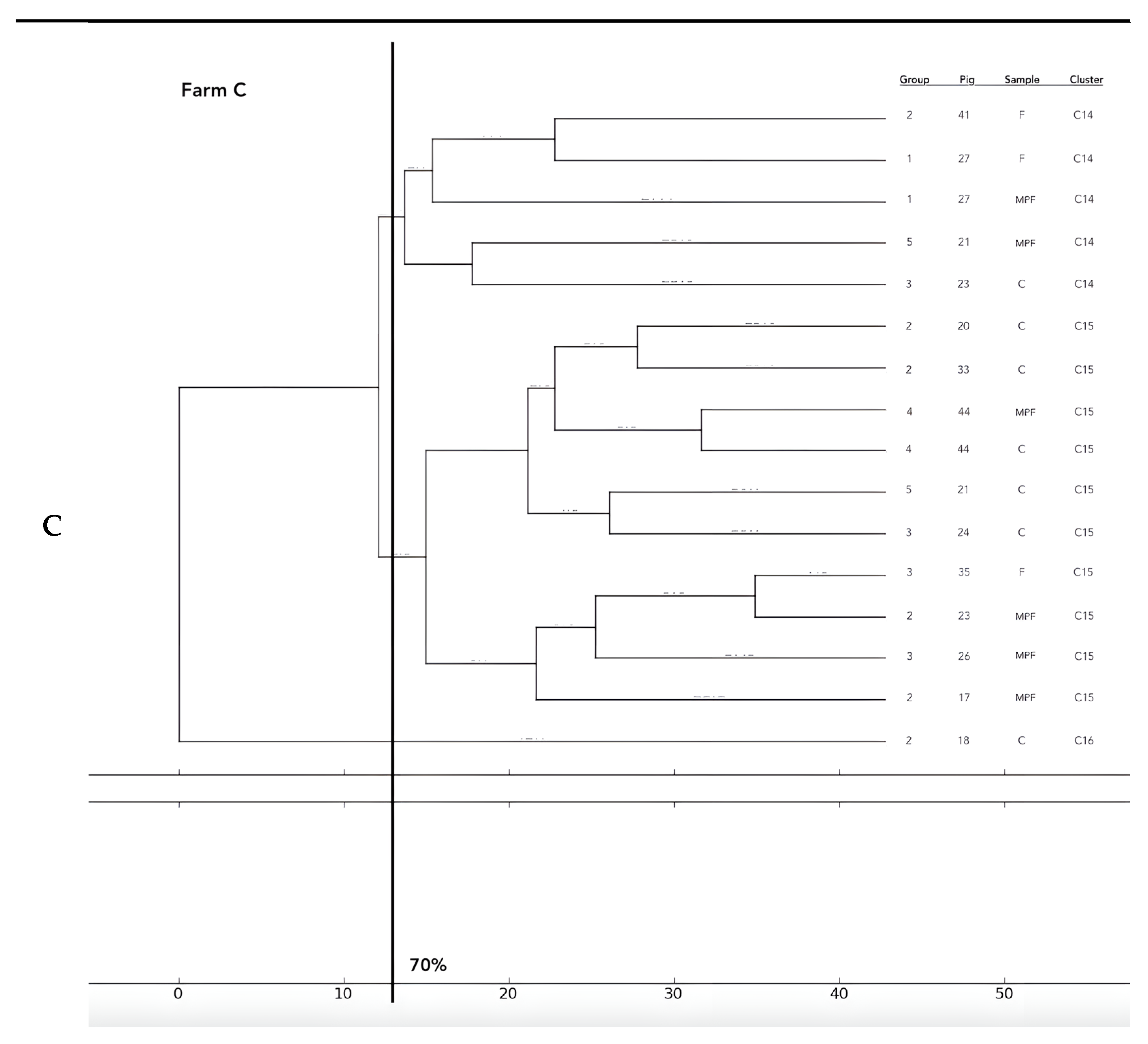
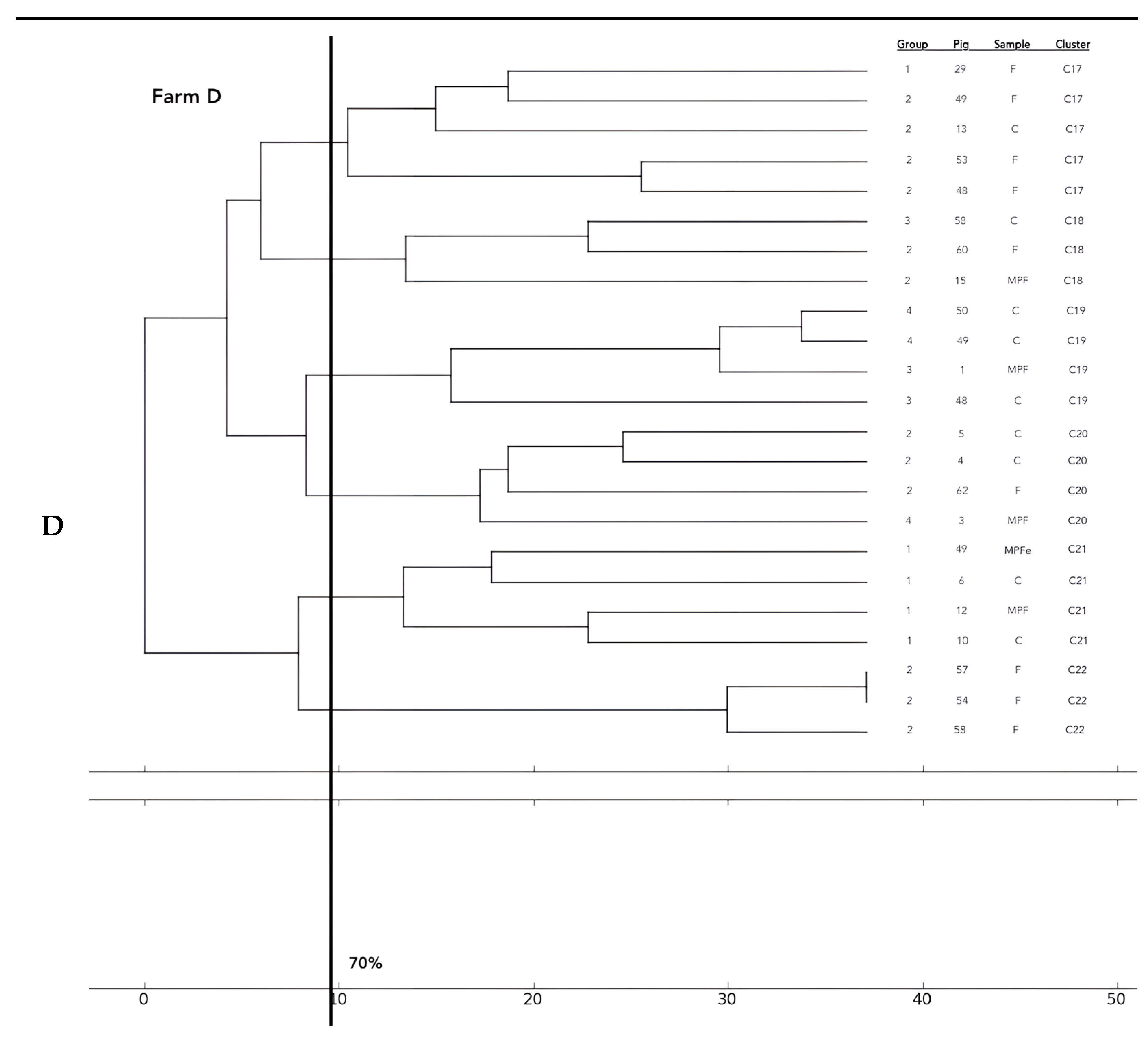
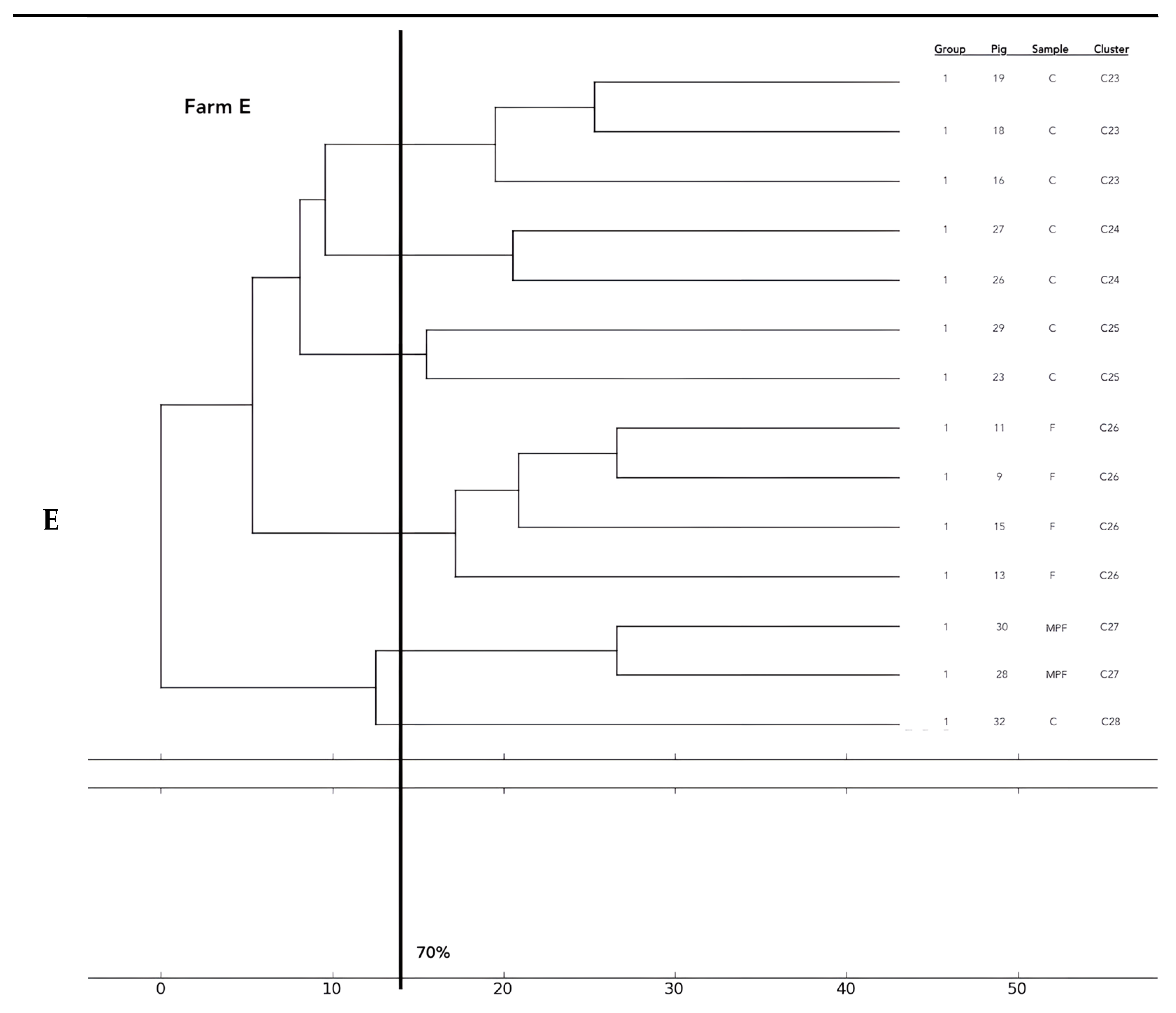
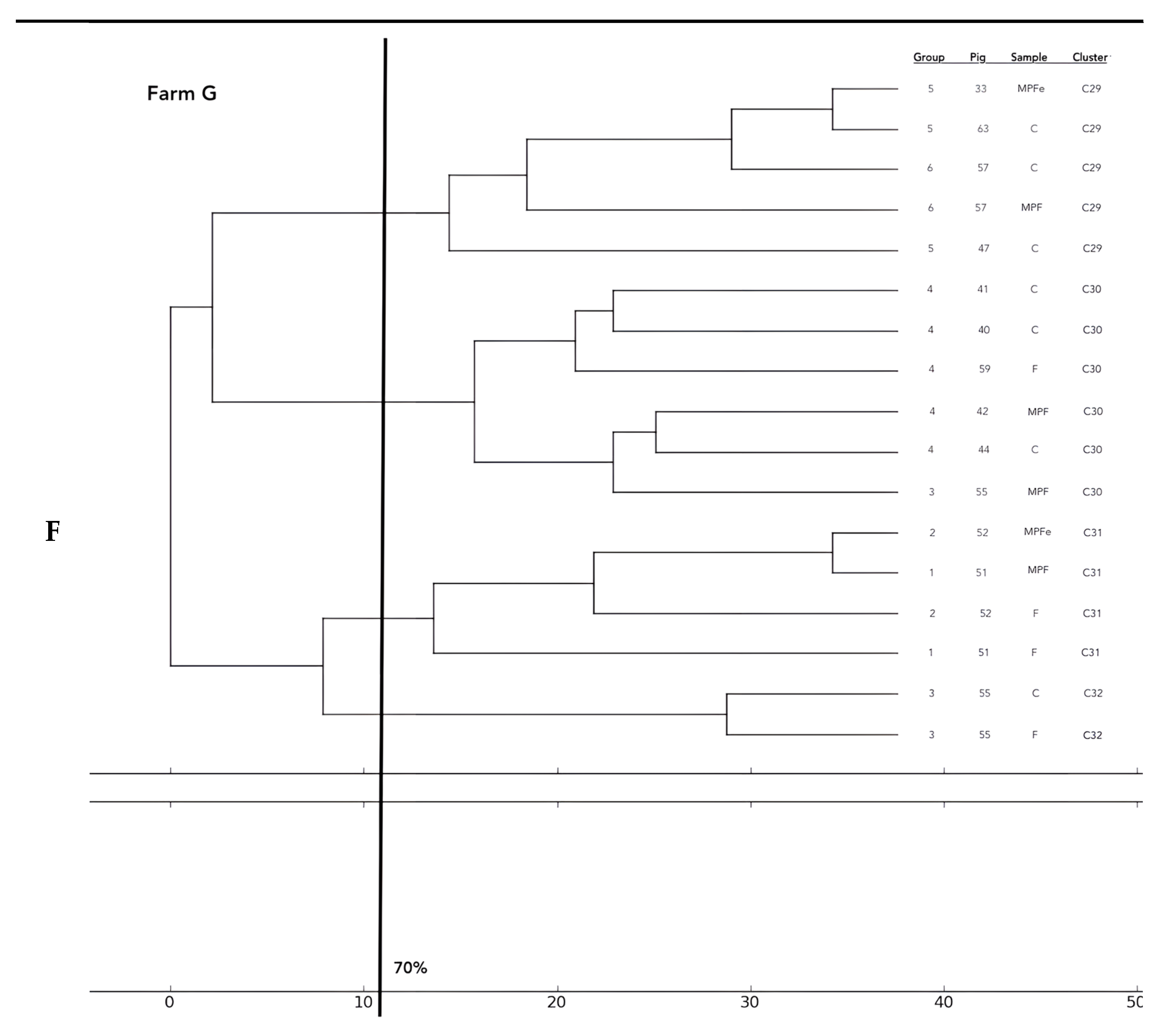
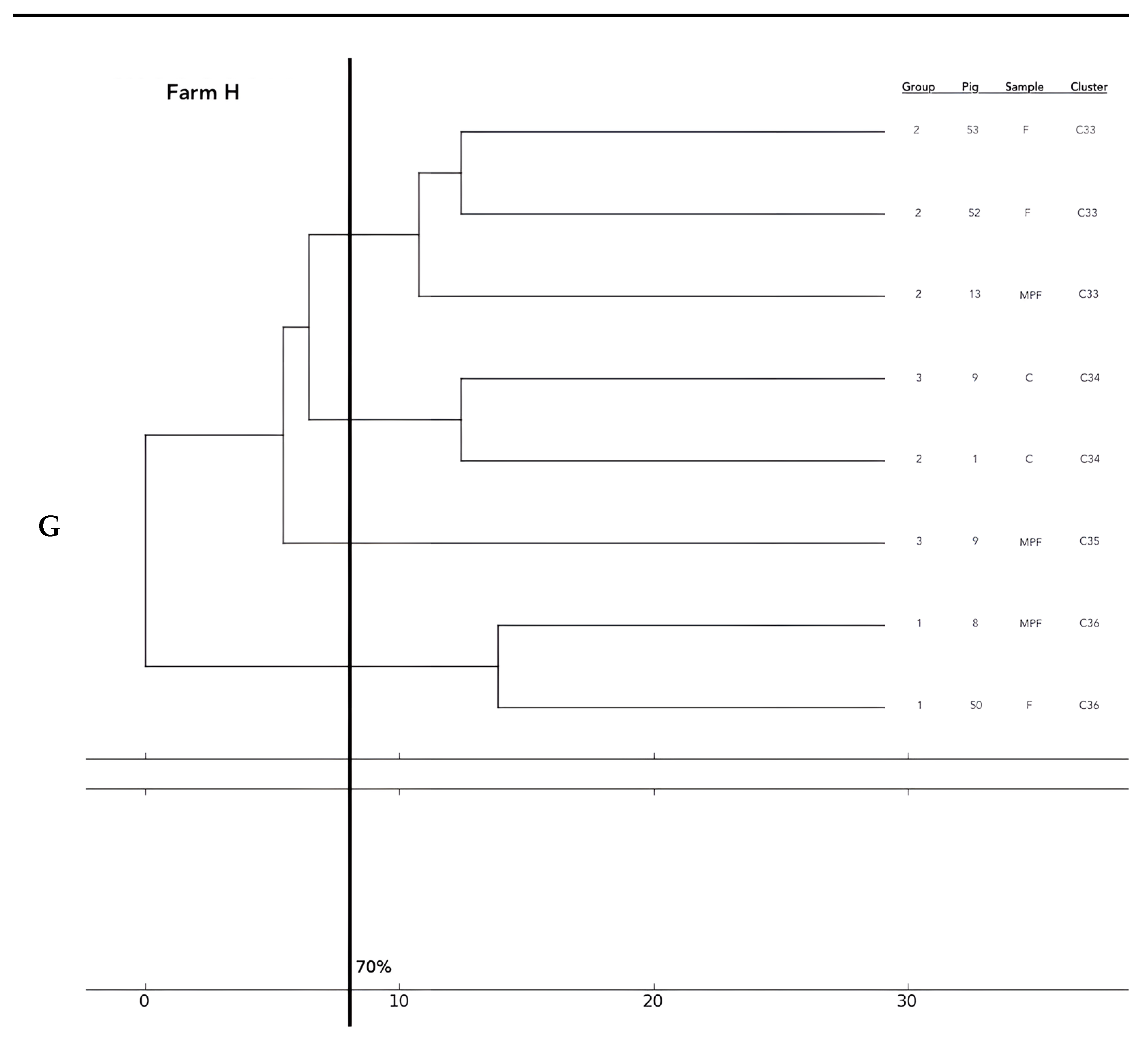
Analysis of the 12 food chains highlighted the relationship between E. coli isolated in Pig 3 (farm A) from feces, fresh meat product, and seasoned meat product in Cluster 6 (C6); in C5 and C32, only feces and carcass E. coli (Pig 8 and Pig 55, respectively) were phylogenetically related despite their related fresh meat E. coli isolates belonging to another cluster. Feces and fresh meat isolates of the same food chain were related in C14 (Pig 27) and C31 (Pig 51), where they were related to the fermented product, too (Pig 52). Carcasses and fresh meat isolates were related in C15 (Pig 44) and C29 (Pig 57). The other 4 pork food chains were not phylogenetically related (Pig 9, 21, 34). Phylogenetic relations of isolates from Pig 46 (farm F) were not calculated by the software and the UPGMA because the number of considered strains (n° 2) was not coherent with the methodology.
Considering the phylogentic analysis of 15 groups of pigs along the different food chains, E. coli isolated from feces belonging to the same groups harbored phylogenetic similarities in 6/36 clusters (C 2, 5, 7, 13, 22, 26). Moreover, isolates from carcasses of the same groups belonged to the same cluster in 6/36 cases (C1, 12, 19, 23, 24, 25), and E. coli of meat products from the same groups showed genetic similarities in only one group (C 27). Frequently, phylogenetic similarities were found in E. coli from carcasses and meat products of the same groups (C1, 7, 15, 19, 21, 29) and, in 3/36 cluster (C 3, 15, 30), E. coli isolated from feces, carcasses and meat products of the same groups were similar. In 2/36, cluster similarities were found in fecal and carcasses E. coli of the same groups (C 17, 20) and in 3/36 in fecal and fresh meat products isolates (C 18, C 33, C 36). All data are reported in Figure 2.







Figure 2.
Phylogenetic relations of resistant E. coli highlighted in 11 pork food chains and 15 groups of food chain analysis (F = feces, C = carcasses, MPF = fresh meat product, MPFe = fermented meat product, MFS= seasoned meat product). Each subfigure (from (A) to (G)) represent the phylogenetic E. coli similarities in the different pig farms selected.
3. Discussion
Commensal bacteria in animals are currently recognized as a reservoir of AMR and, at the same time, as a source of AMR transmission. However, the role of longitudinal transmission of those bacteria directly from livestock to humans through meat products is still poorly understood [22]. This study monitored the AMR pattern and phylogenetic relations in E. coli isolated from feces, carcasses, and meat products following the pigs along the entire food-producing chain, evaluating the risk of AMR transmission to consumers. According to Commission Implementing Decision 2013/652/EU [23], AMR monitoring in indicator commensal E. coli is mandatory in the major domestic producing animal populations and their derived meat. Specific monitoring of extended-spectrum-β-lactamase (ESBL)-, AmpC-, and carbapenemase-producing indicator commensal E. coli is also required. According to the EFSA/ECDC 2022 report [24], resistance to ampicillin, sulfamethoxazole, trimethoprim, and tetracycline is high in all European animal categories, frequently causing the development of MDR bacterial profile. Results highlighted in the present study confirm those data, particularly for sulphamethoxazole-resistant E. coli, although high levels of resistance to aminoglycosides were also found (GEN and TOB). In fact, in previous studies, aminoglycoside-resistant E. coli were frequently detected in pork at slaughter associated with penicillin and tetracycline resistances [25,26]. As reported in other European countries, resistance to colistin, azithromycin, cefotaxime, and ceftazidime is less common, particularly to meropenem. Notably, in this study, only one meropenem-resistant strain isolated from feces was found, while no strain was detected Europe-wide [24]. The MDR E. coli pattern is frequently characterized by tetracycline, ampicillin, sulphamethoxazole, and trimethoprim across Europe [24]. In this study, the most frequently highlighted pattern included AUGC-GEN-TOB-SXT and MDR strains detected in feces and carcasses. Fortunately, no MDR strains were isolated from pork meat products. Bacterial isolation along the food production chain in the present study was essential to evaluate the farm-to-fork involvement in disseminating antimicrobial-resistant bacteria. Phylogenetic analysis allowed us to divide strains into 36 clusters and to understand their phylogenetic similarities along the food chain. Data showed that 50% of phenotypical AMR observed along the pork food chain were related phylogenetically. The contamination of fresh meat, in half of the cases, is not directly related to contamination from feces or carcasses. Several studies have reported the transmission from livestock and/or retail meat to humans of ESBL and AmpC β-lactamases plasmid-related genes harbored by E. coli strains [26]. Linkages between poultry meat, pork, and humans were detected in the USA. Clones of gentamicin and vancomycin-resistant genes by Enterococcus spp. were detected in the feces of healthy humans in Europe and the USA [27,28]. Despite this, some similarities were found in isolates in feces and carcasses. The analysis of groups along the different food chains showed that the most frequent relation was found between E. coli isolated from carcasses and meat products of the same groups (16.7%). The frequent relations found between E. coli isolated from feces (16.7%) and E. coli from carcasses of the same groups (16.7%) highlight possible cross-contamination during farming and processing at slaughter, respectively. In group analysis, phylogenetic similarities from farm-to-fork were detected in the 3/36 cluster (8.3%). Pork can be a reservoir of antimicrobial-resistant bacteria that can be transferred intra and inter-species [25]. This study showed that in the eight selected farms, antimicrobial-resistant bacteria were easily found along the entire food chain, from farm to slaughterhouse to meat products. It is well known that food can be a source of transmission for pathogens. The high demand for ready-to-eat foods and raw or inadequately cooked meals amplifies this phenomenon [29]. To the author’s knowledge, the present study is the first to report a food chain analysis following the same animals directly from farm to meat product. It is necessary to highlight that processed meat products were not frequently found as antimicrobial-resistant E. coli carriers. This suggests that, rather than fresh meat, proper seasoning of meat products is still a good method to reduce bacterial load. The study, however, could not evaluate any potential cross-contamination during the handling and processing of food by consumers, which can be an added risk factor [30,31]. At the same time, the contribution of food in the transmission of live bacterial strains (both commensal and pathogen) or resistance genes to humans is still poorly established and underestimated worldwide [14,32]. AMR surveillance in the pig production chain has provided evidence of genetic fingerprint similarities for human nosocomial infection using multi-locus sequence typing (MLST) and whole-genome sequencing [33,34]. In conclusion, this study highlighted the phylogenetic similarities in E. coli isolated in all food products, particularly fresh meat. Fresh meat showed a higher risk of AMR transmission than seasoned and fermented meat products. Isolates from seasoned products in this study had a few AMR phenotypic profiles and fewer phylogenetically similarities with isolates obtained from other pork products along the food production chain. Consumers’ awareness of the antimicrobial resistance phenomenon and its possible spread from animals to humans is low. Less than half the consumer population in Europe identified the consumption of meat products as a transfer route of antibiotic-resistant bacteria from animals to humans [35,36,37]. For those reasons, further scientific evidence must be reported.
4. Materials and Methods
4.1. Sample Collection
Samples were collected from eight different farms (A, B, C, D, E, F, G, H) located in the Emilia Romagna region, North Italy, from 2020 to 2022. Samples included pig feces, pig carcasses, and pork meat products (fresh, seasoned, and fermented meat). Fecal samples were first collected using fecal swabs, and 30 pigs per farm were selected except for farms B and E, where 32 pigs were sampled, and farm H, where 31 pigs were sampled. Each pig was marked with an ear tag and followed along the food production chain. Fecal samples were collected at least 30/40 days before slaughter. The use of antimicrobial treatments on animals was monitored for four months before slaughtering by electronic prescription collection to compare the AMR found and the antimicrobial usage on the selected farms.
The same pigs (30 pigs per farm) were followed to slaughterhouses, and carcass samples were collected using pre-wetted sponges, following ISO 17604:2015 [38] and Reg. CE 2073/05 [39] after the evisceration and half-carcass portioning. Some carcasses could not be sampled for organizational reasons. At the slaughterhouses, fresh meat samples were collected at the end of carcass sampling. Meat products were sampled only after the fecal swab processing and resulting evaluation: pigs that harbored resistant fecal E. coli were considered for meat sampling. Only a few of the food chains identified could be sampled for organizational reasons. This procedure was set up so as not to interfere excessively with the production chain. A portion (at least 25 g) was collected as fresh meat, and others were destinated for food transformation. Samples were analyzed at the end of seasoning (coppa, pancetta) and fermentation (salami) processes, which last from 30 to 70 days. The total number of samples collected was 245 fecal swabs, 225 carcass sponges, 62 meat samples, 15 seasoned products, and 7 fermented products. Fermented products did not correspond to one single pig because production foresees using parts of different carcasses together. All the samples were sent to the laboratory of Food Hygiene and Inspection of the Veterinary Science Department, University of Parma.
4.2. Escherichia coli Isolation
E. coli isolation was characterized by a sample enrichment phase, an isolation phase, and an identification phase following UNI EN ISO 16649-2:2001 [40]. Fecal swabs were enriched via adding 9 mL of Buffered Peptone Water (BPW; Biolife Italiana, Milan, Italy) to the swab in a stile tube and incubated at 37 °C overnight. The enrichment phase of carcass sponges was done by adding the sponge with 225 mL of BPW to a sterile bag and overnight incubation at 37 °C. For fresh meat, seasoned and fermented products, 25 g of representative cross-sections of the samples were incubated at 37 °C overnight in a sterile bag with 225 mL of BPW. The isolation and identification phases were analogous for all the different sample matrices. Using a sterile calibrated handle, the broth culture was streaked onto Triptone Bile X-gluc (TBX; Biolife Italiana, Milan, Italy) agar and incubated at 42 °C overnight. A typical colony was selected and subjected to an indole test. The indole positive colonies were finally confirmed as E. coli using the conventional miniaturized API 20E system (bioMérieux, Marcy l’Etoile, France).
4.3. Antimicrobial Resistance Evaluation
All E. coli were tested for susceptibility to a set of molecules on Sensititre plates™ (Thermofisher Scientific, Milan, Italy), defining the Minimal Inhibitory Concentration (MIC) following the manufacturer’s instructions. The bacterial suspension inoculated was 5 × 105 CFU/mL, and plates were incubated at 35 ± 1 °C for 18 ± 2 h as defined by European Committee on antimicrobial susceptibility testing (EUCAST), 2020 [41].
Each plate was customized with the following:
- -
- β-lactams: meropenem (MERO: Sensible (S) ≤ 0.25–Resistant (R) > 8), piperacillin/tazobactam (P/T4: S ≤ 8–R > 16), amoxicillin/clavulanic (AUGC: S < 8–R > 8), ceftolozane/tazobactam (C/T4: S ≤ 1–R > 1), cefotaxime (FOT: S < 1–R > 2), ceftazidime (TAZ: S < 1–R > 4), ceftazidime/tazobactam (CZA: S ≤ 8–R > 8), imipenem (IMI: S≤ 2–R > 8), ertapenem (ETP: S≤ 0.5–R > 1); aztreonam (AZT: S ≤ 1–R > 4);
- -
- aminoglycosides: amikacin (AMI: S ≤ 8–R > 16), gentamicin (GEN: S ≤ 2–R > 4), tobramycin (TOB: S ≤ 2–R > 4);
- -
- quinolones: ciprofloxacin (CIP: S ≤ 0.25–R > 0.5);
- -
- polymixin: colistin (COL: S ≤ 2–R > 2);
- -
- glycylcyclines: tigecycline (TGC: S ≤ 1–R > 2);
- -
- sulphonamides: sulphamethoxazole/trimethoprim (SXT: S ≤ 2–R > 4).
The optical density of bacterial growth was recorded at 620 nm by Multiskan FC Version 1.00.75 by Thermofisher Scientific. Resistance to three or more antimicrobial classes defines Multidrug Resistance (MDR) bacterial profile [4].
4.4. Phylogenetic Analysis of Antimicrobial Resistant Bacteria
Bacteria belonging to each food production chain were clustered by their AMR pattern considering their sample origin. All the strains that showed the same AMR in feces, carcasses, and meat products of the same pig or the animals originating from the same farm were considered for performing phylogenetic analysis to evaluate whether antimicrobial-resistant bacteria can be transmitted from farm-to-fork. Determination of the E. coli isolates’ phylogenetic relatedness was performed using Enterobacterial Repetitive Intergenic Consensus (ERIC-PCR) as described by Ventura et al. 2003 [42]. DNA extraction involved heating at 95 °C for 10 min. The amplification was carried out with a GoTaq G2 Flexi DNA Polymerase kit (Promega Italia S.r.l., Milan, Italy). The master mix was prepared for 25 μL of final volume reaction containing 5x Green GoTaq Flexi Buffer at a final concentration of 1×, 3 mM of MgCl2, 0.2 mM of dNTPs, and 2.5 U of GoTaq G2 Flexi DNA Polymerase. Primers ERIC-1 primer (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC-2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) [42] were added at a final concentration of 1 μM. A total of 3 μL of sample lysate was added to the reaction mixture and Nuclease Free Water to reach final volume. The PCR protocol and PCR evaluation product are reported by Ventura et al., 2003 [42]. Images were analyzed using Pyelph software 1.4 (Python Software Foundation, Wilmington, DE, USA) through the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) to draw phylogenetic trees from a distance matrix using arithmetic averages of the measure of dissimilarity. Genotyping data were evaluated based on a 70% similarity threshold.
Author Contributions
Conceptualization, C.B. and M.R.; methodology, L.A.; software, M.R.; validation, M.R.; formal analysis, M.R.; investigation, M.R. and L.A.; resources, A.P.; data curation, M.R. writing original draft preparation, M.R.; writing-review and editing, M.C.; visualization, C.B.; supervision, C.B. and S.B.; project administration, C.B.; funding acquisition, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Regione Emilia-Romagna, Italy (DGR 449/2019, DGR 912/2020).
Institutional Review Board Statement
Ethical review and approval were waived for this study since no live animal manipulation or injury was performed to collect samples needed.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are reported within the article.
Acknowledgments
The authors thank Laura Helen Kramer for the linguistic revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Infect Dis. Trop. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- George, A. Antimicrobial Resistance (AMR) in the Food Chain: Trade, One Health and Codex. Infect. Dis. Trop. Med. 2019, 4, 54. [Google Scholar] [CrossRef]
- de Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Bacci, C.; Barilli, E.; Frascolla, V.; Rega, M.; Torreggiani, C.; Vismarra, A. Antibiotic Treatment Administered to Pigs and Antibiotic Resistance of Escherichia coli Isolated from Their Feces and Carcasses. MDR 2020, 26, 1081–1089. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A Review of Current Bacterial Resistance to Antibiotics in Food Animals. Front. Microbiol. 2022, 13, 1458. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef]
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef]
- Directive 2003/99/EC of the European Parliament and of the Council. Monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. 2003, L325, pp. 31–40. Available online: http://data.europa.eu/eli/dir/2003/99/oj (accessed on 31 October 2022).
- Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.A.; Veldman, K.; Wasyl, D.; Guerra, B.; Liébana, E.; et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019, 17, e05709. [Google Scholar] [CrossRef]
- Paitan, Y. Current trends in antimicrobial resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Verraes, C.; van Boxstael, S.; van Meervenne, E.; van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.A.; van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. IJERPH 2013, 10, 2643. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microbiol. Spectr. 2016, 8. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric Food Sec 2017, 6, 51. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Critically Important Antimicrobials for Human Medicine, 6th ed.; WHO: Switzerland, Geneva, 2018; ISBN 9789241515528. [Google Scholar]
- World Health Organisation (WHO). WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA List). Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 28 February 2020).
- European Medicine Agency (EMA)- Veterinary Medicine Division. Sales Trends (MG/PCU) of Antimicrobials VMPs for Food-Producing Animals. Available online: https://www.ema.europa.eu/en/documents/report/italy-sales-trends-mg/pcu-antibiotic-veterinary-medicinal-products-food-producing-animals-2010-2021_en.pdf (accessed on 31 October 2022).
- European Commission-Directorate-General for Health and Food Safety (DG SANTE). Final Report of a Fact-Finding Mission Carried Out in Italy from 08 November 2018 to 16 November 2018 in Order to Gather Information on the Prudent Use of Antimicrobials in Animals. Ref. Ares(2019)905480; European Commission: Brussels, Belgium, 2019. Available online: https://veterinariaalimenti.sanita.marche.it/Portals/0/OldFiles/Relazione%20DG%20SANTE%20PSA%20Sardegna%2014_02_20.pdf (accessed on 31 October 2022).
- European Medicine Agency (EMA). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf (accessed on 31 October 2022).
- Otokunefor, K.; Ogugbue, C.J.; Fajoyomi, B.U. Enterobacteria Repetitive Intragenic Consensus (ERIC) as a tool for genetic charachterisation of bacterial isolates in Nigeria. Nig. J. Biotechnol. 2020, 37, 122–128. [Google Scholar] [CrossRef]
- Lugsomya, K.; Yindee, J.; Niyomtham, W.; Tribuddharat, C.; Tummaruk, P.; Hampson, D.J.; Prapasarakul, N. Antimicrobial Resistance in Commensal Escherichia coli Isolated from Pigs and Pork Derived from Farms Either Routinely Using or Not Using In-Feed Antimicrobials. Microb. Drug Resist. 2018, 24, 1054–1066. [Google Scholar] [CrossRef]
- 2013/652/EU: Commission Implementing Decision of 12 November 2013 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria (Notified under Document C(2013) 7145). 2013, L303, pp. 26–39. Available online: https://eur-lex.europa.eu/eli/dec_impl/2013/652/oj (accessed on 31 October 2022).
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 7209. [Google Scholar] [CrossRef]
- Heidemann Olsen, R.; Yan, H.; Nie, Q.; Meng, H.; Shi, L. Antimicrobial Resistance and Resistance Genes in Aerobic Bacteria Isolated from Pork at Slaughter. J. Food Prot. 2016, 79, 589–597. [Google Scholar] [CrossRef]
- Dorado-García, A.; Smid, J.H.; van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; van den Bunt, G.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: A pooled analysis. J. Antimicrob. Chemoter. 2018, 73, 339–347. [Google Scholar] [CrossRef]
- Novais, C.; Coque, T.M.; Sousa, J.C.; Peixe, L.V. Antimicrobial resistance among faecal enterococci from healthy individuals in Portugal. Clin. Microbiol. Infect. 2006, 12, 1131–1134. [Google Scholar] [CrossRef]
- Donabedian, S.M.; Thal, L.A.; Hershberger, E.; Perri, M.B.; Chow, J.W.; Bartlett, P.; Jones, R.; Joyce, K.; Rossiter, S.; Gay, K.; et al. Molecular characterization of gentamicin-resistant Enterococci in the United States: Evidence of spread from animals to humans through food. J. Clin. Microbiol. 2003, 41, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Rega, M.; Carmosino, I.; Bonilauri, P.; Frascolla, V.; Vismarra, A.; Bacci, C. Prevalence of ESβL, AmpC and Colistin-Resistant E. coli in Meat: A Comparison between Pork and Wild Boar. Microorganisms 2021, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Sacher-Pirklbauer, A.; Klein-Jöbstl, D.; Sofka, D.; Blanc-Potard, A.B.; Hilbert, F. Phylogenetic groups and antimicrobial resistance genes in Escherichia coli from different meat species. Antibiotics 2021, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Rega, M.; Andriani, L.; Cavallo, S.; Bonilauri, P.; Bonardi, S.; Conter, M.; Carmosino, I.; Bacci, C. Antimicrobial Resistant E. coli in Pork and Wild Boar Meat: A Risk to Consumers. Foods 2022, 11, 3662. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Sirichokchatchawan, W.; Apiwatsiri, P.; Pupa, P.; Saenkankam, I.; Khine, N.O.; Lekagul, A.; Lugsomya, K.; Hampson, D.J.; Prapasarakul, N. Reducing the Risk of Transmission of Critical Antimicrobial Resistance Determinants From Contaminated Pork Products to Humans in South-East Asia. Front. Microbiol. 2021, 12, 689015. [Google Scholar] [CrossRef]
- Bonardi, S.; Cabassi, C.S.; Manfreda, G.; Parisi, A.; Fiaccadori, E.; Sabatino, A.; Cavirani, S.; Bacci, C.; Rega, M.; Spadini, C.; et al. Survey on Carbapenem-Resistant Bacteria in Pigs at Slaughter and Comparison with Human Clinical Isolates in Italy. Antibiotics 2022, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Ritter, G.D.; Acuff, G.R.; Bergeron, G.; Bourassa, M.W.; Chapman, B.J.; Dickson, J.S.; Opengart, K.; Salois, M.J.; Singer, R.S.; Storrs, C. Antimicrobial-resistant bacterial infections from foods of animal origin: Understanding and effectively communicating to consumers. Ann. NY Acad. Sci. 2019, 1441, 40–49. [Google Scholar] [CrossRef]
- German Federal Institute for Risk Assessment (BfR). BfR Study on Risk Perception: The Majority of German Consumers Believe That Animal Farming Is the Cause of Antimicrobial Resistance. Available online: https://www.bfr.bund.de/en/press_information/2015/03/bfr_study_on_risk_perception__the_majority_of_german_consumers_believe_that_animal_farming_is_the_cause_of_antimicrobial_resistance-192759.html (accessed on 31 October 2022).
- European Food Safety Authority (EFSA). EU Insights—Perceptions on the human health impact of antimicrobial resistance (AMR) and antibiotics use in animals across the EU. Efsa sp 2017, 14, 1183E. [Google Scholar] [CrossRef]
- EN ISO 17604:2015; Microbiology of the Food Chain-Carcass Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Switzerland, 2001.
- Regolamento (CE) n. 2073/2005 della Commissione, del 15 novembre 2005, sui criteri microbiologici applicabili ai prodotti alimentari. 2005; L388, pp. 1–26. Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj (accessed on 31 October 2022).
- EN ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). v_10.0_Breakpoint_Tables 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 31 January 2020).
- Ventura, M.; Meylan, V.; Zink, R. Identification and tracing of Bifidobacterium species by use of enterobacterial repetitive intergenic consensus sequences. Appl. Environ. Microbiol. 2003, 69, 4296–4301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).